Chemical Composition and Biological Activities of Essential Oils of Curcuma Species
Abstract
:1. Introduction
2. Volatile Components of Curcuma spp.
2.1. Curcuma longa L.
2.2. Curcuma zedoaria (Christm.) Roscoe
2.3. Curcuma aeruginosa Roxb.
2.4. Curcuma zanthorrhiza Roxb.
2.5. Curcuma aromatica Salisb.
2.6. Curcuma phaeocaulis Valeton
2.7. Curcuma amada Roxb.
2.8. Curcuma caesia Roxb.
2.9. Other Curcuma Species
3. Biological Activities of Curcuma Oils
3.1. Turmeric (C. longa) Essential Oil
3.2. Zedoary (C. zedoaria) Essential Oil
3.3. Curcuma aeruginosa Essential Oil
3.4. Curcuma zanthorrhiza Essential Oil
3.5. Wild Turmeric (Curcuma aromatica) Essential Oil
3.6. Curcuma phaeocaulis Essential Oil
3.7. Curcuma amada Essential Oil
3.8. Bioactivities of Other Curcuma Essential Oils
4. Toxicity and Safety
5. Bioactivity and Safety of Individual Key Components
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | human lung-cancer cells |
| ABTS | 2,2’-azinodi (3-ethyl benz-thiazoline sulfonic acid) diammonium salt |
| AGS | human gastric adenocarcinoma cells |
| Akt | protein kinase B |
| ASTC-a-1 | human lung-adenocarcinoma cells |
| B16 | melanoma cells |
| B16BL6 | mouse melanoma cells |
| B16F10 | melanoma cells |
| Ca Ski | human cervical cancer |
| CD14 | cluster of differentiation 14 |
| COX | Cyclooxygenase |
| CRTO | curcumin-removed turmeric oleoresin |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC50 | half maximal effective concentration |
| EO | essential oil |
| FRAP | ferric-reducing/antioxidant power |
| GRAS | generally recognized as safe |
| H1299 | human lung-cancer cells |
| HCT116 | human colon-cancer cells |
| HD | hydrodistillation |
| HDL | high-density lipoprotein |
| HeLa | human cervical-adenocarcinoma cells |
| Hep-2 | laryngeal-cancer cells |
| HepG2 | human hepatoma cell line |
| HFD | high-fat diet |
| HL-60 | human myeloid leukemia cells |
| Hs578T | breast-tumor cells |
| HSME | headspace solvent microextraction |
| HT-29 | human colorectal-cancer cells |
| i.p. | intraperitoneal |
| IC50 | median inhibitory concentration |
| IKK | IκB kinase |
| iNOS | inducible nitric oxide synthase |
| J774.2 | murine macrophages |
| K-562 | Human erythroleukemia cells |
| KB | human mouth epidermal carcinoma cells |
| L1210 | mouse lymphocytic leukemia cells |
| LC50 | median lethal concentration |
| LD100 | absolute lethal dose |
| LD50 | median lethal dose |
| LDL | low-density lipoprotein |
| LNCaP | human prostate acedocarcinoma cells |
| LPS | lipopolysaccharide |
| MCF-7 | human breast-cancer cells |
| MDA-MB-231 | human breast-cancer cells |
| MIC | Minimal inhibitory concentration |
| NDMA | N-nitrosodimethylamine |
| NF-κB | nuclear factor-kappa B |
| NG108-15 | mouse neuroblastoma cells |
| NSCLC | non-small-cell lung carcinoma cells |
| p.o. | per os (oral administration) |
| P388 | mouse leukemia cells |
| PANC-1 | pancreatic-cancer cells |
| PC-3 | prostate-tumor cells |
| PGE2 | prostaglandin E2 |
| PKC | protein kinase C |
| PLE | pressurized liquid extraction |
| ppm | parts per million |
| RAW 264.7 | mouse macrophage cells |
| RBL-2H3 | rat leukemia cells |
| SD | steam distillation |
| SE | solvent extract |
| SFE | supercritical fluid extraction |
| SiHa | human cervical-cancer cells |
| SKOV3 | human ovarian-cancer cells |
| SMMC-7721 | human hepatoma cells |
| SNU-1 | colorectal-cancer cells |
| SPME | solid phase microextraction |
| THP-1 | human monocytes |
| TNF-α | tumor necrosis factor-α |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| U-251 | human glioblastoma cells |
| U-87 | human glioblastoma cells |
| U937 | human lymphoma |
| VEGF | vascular endothelial growth factor |
| ZOI | zone of inhibition |
References
- Ravindran, P.N.; Babu, K.N.; Shiva, K.N. Botany and crop improvement of tumeric. In Turmeric The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–70. [Google Scholar]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Leong-Skornikova, J.; Newman, M. Gingers of Cambodia, Laos & Vietnam; Oxford Graphic Printers Pte Ltd.: Singapore, 2015. [Google Scholar]
- Chuakul, W.; Boonpleng, A. Ethnomedical uses of Thai Zingiberaceous plant (1). Thai. J. Phytopharm. 2003, 10, 33–39. [Google Scholar]
- Basaka, S.; Sarma, G.C.; Rangan, L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J. Ethnopharmacol. 2010, 132, 286–296. [Google Scholar]
- Jayaprakasha, G.K.; Jagan, L.; Rao, M.; Sakariah, K.K. Chemistry and biological activity of Curcuma longa. Trend Food Sci. Technol. 2005, 16, 533–548. [Google Scholar] [CrossRef]
- Mau, J.; Lai, E.Y.C.; Wang, N.P.; Chen, C.C.; Chang, C.H.; Chyau, C.C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003, 82, 583–591. [Google Scholar] [CrossRef]
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A. Curcuma zedoaria Rosc (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Sikha, A.; Harini, A.; Prakash, H. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J. Pharmacogn. Phytochem. 2015, 3, 1–4. [Google Scholar]
- Afzal, A.; Oriqat, G.; Khan, M.A.; Jose, J.; Afzal, M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J. Biol. Act. Prod. Nat. 2013, 3, 1–55. [Google Scholar]
- Krup, V.; Prakash, H.L.; Harini, A. Pharmacological activities of turmeric (Curcuma longa Linn): A review. J. Tradit. Med. Clin. Naturop. 2013, 2, 133. [Google Scholar] [CrossRef]
- Herath, H.M.I.C.; Wiyasiriwardene, T.D.C.M.K.; Premakumara, G.A.S. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhunu J. Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Chen, I.N.; Chang, C.C.; Ng, C.C.; Wang, C.Y.; Shyu, Y.T.; Chang, T.L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plants Food Hum. Nutr. 2008, 63, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Reanmongkol, W.; Subhadhirasakul, S.; Khaisombat, N.; Fuengnawakit, P.; Jantasila, S.; Khamjun, A. Investigation the antinociceptive, antipyretic and anti-inflammatory activities of Curcuma aeruginosa Roxb. extracts in experimental animals. Songklanakarin J. Sci. Technol. 2006, 28, 999–1008. [Google Scholar]
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005, 99, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Angel, G.R.; Menon, N.; Vimala, B.; Nambisan, B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014, 60, 233–238. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maielti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Weiss, E.A. Spice Crops; CAB International Publishing: Oxon, UK, 2002. [Google Scholar]
- Gopalan, B.; Goto, M.; Kodama, A.; Hirose, T. Supercritical carbon dioxide extraction of turmeric (Curcuma longa). J. Agric. Food Chem. 2000, 48, 2189–2192. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Negi, P.S.; Anandharamakrishnan, C.; Sakariah, K.K. Chemical composition of turmeric oil—A byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Z. Naturforsch. Sect. C J. Biosci. 2001, 56, 40–44. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A.N. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chem. Toxicol. 2010, 48, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S. Isolation and Identification of Bioactive Compounds from Conradina canescens Gray. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, December 2015. [Google Scholar]
- Sanghamitra, N.; Sujata, M.; Nagar, K. Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma). Indian J. Exp. Biol. 2015, 53, 406–411. [Google Scholar]
- Srinivasan, V.; Thankamani, C.K.; Dinesh, R.; Kandiannan, K.; Zachariah, T.J.; Leela, N.K.; Hamza, S.; Shajina, O.; Ansha, O. Nutrient management systems in turmeric: Effects on soil quality, rhizome yield and quality. Ind. Crops Prod. 2016, 85, 241–250. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kamazeri, T.S.A.T.; Samah, O.A.; Taher, M.; Susanti, D.; Qaralleh, H. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med. 2012, 5, 202–209. [Google Scholar] [CrossRef]
- Theanphong, O.; Mingvanish, W.; Kirdmanee, C. Chemical constituents and biological activities of essential oil from Curcuma aeruginosa Roxb. rhizome. Bull. Heal. Sci. Technol. 2015, 13, 6–16. [Google Scholar]
- Srivilai, J.; Waranuch, N.; Tangsumranjit, A.; Khorana, N.; Ingkaninan, K. Germacrone and sesquiterpene-enriched extracts from Curcuma aeruginosa Roxb. increase skin penetration of minoxidil, a hair growth promoter. Drug Deliv. Transl. Res. 2018, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.B.; Ahmad, A.S.; Ali, N.A.M.; Ahmad, A.R.; Ibrahim, H. Chemical composition of the rhizome oils of four Curcuma species from Malaysia. J. Essent. Oil Res. 1999, 11, 719–723. [Google Scholar] [CrossRef]
- Sirat, M.H.; Jamil, S.; Hussain, J. Essential oil of Curcuma aeruginosa Roxb. from Malaysia. J. Essent. Oil Res. 1998, 10, 453–458. [Google Scholar] [CrossRef]
- Simoh, S.; Zainal, A. Chemical profiling of Curcuma aeruginosa Roxb. rhizome using different techniques of solvent extraction. Asian Pac. J. Trop. Biomed. 2015, 5, 412–417. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Puschmann, C.; Shafi, M.P.; Geetha Nambiar, M.K. Essential oil analysis of Curcuma aeruginosa Roxb. leaves from South India. J. Essent. Oil Res. 2000, 12, 47–49. [Google Scholar] [CrossRef]
- Dũng, N.X.; Tuyêt, N.T.B.; Leclercq, P.A. Characterization of the leaf oil of Curcuma aeruginosa Roxb. from Vietnam. J. Essent. Oil Res. 1995, 7, 657–659. [Google Scholar] [CrossRef]
- Theanphong, O. Chemical constituents and antioxidant activities of essential oils from roots and rhizomes of Curcuma alismatifolia Gagnap. from Thailand. J. Appl. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Singh, G.; Singh, O.P.; Maurya, S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Prog. Cryst. Growth Charact. Mater. 2002, 45, 75–81. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Sundaresan, V.; Chauhan, A.; Chanotiya, C.S.; Yadav, A. Volatile terpenoid compositions of leaf and rhizome of Curcuma amada Roxb. from northern India. J. Essent. Oil Res. 2013, 25, 17–22. [Google Scholar] [CrossRef]
- Choudhury, S.N.; Rabha, L.C.; Kanjilal, P.B.; Ghosh, A.C.; Leclercq, P.A. Essential oil of Curcuma amada Roxb. from northeastern India. J. Essent. Oil Res. 1996, 8, 79–80. [Google Scholar] [CrossRef]
- Mustafa, A.; Ali, M.; Khan, N.Z. Volatile oil constituents of the fresh rhizomes of Curcuma amada Roxb. J. Essent. Oil Res. 2005, 17, 490–491. [Google Scholar] [CrossRef]
- Rao, A.S.; Rajanikanth, B.; Seshadri, R. Volatile aroma components of Curcuma amada Roxb. J. Agric. Food Chem. 1989, 37, 740–743. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, S.K.; Shah, N.C. Constituents of the rhizome essential oil of Curcuma amada Roxb. from India. J. Essent. Oil Res. 2001, 13, 63–64. [Google Scholar] [CrossRef]
- Srivastava, S.; Chitranshi, N.; Srivastava, S.; Dan, M.; Rawat, A.K.S.; Pushpangadan, P. Pharmacognostic evaluation of Curcuma aeruginosa Roxb. Nat. Prod. Sci. 2006, 12, 162–165. [Google Scholar]
- Thongkhwan, P.; Chaibunga, T.; Kwanboonjan, H.; Theanphong, O. Essential oil constituents of the fresh root and rhizome of Curcuma angustifolia Roxb. from Thailand. Bull. Heal. Sci. Technol. 2017, 15, 52–53. [Google Scholar]
- Jena, S.; Ray, A.; Banerjee, A.; Sahoo, A.; Nasim, N.; Sahoo, S.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S. Chemical composition and antioxidant activity of essential oil from leaves and rhizomes of Curcuma angustifolia Roxb. Nat. Prod. Res. 2017, 31, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, A.K.; Sperkova, J.; Leclercq, P.A. Essential oils of Curcuma aromatica Salisb. from northeast India. J. Essent. Oil Res. 1999, 11, 537–540. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Yang, Z.; Chen, F.; Zheng, X.; Liu, X. Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crops Prod. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Choudhury, S.N.; Ghosh, A.C.; Saikia, M.; Choudhury, M.; Leclercq, P.A. Volatile constituents of the aerial and underground parts of Curcuma aromatica Salisb from India. J. Essent. Oil Res. 1996, 8, 633–638. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Xi, L.; Yang, Y.; Wang, X.; Lei, D.; Zheng, X.; Liu, X. Phytochemical profiles and bioactivities of essential oils extracted from seven Curcuma herbs. Ind. Crops Prod. 2018, 111, 298–305. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Huang, S.J.; Chyau, C.C.; Tsai, C.H.; Weng, C.C.; Mau, J.L. Composition and antioxidant properties of essential oils from Curcuma rhizome. Asian J. Arts Sci. 2011, 2, 57–66. [Google Scholar]
- Nampoothiri, S.V.; Philip, R.M.; Kankangi, S.; Kiran, C.R.; Menon, A.N. Essential oil composition, α-amylase inhibition and antiglycation potential of Curcuma aromatica Salisb. J. Essent. Oil Bear. Plants 2015, 18, 1051–1058. [Google Scholar] [CrossRef]
- Choochote, W.; Chaiyasit, D.; Kanjanapothi, D.; Rattanachanpichai, E.; Jitpakdi, A.; Tuetun, B.; Pitasawat, B. Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae). J. Vector. Ecol. 2005, 30, 302–309. [Google Scholar] [PubMed]
- Cao, J.; Qi, M.; Zhang, Y.; Zhou, S.; Shao, Q.; Fu, R. Analysis of volatile compounds in Curcuma wenyujin Y.H. Chen et C. Ling by headspace solvent microextraction-gas chromatography-mass spectrometry. Anal. Chim. Acta 2006, 561, 88–95. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Rahman, A.; Sattar, M.A.; Rahman, M.O.; Fida, H.M. Essential oil composition and antioxidant activities of Curcuma aromatica Salisb. Food Chem. Toxicol. 2010, 48, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, K.B.; Sheeja, D.B.A.; Nair, M.S.; George, V. Curcuma ecalcarata—New natural source of pinocembrin and piperitenone. Nat. Prod. Res. 2015, 29, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Zhao, N.N.; Liu, C.M.; Zhou, L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Curcuma wenyujin rhizomes active against Liposcelis bostrychophila Badonnel. Molecules 2012, 17, 12049–12060. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Chowdhury, A.R. Volatile constituents of the rhizome oil of Curcuma caesia Roxb. from central India. Flavor Fragr. J. 2003, 18, 463–465. [Google Scholar] [CrossRef]
- Behura, S.; Srivastava, V.K. Essential oils of leaves of Curcuma species. J. Essent. Oil Res. 2004, 16, 109–110. [Google Scholar] [CrossRef]
- Raj, G.; Baby, S.; Dan, M.; Thaha, A.R.M.; Sethuraman, M.G.; George, V. Volatile constituents from the rhizomes of Curcuma haritha Mangaly and Sabu from southern India. Flavour Fragr. J. 2008, 23, 348–352. [Google Scholar] [CrossRef]
- Dũng, N.; Truong, P.X.; Ky, P.T.; Leclercq, P.A. Volatile constituents of the leaf, stem, rhizome, root and flower oils of Curcuma harmandii Gagnep. from Vietnam. J. Essent. Oil Res. 1997, 9, 677–681. [Google Scholar] [CrossRef]
- Malek, S.N.; Seng, C.K.; Zakaria, Z.; Ali, N.A.; Ibrahim, H.; Jalil, M.N. The essential oil of Curcuma inodora aff. Blatter from Malaysia. J. Essent. Oil Res. 2006, 18, 281–283. [Google Scholar] [CrossRef]
- Zeng, J.H.; Xu, G.B.; Chen, X. Application of the chromatographic fingerprint for quality control of essential oil from GuangXi Curcuma kwangsiensis. Med. Chem. Res. 2009, 18, 158–165. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Huang, Z.; Zhao, M.; Li, P.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; Tang, J.; et al. Variation in essential oil and bioactive compounds of Curcuma kwangsiensis collected from natural habitats. Chem. Biodivers. 2017, 14, 2017. [Google Scholar] [CrossRef] [PubMed]
- Naveen Kumar, K.; Venkataramana, M.; Allen, J.A.; Chandranayaka, S.; Murali, H.S.; Batra, H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT-Food Sci. Technol. 2016, 69, 522–528. [Google Scholar] [CrossRef]
- Chatterjee, S.; Variyar, P.S.; Gholap, A.S.; Bongirwar, D.R. Effect of γ-irradiation on the volatile oil constituents of turmeric (Curcuma longa). Food Res. Int. 2000, 33, 103–106. [Google Scholar] [CrossRef]
- Awasthi, P.K.; Dixit, S.C. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of northern India. J. Young Pharm. 2009, 1, 312–316. [Google Scholar] [CrossRef]
- Kutti Gounder, D.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Raina, V.K.; Syamsundar, S.K. Srivastava, K.V. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J. Essent. Oil Res. 2005, 17, 556–559. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.A.N.; De Lampasona, M.P. Chemical, antifungal, insecticidal and antioxidant studies on Curcuma longa essential oil and its oleoresin. Indian Perfum. 2005, 49, 441–451. [Google Scholar]
- Leela, N.K.; Tava, A.; Shafi, P.M.; John, S.P.; Chempakami, B. Chemical Composition of essential oils of turmeric (Curcuma longa L.). Acta Pharm. 2002, 52, 137–141. [Google Scholar]
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evid. Based Complement. Altern. Med. 2012, 2012, 438356. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ilyas, S.; Parveen, Z.; Javed, S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010, 22, 3153–3158. [Google Scholar]
- Naz, S.; Ilyas, S.; Jabeen, S.; Parveen, Z. Composition and antibacterial activity of the essential oil from the rhizome of turmeric (Curcuma longa L.). Asian J. Chem. 2011, 23, 1639–1642. [Google Scholar]
- Zhang, L.; Yang, Z.; Chen, F.; Su, P.; Chen, D.; Pan, W.; Fang, Y.; Dong, C.; Zheng, X.; Du, Z. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Ind. Crops Prod. 2017, 109, 60–73. [Google Scholar] [CrossRef]
- Ling, J.; Wei, B.; Lv, G.; Ji, H.; Li, S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012, 130, 229–235. [Google Scholar] [CrossRef]
- Ferreira, F.D.; Kemmelmeier, C.; Arrotéia, C.C.; Da Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.D.; Mossini, S.A.G.; Silva, E.L.; Machinski, M. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; Andréia de Souza Rodrigues dos Santos, P.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Leal, P.F.; Carvalho, J.E.; Meireles, M.A.A. Comparison of yield, composition and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem. 2003, 51, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Mostajeran, A.; Shebli, M. Curcuminoid and essential oil components of turmeric at different stages of growth cultivated in Iran. Res. Pharm. Sci. 2009, 4, 55–61. [Google Scholar]
- Marongiu, B.; Porcedda, S.; Caredda, A.; Gioannis, B.D.; Piras, A. Supercritical CO2 extraction of curcumin and essential oil from the rhizomes of turmeric (Curcuma longa L.). J. Essent. Oil Bear. Plants 2002, 5, 144–153. [Google Scholar]
- Oyemitan, I.A.; Elusiyan, C.A.; Onifade, A.O.; Akanmu, M.A.; Oyedeji, A.O.; McDonald, A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in southwest Nigeria. Toxicol. Rep. 2017, 4, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Belletti, N.; Ndagijimana, M.; Guerzoni, M.E.; Tchoumbougnang, F.; Zollo, P.H.A.; Micci, C.; Lanciotti, R.; Kamdem, S.L.S. Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria monocytogenes, Salmonella enteritidis and Staphylococcus aureus. Afr. J. Microbiol. Res. 2009, 3, 264–271. [Google Scholar]
- Sharma, R.K.; Mishra, B.P.; Sharma, T.C.; Bordloi, A.K.; Pathak, M.G.; Leclercq, P.A. Essential oil of Curcuma longa L. from Bhutan. J. Essent. Oil Res. 1997, 9, 589–592. [Google Scholar] [CrossRef]
- Chane-Ming, J.; Vera, R.; Chalchat, J.-C.; Cabassu, P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251. [Google Scholar] [CrossRef]
- Usman, L.A.; Hamid, A.A.; George, O.C.; Ameen, O.M.; Muhammad, N.O.; Zubair, M.F.; Lawal, A. Chemical composition of rhizome essential oil of Curcuma longa L. growing in north central Nigeria. World J. Chem. 2009, 4, 178–181. [Google Scholar]
- Raina, V.K.; Srivastava, S.K.; Jain, N.; Ahmad, A.; Syamasundar, K.V.; Aggarwal, K.K. Essential oil composition of Curcuma longa L. cv. Roma from the plains of northern India. Flavour Fragr. J. 2002, 17, 99–102. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Gonçalves, M.J.; Da Cunha, A.P.; Vila, R.; Canigueral, S.; Mazzoni, V.; Tomi, F.; Casanova, J. Essential oil composition and antimicrobial activity of three zingiberaceae from S. Tomé e Principe. Planta Med. 2001, 67, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Jamil, S.; Rahman, A.A. Rhizome oil of Curcuma ochrorhiza Val. J. Essent. Oil Res. 1997, 9, 351–353. [Google Scholar] [CrossRef]
- Sindhu, S.; Chempakam, B.; Leela, N.K.; Suseela Bhai, R. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem. Toxicol. 2011, 49, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.N.; Mengi, N.; Patra, N.K.; Charles, R.; Kumar, S. Chemical examination of the leaf essential oil of Curcuma longa L. from the north Indian plains. Flavour Fragr. J. 2002, 17, 103–104. [Google Scholar] [CrossRef]
- Oguntimein, B.O.; Weyerstahl, P.; Marschall-Weyerstahl, H. Essential oil of Curcuma longa L. leaves. Flavour Fragr. J. 1990, 5, 80–90. [Google Scholar] [CrossRef]
- Priya, R.; Prathapan, A.; Raghu, K.G.; Nirmala Menon, A. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L. leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S695–S699. [Google Scholar] [CrossRef]
- Pande, C.; Chanotiya, C.S. Constituents of the leaf oil of Curcuma longa L. from Uttaranchal. J. Essent. Oil Res. 2006, 18, 166–167. [Google Scholar] [CrossRef]
- Essien, E.; Newby, J.; Walker, T.; Setzer, W.; Ekundayo, O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in southern Nigeria. Medicines 2015, 2, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Zaibunnisa, A.H.; Norashikin, S.; Mamot, S.; Osman, H. An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurised liquid extraction (PLE). LWT-Food Sci. Technol. 2009, 42, 233–238. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Londero, É.P.; Bressan, C.A.; Salbego, J.; Gressler, L.T.; Silva, L.V.F.; Mourão, R.H.V.; Oliveira, R.B.; Llesuy, S.F.; Baldisserotto, B.; et al. Oxidative and biochemical responses in Brycon amazonicus anesthetized and sedated with Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. essential oils. Vet. Anaesth. Analg. 2017, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Dũng, N.X.; Tuyecirct, N.T.B.; Leclercq, P.A. Constituents of the leaf oil of Curcuma domestica L. from Vietnam. J. Essent. Oil Res. 1995, 7, 701–703. [Google Scholar] [CrossRef]
- Wahab, I.R.; Blagojević, P.D.; Radulović, N.S.; Boylan, F. Volatiles of Curcuma mangga Val. & Zijp (Zingiberaceae) from Malaysia. Chem. Biodivers. 2011, 8, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Chong, T.C.; Chee, S.G. Essential oil of Curcumma mangga Val. and Van Zijp. rhizome. J. Essent. Oil Res. 1999, 11, 349–351. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Wei, J.; Su, P.; Pan, W.; Zheng, X.; Zhang, K.; Lin, L.; Tang, J.; Fang, Y.; et al. Essential oil composition and bioactivity variation in wild-growing populations of Curcuma phaeocaulis Valeton collected from China. Ind. Crops Prod. 2017, 103, 274–282. [Google Scholar] [CrossRef]
- Dũng, N.X.; Tuyêt, N.T.B.; Van Khlên, P.; Barthel, A.; Leclercq, P.A. Chemical composition of the flower oil of Curcuma pierreana Gagnep. from Vietnam. J. Essent. Oil Res. 1998, 10, 527–528. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N.S. Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. J. Environ. Chem. Eng. 2014, 2, 2037–2044. [Google Scholar] [CrossRef]
- Hong, S.L.; Lee, G.S.; Syed Abdul Rahman, S.N.; Ahmed Hamdi, O.A.; Awang, K.; Aznam Nugroho, N.; Abd Malek, S.N. Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Theanphong, O.; Jenjittikul, T.; Mingvanish, W. The rhizome oil of Curcuma rhabdota Sirirugsa & M.F. Newman from Thailand. In Plant Science: International Proceedings; The 2nd International Conference on Advanced Pharmaceutical Research Strategies and Innovation in Pharmaceutical Research: Safety, Efficacy and Quality; Mahidol University: Bangkok, Thailand, 2015; pp. 146–150. [Google Scholar]
- Zhou, X.; Li, Z.; Liang, G.; Zhu, J.; Wang, D.; Cai, Z. Analysis of volatile components of Curcuma sichuanensis X. X. Chen by gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.M.; Ha, V.T.; Khanh, P.N.; Van, D.T.; Cuong, T.D.; Huong, T.T.; Thuy, D.T.T.; Nhan, N.T.; Hanh, N.P.; Toan, T.Q.; et al. Chemical compositions and antimicrobial activity of essential oil from the rhizomes of Curcuma singularis growing in Vietnam. Am. J. Essent. Oils Nat. Prod. 2017, 5, 20–25. [Google Scholar]
- Ky, P.T.; Van De Ven, L.J.M.; Leclercq, P.A.; Dũng, N.X. Volatile constituents of the essential oil of Curcuma trichosantha Gagnep. from Vietnam. J. Essent. Oil Res. 1994, 6, 213–214. [Google Scholar]
- Yasni, S.; Imaizumi, K.; Sin, K.; Sugano, M.; Nonaka, G. Sidik Identification of an active principle in essential oils and hexane-soluble fractions of Curcuma xanthorrhiza Roxb. showing triglyceride-lowering action in rats. Food Chem. Toxicol. 1994, 32, 273–278. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Li, J.; Cui, X.; Wang, A.; Huang, S.; Zheng, S.; Lu, Y.; Chen, W. Inhibition of vascular endothelial growth factor-mediated angiogenesis involved in reproductive toxicity induced by sesquiterpenoids of Curcuma zedoaria in rats. Reprod. Toxicol. 2013, 37, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.Y.C.; Chyau, C.-C.; Mau, J.-L.; Chen, C.-C.; Lai, Y.-J.; Shih, C.-F.; Lin, L.-L. Antimicrobial activity and cytotoxicity of the essential oil of Curcuma zedoaria. Am. J. Chin. Med. 2004, 32, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.; Kapoor, I.P.S.; Singh, G.; Isidorov, V.; Szczepaniak, L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013, 3, 42–48. [Google Scholar] [CrossRef]
- Purkayastha, J.; Nath, S.C.; Klinkby, N. Essential oil of the rhizome of Curcuma zedoaria (Christm.) Rosc. native to northeast India. J. Essent. Oil Res. 2006, 18, 154–155. [Google Scholar] [CrossRef]
- Jarikasem, S.; Thubthimthed, S.; Chawananoraseth, K.; Suntorntanasat, T. Essential oils from three Curcuma species collected in Thailand. Acta Hortic. 2005, 677, 37–41. [Google Scholar] [CrossRef]
- Shi, H.; Tan, B.; Ji, G.; Lu, L.; Cao, A.; Shi, S.; Xie, J. Zedoary oil (Ezhu You) inhibits proliferation of AGS cells. Chin. Med. 2013, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.N.; Naquvi, A.A.; Bansal, R.P.; Bahl, J.R.; Kumar, S. Chemical composition of the essential oil from the leaves of Curcuma zedoaria Rosc. of Indian origin. J. Essent. Oil Res. 2005, 17, 29–31. [Google Scholar] [CrossRef]
- Dahal, K.R.; Idris, S. Plant Resources of South-East Asia No 13. Spices; De Guzman, C.C., Siemonsma, J.S., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 111–116. [Google Scholar]
- Zachariah, T.J.; Babu, K.N. Effect of storage of fresh turmeric rhizome on oleoresin and curcumin contents. J. Spice Arom. Crop 1992, 1, 55–58. [Google Scholar]
- Jayashree, E.; John Zachariah, T. Processing of turmeric (Curcuma longa) by different curing methods and its effect on quality. Indian J. Agric. Sci. 2016, 86, 696–698. [Google Scholar]
- Govindarajan, V.S. Turmeric-Chemistry, technology and quality. CRC Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef] [PubMed]
- Helen, C.F.; Su, R.H.; Ghulam, J. Isolation, purification and characterization of insect repellents from Curcuma longa L. J. Agric. Food Chem. 1982, 30, 290–292. [Google Scholar]
- Srimal, R.C. Turmeric: A brief review of medicinal properties. Fitoterapia 1997, 68, 483–493. [Google Scholar]
- Narayana, D.B.A.; Brindavanam, N.B.; Dobriyal, R.M.; Katuyar, K.C. Indian spices: An overview with special reference to neutraceuticals. J. Med. Aromat. Plant Sci. 2000, 22, 236–246. [Google Scholar]
- Paranjpe, P. Herbs for Beauty; Chaukhambha Sanskrit Pratishthan: New Delhi, India, 2001. [Google Scholar]
- Chempakam, B.; Parthasarathy, V.A. Chemistry of Spices; Parthasarathy, V.A., Chempakam, B., Zachariah, T.J., Eds.; CABI: Oxfordshire, UK, 2008; pp. 97–123. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Safety, 2nd ed.; Elsevier: Edinburgh, UK, 2014. [Google Scholar]
- Balakrishnan, K.V. Post harvest technology and processing of turmeric. In Turmeric, The Genus Curcuma; Ravindra, P.N., Nirmal Babu, K., Sivaraman, K., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2007; Volume 45, pp. 193–256. [Google Scholar]
- Parveen, Z.; Nawaz, S.; Siddique, S.; Shahzad, K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Indian J. Pharm. Sci. 2013, 75, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.P.; Bahl, J.R.; Garg, S.N.; Naqvi, A.A.; Sushil, K.; Kumar, S. Differential chemical composition of the essential oils of the shoot organs, rhizomes and rhizoids in the turmeric Curcuma longa grown in Indo-Gangetic plains. Pharm. Biol. 2002, 40, 384–389. [Google Scholar] [CrossRef]
- Pitasawat, B.; Champakaew, D.; Choochote, W.; Jitpakdi, A.; Chaithong, U.; Kanjanapothi, D.; Rattanachanpichai, E.; Tippawangkosol, P.; Riyong, D.; Tuetun, B.; et al. Aromatic plant-derived essential oil: An alternative larvicide for mosquito control. Fitoterapia 2007, 78, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.G.; Hwang, J.C.; Kang, S.K.; Jin, U.H.; Suh, S.J.; Moon, S.K.; Kim, C.H. Suppressive effect of Zedoariae rhizome on pulmonary metastasis of B16 melanoma cells. J. Ethnopharmacol. 2005, 101, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Makabe, H.; Maru, N.; Kuwabara, A.; Kamo, T.; Hirota, M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria. Nat. Prod. Res. 2006, 20, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Navarro Dde, F.; De Souza, M.M.; Neto, R.A.; Golin, V.; Niero, R.; Yunes, R.A.; Delle Monache, F.; Filho, V.C. Phytochemical analysis and analgesic properties of Curcuma zedoaria grown in Brazil. Phytomedicine 2002, 9, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Nakamura, A.; Yoshikawa, M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 5891–5898. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.H.; Ahmad, S. Screening of some medicinal plants for antiamoebic action. Fitoterapia 1991, 62, 171–175. [Google Scholar]
- Thaina, P.; Tungcharoena, P.; Wongnawaa, M.; Reanmongkol, W.; Subhadhirasakul, S. Uterine relaxant effects of Curcuma aeruginosa Roxb. rhizome extracts. J. Ethnopharmacol. 2009, 121, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Sookchot, T. Chemotaxonomy of Medicinal and Auspicious Plants in Zingiberaceae Sold at Ban Thum, Chiang Dao District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2005. [Google Scholar]
- Suphrom, N.; Pumthong, G.; Khorana, N.; Waranuch, N.; Limpeanchob, N.; Ingkaninan, K. Anti-androgenic effect of sesquiterpenoids isolated from the rhizomes of Curcuma aeruginosa Roxb. Fitoterapia 2012, 83, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Nanda, Y.; Singson, N.; Rao, A.N. Ethnomedicinal plants of Thadou tribe of Manipur (India)-1. Pleione 2013, 7, 138–145. [Google Scholar]
- Neamsuvan, O.; Tuwaemaengae, T.; Bensulong, F.; Asae, A.; Mosamae, K. A survey of folk remedies for gastrointestinal tract diseases from Thailand’s three southern border provinces. J. Ethnopharmacol. 2012, 144, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Ghosal, M.; Das, A.P.; Mandal, P. Development of single node cutting propagation techniques and evaluation of antioxidant activities of Curcuma aeruginosa Roxburgh rhizome. Int. J. Pharm. Pharm. Sci. 2013, 5, 227–234. [Google Scholar]
- Salleh, N.M.; Ismail, S.; Ab Halim, M. Effects of Curcuma xanthorrhiza extracts and their constituents on phase II drug-metabolizing enzymes activity. Pharmacognosy Res. 2016, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chen, Y.P.; Shen, S.J.; Hsu, C.S.; Chen, C.C.; Chang, H.C. Oriental Materia Medica, A Concise Guide; Oriental Healing Arts Institute: Long Beach, CA, USA, 1986. [Google Scholar]
- Perry, M.L. Medicinal Plants of East and South East Asia, Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Mary, H.P.A.; Susheela, G.K.; Jayasree, S.; Nizzy, A.M.; Rajagopal, B.; Jeeva, S. Phytochemical characterization and antimicrobial activity of Curcuma xanthorrhiza Roxb. Asian Pac. J. Trop. Biomed. 2012, 2, S637–S640. [Google Scholar] [CrossRef]
- Salea, R.; Widjojokusumo, E.; Veriansyah, B.; Tjandrawinata, R.R. Optimizing oil and xanthorrhizol extraction from Curcuma xanthorrhiza Roxb. rhizome by supercritical carbon dioxide. J. Food Sci. Technol. 2014, 51, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Sukari, M.A.H.; Saad, S.M.; Lajis, N.H.; Rahman, M.; Muse, R.; Yusuf, U.K.; Riyanto, S. Chemical constituents and bioactivity of Curcuma aeruginosa Roxb. Nat. Prod. Sci. 2007, 13, 175–179. [Google Scholar]
- Li, S.Y.; Li, S.P. Antioxidant activities of essential oil of Curcuma longa and Curcuma wenyujin. Int. J. Essent. Oil Ther. 2009, 3, 31–34. [Google Scholar]
- Shi, J.H.; Li, C.Z.; Liu, D.L. Experimental research on the pharmacology of Curcuma aromatica volatile oil. Zhongyao Tongbao 1981, 6, 36–38. [Google Scholar]
- Chadha, Y.R. The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products; NISCOM (CSIR): New Delhi, India, 2001. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants, 1st ed.; CSIR: New Delhi, India, 1956. [Google Scholar]
- Kim, J.K.; Jo, C.; Hwang, H.J.; Park, H.J.; Kim, Y.J.; Byun, M.W. Color improvement by irradiation of Curcuma aromatica extract for industrial application. Radiat. Phys. Chem. 2006, 75, 449–452. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Zhen, H.; Zhong, X.M.; Feng, C.R.; Pan, A.J.; Li, Z.Y.; Ip, S.P.; Che, C.T. Effects of SYJN, a Chinese herbal formula, on chronic unpredictable stress-induced changes in behavior and brain BDNF in rats. J. Ethnopharmacol. 2010, 128, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Zhang, M.; Zhang, C.F.; Wang, Z.T. Diaryl derivatives from the root tuber of Curcuma longa. Biochem. Syst. Ecol. 2008, 36, 476–480. [Google Scholar] [CrossRef]
- Wu, D.L. Zingiberaceae Resource of China; Huazhong University of Science and Technology Press: Wuhan, China, 2015. [Google Scholar]
- Alonso-Amelot, M.E. Multitargeted bioactive materials of plants in the Curcuma genus and related compounds: Recent advances. Studi. Nat. Prod. Chem. 2016, 47, 111–200. [Google Scholar]
- Varadarajan, R.; Mathew, M.C.R.; Souprayan, S. Hepatoprotective efficacy of ethanolic extracts of rhizome Curcuma amada Roxb. in experimental rats. Ann. Plant Sci. 2018, 71, 1966–1972. [Google Scholar] [CrossRef]
- Policegoudra, R.S.; Aradhya, S.M.; Singh, L. Mango ginger (Curcuma amada Roxb.)—A promising spice for phytochemicals and biological activities. J. Biosci. 2011, 36, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, P.K.; Rout, S.D.; Sahu, H.K. Ethnomedicinal plant resources of Similipal Biosphere Reserve, Orissa, India. Zoo’s Print J. 2006, 21, 2372–2374. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Shrestha, K.P.; Bussmann, R.W. Traditional herbal medicine in far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomed. 2010, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, M.B.; Munzbergova, Z.; Timsina, B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J. Ethnopharmacol. 2010, 130, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.R.; Rana, V.S.; Devi, S.I.; Verdeguer, M.; Blázquez, M.A. Chemical composition and antimicrobial activity of the essential oil of Curcuma leucorhiza Roxb. J. Essent. Oil Res. 2012, 24, 533–538. [Google Scholar] [CrossRef]
- Lakshmi, S.; Padmaja, G.; Remani, P. Antitumour effects of isocurcumenol isolated from Curcuma zedoaria rhizomes on human and murine cancer cells. Int. J. Med. Chem. 2011, 1–13. [Google Scholar] [CrossRef]
- Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Rana, M.; Prakash, P.; Malasoni, R.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br. J. Nutr. 2013, 110, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Aoki, F.; Tanaka, H.; Kishida, H.; Nishiyama, T.; Okada, S.; Matsumoto, I.; Abe, K.; Mae, T. Effects of ingested turmeric oleoresin on glucose and lipid metabolisms in obese diabetic mice: A DNA microarray study. J. Agric. Food Chem. 2006, 54, 9055–9062. [Google Scholar] [CrossRef] [PubMed]
- Omosa, L.K.; Midiwo, J.O.; Kuete, V. Curcuma longa; Elsevier: Edinburgh, UK, 2012. [Google Scholar]
- Lekshmi, P.C.; Arimboor, R.; Indulekha, P.S.; Menon, A.N. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutr. 2012, 63, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.S.; Yang, B.; Lv, G.P.; Li, S.P. Free radical scavenging activity and characterization of sesquiterpenoids in four species of Curcuma using a TLC bioautography assay and GC–MS analysis. Molecules 2010, 15, 7547–7557. [Google Scholar] [CrossRef] [PubMed]
- Himaja, M.; Ranjitha, A.; Ramana, M.V.; Anand, M.; Karigar, A. Phytochemical screening and antioxidant activity of rhizome part Curcuma zedoaria. Int. J. Res. Ayurveda Pharm. 2010, 1, 414–417. [Google Scholar]
- Dohare, P.; Garg, P.; Sharma, U.; Jagannathan, N.R.; Ray, M. Neuroprotective efficacy and therapeutic window of Curcuma oil: In rat embolic stroke model. BMC Complement. Altern. Med. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Dohare, P.; Varma, S.; Ray, A.; Sharma, U.; Jaganathanan, N.R.; Ray, M. Curcuma oil: Reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008, 33, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Manhas, A.; Khanna, V.; Prakash, P.; Goyal, D.; Malasoni, R.; Naqvi, A.; Dwivedi, A.K.; Dikshit, M.; Jagavelu, K. Curcuma oil reduces endothelial cell-mediated inflammation in postmyocardial ischemia/reperfusion in rats. J. Cardiovasc. Pharmacol. 2014, 64, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Misra, A.; Surin, W.R.; Jain, M.; Bhatta, R.S.; Pal, R.; Raj, K.; Barthwal, M.K.; Dikshit, M. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb. Res. 2011, 127, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006, 97, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.N. Comparative studies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile rractions of turmeric (C. longa) and ginger (Z. officinale). Stud. Nat. Prod. Chem. 2016, 48, 101–135. [Google Scholar]
- Arora, R.; Basu, N.; Kapoor, V.; Jain, A. Anti-inflammatory studies on Curcuma longa (turmeric). Indian J. Med. Res. 1971, 59, 1289–1295. [Google Scholar] [PubMed]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jena, B.S.; Negi, P.S.; Sakariah, K.K. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: A byproduct from curcumin production. Z. Naturforsch. 2002, 57, 828–835. [Google Scholar] [CrossRef]
- Hastak, K.; Lubri, N.; Jakhi, S.D.; More, C.; John, A.; Ghaisas, S.D.; Bhide, S.V. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997, 116, 265–269. [Google Scholar] [CrossRef]
- Joshi, J.; Ghaisas, S.; Vaidya, A.; Vaidya, R.; Kamat, D.V.; Bhagwat, A.N.; Bhide, S. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J. Assoc. Physicians India 2003, 51, 1055–1060. [Google Scholar] [PubMed]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Zhang, H.; Barbara, N. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.). J. Agric. Food Chem. 2010, 58, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Suzuki, Y.; Watarable, N. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983, 49, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Rana, M.; Jain, M.; Singh, N.; Naqvi, A.; Malasoni, R.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. Curcuma oil attenuates accelerated atherosclerosis and macrophage foam-cell formation by modulating genes involved in plaque stability, lipid homeostasis and inflammation. Br. J. Nutr. 2015, 113, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Shafreen, R.B.; Lubinska, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. Human serum interactions with phenolic and aroma substances of Kaffir (Citrus hystrix) and Key lime (Citrus aurantifolia) juices. J. Lumin. 2018. [Google Scholar] [CrossRef]
- Nwozo, S.O.; Osunmadewa, D.A.; Oyinloye, B.E. Anti-fatty liver effects of oils from Zingiber officinale and Curcuma longa on ethanol-induced fatty liver in rats. J. Integr. Med. 2014, 12, 59–65. [Google Scholar] [CrossRef]
- Palasa, K.; Scsikaran, B.; Krishna, T.P.; Krishnaswamy, K. Effect of turmeric on urinary mutagens in smokers. Mutagenesis 1992, 7, 107–109. [Google Scholar] [CrossRef]
- Ferreira, L.A.F.; Henriques, O.B.; Andreoni, A.A.S.; Vital, G.R.F.; Campos, M.M.C.; Habermehl, G.G.; De Moraes, V.L.G. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon 1992, 30, 1211–1218. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef] [PubMed]
- Behura, C.; Ray, P.; Rathi, C.C.; Mishra, R.K.; Ramachandraiah, O.S.; Charyulu, J.K. Antifungal activity of essential oil of Curcuma longa against five rice pathogens in vitro. J. Essent. Oil Bear. Plants 2000, 3, 79–84. [Google Scholar]
- Apisariyakul, A.; Vanittanakom, N.; Buddhasukh, D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J. Ethnopharmacol. 1995, 49, 163–169. [Google Scholar] [CrossRef]
- Fouad, H.A.; Da Camara, C.A.G. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Roth, G.N.; Chandra, A.; Nair, M.G. Novel bioactivities of Curcuma longa constituents. J. Nat. Prod. 1998, 61, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techadamrongsin, Y. Repellency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 2001, 26, 76–82. [Google Scholar] [PubMed]
- Sumathi, S.; Iswariya, G.T.; Sivaprabha, B.; Dharani, B.; Radha, P.; Padma, P.R. Comparative study of radical scavenging activity and phytochemical analysis of fresh and dry rhizomes of Curcuma zedoaria. Int. J. Pharm. Sci. Res. 2013, 4, 1069–1073. [Google Scholar]
- Angel, G.R.; Vimala, B.; Bala, N. Antioxidant and antimicrobial activity of essential oils from nine starchy Curcuma species. Int. J. Curr. Pharm. Res. 2012, 4, 45–47. [Google Scholar]
- Myoungae, K. Cytotoxic activity of the extracts from Curcuma zedoaria. J. Toxicol. Environ. Health 2003, 19, 293–296. [Google Scholar]
- Chen, C.; Chen, Y.; Hsi, Y.T.; Chang, C.S.; Huang, L.F.; Ho, C.T.; Way, T.D.; Kao, J.Y. Chemical constituents and anticancer activity of Curcuma zedoaria Roscoe essential oil against non-small cell lung carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 11418–11427. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Gao, M.; Wu, J.; Wang, A.; Shi, R. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Chang, G.; Wu, W.Y. A controlled clinical study between hepatic arterial infusion with embolized Curcuma aromatic oil and chemical drugs in treating primary liver cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001, 21, 165–167. [Google Scholar] [PubMed]
- Zhou, Y.; Shen, J.; Xia, L.; Wang, Y. Curcuma zedoaria (Berg.) Rosc. essential oil and paclitaxel synergistically enhance the apoptosis of SKOV3 cells. Mol. Med. Rep. 2015, 12, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Luo, Y.J.; Cheng, J.H. Zedoary turmeric oil inhibits transplantal hepatoma in rat via hepatic artery perfusion. World Chin. J. Dig. 1998, 11, 260–263. [Google Scholar] [CrossRef]
- Handajani, J.; Narissi, D.H. The effects of Curcuma zedoaria oil on high blood sugar level and gingivitis. Dent. J. 2015, 69, 69–73. [Google Scholar] [CrossRef]
- Pumthong, G.; Asawanonda, P.; Varothai, S.; Jariyasethavong, V.; Triwongwaranat, D.; Suthipinittharm, P.; Ingkaninan, K.; Leelapornpisit, P.; Waranuch, N. Curcuma aeruginosa, a novel botanically derived 5-α-reductase inhibitor in the treatment of male-pattern baldness: A multicenter, randomized, double-blind, placebo-controlled study. J. Dermatolog. Treat. 2012, 23, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Srivilai, J.; Phimnuan, P.; Jaisabai, J.; Luangtoomma, N.; Waranuch, N.; Khorana, N.; Wisuitiprot, W.; Scholfield, C.N.; Champachaisri, K.; Ingkaninan, K. Curcuma aeruginosa Roxb. essential oil slows hair-growth and lightens skin in axillae; a randomised, double blinded trial. Phytomedicine 2017, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, W.T.; Batubara, I.; Tambunan, D.Y. Antibacterial and teeth biofilm degradation activity of Curcuma aeruginosa essential oil. J. Biol. Sci. 2017, 17, 84–90. [Google Scholar] [CrossRef]
- Ma, J.W.; Tsao, T.C.; His, Y.T.; Lin, Y.C.; Chen, Y.; Chen, Y.; Ho, C.T.; Kao, J.Y.; Way, T.D. Essential oil of Curcuma aromatica induces apoptosis in human non-small-cell lung carcinoma cells. J. Funct. Foods 2016, 30, 101–112. [Google Scholar] [CrossRef]
- Li, Y.; Wo, J.M.; Liu, Q.; Li, X.; Martin, R.C. Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann. Surg. Oncol. 2009, 16, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhou, L.; Zheng, L.; Yao, M. Elemene displays anti-cancer ability on laryngeal cancer cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2006, 58, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Luo, Y.J.; Cheng, J.H.; Chang, G.; Liu, W.S.; Li, R.X. Therapeutic effect of Curcuma aramatica oil infused via hepatic artery against transplanted hepatoma in rats. Huaren Xiaohua Zazhi 1998, 6, 859–861. [Google Scholar]
- Wu, W.Y.; Xu, Q.; Shi, L.C.; Zhang, W.B. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J. Gastroenterol. 2000, 6, 216–219. [Google Scholar] [PubMed]
- Cheng, J.H.; Wu, W.Y.; Liu, W.S.; Chang, G.; Liu, Y.L.; Yang, Z.G.; Li, L.N.; Zhou, H. Treatment of 17 cases of patients with primary liver cancer with Curcuma aromatica oil infused via hepatic artery. Shijie Huaren Xiaohua Zazhi 1999, 7, 92. [Google Scholar]
- Li, Y.; Shi, X.; Zhang, J.; Zhang, X.; Martin, R.C.G. Hepatic protection and anticancer activity of Curcuma: A potential chemopreventive strategy against hepatocellular carcinoma. Int. J. Oncol. 2014, 44, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Yang, Y.; Zheng, Y.; Dong, M.; Wang, Y.; Bai, G.; Ye, X.; Yan, Z.; Gao, H. Serum metabonomic analysis of protective effects of Curcuma aromatica oil on renal fibrosis rats. PLoS ONE 2014, 9, e108678. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, X.; Xu, D.J.; Chen, X.H.; Chen, F.H. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential oil. Thromb. Res. 2012, 130, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Al-Reza, S.M.; Rahman, A.; Parvin, T.; Rahman, M.M.; Rahman, M.S. Chemical composition and antibacterial activities of essential oil and organic extracts of Curcuma aromatica Salisb. J. Food Saf. 2011, 31, 433–438. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Mo, Y.; Liu, H.; Yang, B. Concordance between cardio-protective effect on isoproterenol-induced acute myocardial ischemia and phenolic content of different extracts of Curcuma aromatica. Pharm. Biol. 2016, 54, 3226–3231. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Wei, Y.; Cui, J.; Wang, L.H.; Liu, S.G.; Long, Z.F. Antifungal activities of the extracts from Curcuma phaeocaulis against Phoma wasabiae. J. Sichuan Univ. (Nat. Sci. Ed.) 2008, 45, 1235–1238. [Google Scholar]
- Schmidt, E.; Ryabchenko, B.; Wanner, J.; Jäger, W.; Jirovetz, L. Cytotoxic active constituents of essential oils of Curcuma longa and Curcuma zanthorrhiza. Nat. Prod. Commun. 2015, 10, 139–141. [Google Scholar] [PubMed]
- Ozaki, Y. Antiinflammatory effect of Curcuma xanthorrhiza Roxb and its active principles. Chem. Pharm. Bull. 1990, 38, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, A.J.; Yuniarti, N.; Pramono, S. Analgesic effect of combination of essential oil Curcuma xanthorriza Roxb. and its curcuminoids in mice. Trad. Med. J. 2015, 20, 16–23. [Google Scholar]
- Yasni, S.; Imaizumi, K.; Sugano, M. Effects of an Indonesian medicinal plant, Curcuma xanthorrhiza Roxb. on the levels of serum glucose and triglyceride, fatty acid desaturation, and bile acid excretion in streptozotocin-induced diabetic rats. Agric. Biol. Chem. 1991, 55, 3005–3010. [Google Scholar] [CrossRef]
- Yasni, S.; Imaizumi, K.; Nakamura, M.; Aimoto, J.; Sugano, M. Effects of Curcuma xanthorrhiza Roxb. and curcuminoids on the level of serum and liver lipids, serum apolipoprotein A-I and lipogenic enzymes in rats. Food Chem. Toxicol. 1993, 31, 213–218. [Google Scholar] [CrossRef]
- Ramachandran, C.; Quirin, K.W.; Escalon, E.A.; Lollett, I.V.; Melnick, S.J. Therapeutic effect of supercritical CO2 extracts of Curcuma species with cancer drugs in rhabdomyosarcoma cell lines. Phytother. Res. 2015, 29, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Lollett, I.V.; Escalon, E.; Quirin, K.W.; Melnick, S.J. Anticancer potential and mechanism of action of mango ginger (Curcuma amada Roxb.) supercritical CO2 extract in human glioblastoma cells. J. Evid. Based Complement. Altern. Med. 2015, 20, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Portalatin, G.M.; Prado, A.M.; Quirin, K.W.; Escalon, E.; Melnick, S.J. In vivo antitumor effect of supercritical CO2 extract of mango ginger (Curcuma amada Roxb) in U-87MG human glioblastoma nude mice xenografts. J. Evid. Based Complement. Altern. Med. 2017, 22, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, L.; Chomwal, R.; Sawal, R. Anti-microbial potential of the rhizome extracts of Curcuma amada Roxb. J. Pharm. Res. 2009, 2, 339–340. [Google Scholar]
- George, M.; Britto, S.J.; Arulappan, M.T.; Marandi, R.R.; Kindo, I.; Dessy, V.J. Phytochemical, antioxidant and antibacterial studies on the essential oil of the rhizome of Curcuma amada Roxb. Int. J. Curr. Res. 2015, 7, 18098–18104. [Google Scholar]
- Singh, D.; Singh, A.K. Repellent and insecticidal properties of essential oils against housefly, Musca domestica L. Int. J. Trop. Insect Sci. 1991, 4, 487–491. [Google Scholar] [CrossRef]
- Dohare, P.; Garg, P.; Jain, V.; Nath, C.; Ray, M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav. Brain Res. 2008, 193, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Luo, J.; Huang, N. Effect of turmeric volatile oil on the respiratory tract. Zhongguo Zhong Yao Za Zhi. 1998, 23, 624–625. [Google Scholar] [PubMed]
- Murakami, A.; Furukawa, I.; Miyamoto, S.; Tanaka, T.; Ohigashi, H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors 2013, 39, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, F.Q.; Li, S.P.; Hu, G.; Lee, S.M.; Wang, Y.T. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J. Gastroenterol. 2008, 14, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Q.; Zhou, Y.R.; Li, C.Q.; Wang, Z.; Wang, Y.L.; Shen, B.Y.; Dou, J. Zedoary Turmeric oil induces senescence and apoptosis in human colon cancer HCT116 cells. Nat. Prod. Commun. 2018, 13, 907–910. [Google Scholar]
- Wang, Y.F.; Liu, S.Q.; Zhao, J.H. Observation of therapeutic effect of compound zedoary turmeric oil duppositories for treating monilial vaginitis with pregnancy. Hebei Yi Yao 2006, 28, 839–840. [Google Scholar]
- Yang, H.; Wang, X.P.; Yu, L.L.; Zheng, S. The antitumor activity of elemene is associated with apoptosis. Zhonghua Zhongliu Zazhi 1996, 18, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Ray, A.; Sahoo, A.; Kar, B.; Panda, P.C.; Nayak, S. Chemical constituents of leaf essential oil of Curcuma angustifolia Roxb. growing in eastern India. J. Essent. Oil Bear. Plants 2016, 19, 1527–1531. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1973, 11, 855–876. [Google Scholar] [CrossRef]
- Zhou, N.N.; Mao, X.J.; Zhang, J.; Yang, S.W.; Zhang, J.M. Pharmacological investigation on contraindication of Curcuma zedoaria. Chin. Arch. Tradit. Chin. Med. 2004, 22, 2291–2294. [Google Scholar]
- Kong, Y.C.; Xie, J.X.; But, P.P.H. Fertility regulating agents from traditional Chinese medicine. J. Ethnopharmacol. 1986, 15, 1–44. [Google Scholar] [CrossRef]
- Oh, S.; Han, A.; Park, H.R.; Jang, E.J.; Kim, H.K.; Jeong, M.G.; Song, H.; Park, G.H.; Seo, E.K.; Hwang, E.S. Suppression of inflammatory cytokine production by ar-turmerone isolated from Curcuma phacaulis essential oil. Chem. Biodivers. 2014, 11, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Reddy, S.S.; Maurya, P.; Singh, V.; Chaturvedi, S.; Kaur, K.; Agarwal, H.; Ahmad, H.; Naqvi, A.; Dwivedi, A.K.; et al. Turmerone enriched standardized Curcuma longa extract alleviates LPS induced inflammation and cytokine production by regulating TLR4–IRAK1–ROS–MAPK–NFkB axis. J. Funct. Foods 2015, 16, 152–163. [Google Scholar] [CrossRef]
- Hucklenbroich, J.; Klein, R.; Neumaier, B.; Graf, R.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Aromatic turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.H.; Kim, G.J.; Jeong, H.S.; Yum, S.K. Ar-turmerone and β-atlantone induce internucleosomal DNA fragmentation associated with programmed cell death in human myeloid leukemia HL-60 cells. Arch. Pharmacol. Res. 1996, 19, 91–94. [Google Scholar] [CrossRef]
- Baik, K.U.; Jung, S.H.; Ahn, B.Z. Recognition of pharmacophore of ar-turmerone for its anticancer activity. Arch. Pharmacol. Res. 1993, 16, 254–256. [Google Scholar] [CrossRef]
- Ji, M.; Choi, J.; Lee, J.; Lee, Y. Induction of apoptosis by ar-turmerone on various cell lines. Int. J. Mol. Med. 2004, 14, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Hirayama, F.; Funakoshi, K.; Takeya, K. Studies on the antitumor bisabolane sesquiterpenoids isolated from Curcuma xanthorrhiza. Chem. Pharmacol. Bull. 1985, 33, 3488–3492. [Google Scholar] [CrossRef]
- Lee, S.K.; Hong, C.H.; Huh, S.K.; Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, K.K.; Chung, W.Y.; Hwang, J.K. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Sci. Biotechnol. 2006, 15, 559–563. [Google Scholar]
- Dhingra, O.D.; Jham, G.N.; Barcelos, R.C.; Mendonça, F.A.; Ghiviriga, I. Isolation and identification of the principal fungitoxic component of turmeric essential oil. J. Essent. Oil Res. 2007, 19, 387–391. [Google Scholar] [CrossRef]
- Li, J.; Bian, W.H.; Wan, J.; Zhou, J.; Lin, Y.; Wang, J.R.; Wang, Z.X.; Shen, Q.; Wang, K.M. Curdione inhibits proliferation of MCF-7 cells by inducing apoptosis. Asian Pac. J. Cancer Prev. 2014, 15, 9997–10001. [Google Scholar] [CrossRef] [PubMed]
- Oh, O.J.; Min, H.Y.; Lee, S.K. Inhibition of inducible prostaglandin E2 production and cyclooxygenase-2 expression by curdione from Curcuma zedoaria. Arch. Pharm. Res. 2007, 30, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Sampson, J.; Theobald, A.E.; Hart, S.; Lis-Balchin, M.; Hoult, J.R.; Evans, P.; Jenner, P.; Milligan, S.; et al. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2001, 53, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Shiga, A.; Yoshida, Y.; Furuhashi, T.; Fujita, Y.; Niki, E. Effects of a novel gaseous antioxidative system containing a rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci. Biotechnol. Biochem. 2004, 68, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Moteki, H.; Hibasami, H.; Yamada, Y.; Katsuzaki, H.; Imai, K.; Komiya, T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002, 9, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008, 41, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.S.; Ogundajo, A.L.; Dosoky, N.S.; Setzer, W.N. The cytotoxic activity of Annona muricata leaf oil from Badagary, Nigeria. Am. J. Essent. Oils Nat. Prod. 2013, 1, 1–3. [Google Scholar]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. trans-Caryophyllene: An effective antileishmanial compound found in commercial copaiba oil (Copaifera spp.). Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira, A.C.; Ribeiro-Pinto, L.F.; Paumgartten, J.R. In vitro inhibition of CYP2B1 monooxygenase by β-myrcene and other monoterpenoid compounds. Toxicol. Lett. 1997, 92, 39–46. [Google Scholar] [CrossRef]
- Sawamura, M.; Sun, S.H.; Ozaki, K.; Ishikawa, J.; Ukeda, H. Inhibitory effects of Citrus essential oils and their components on the formation of N-nitrosodimethylamine. J. Agric. Food Chem. 1999, 47, 4868–4872. [Google Scholar] [CrossRef] [PubMed]
- Chaouki, W.; Leger, D.Y.; Liagre, B.; Beneytout, J.L.; Hmamouchi, M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Pokharel, S.K.; Setzer, W.N. Leaf essential oil composition, antimicrobial; and cytotoxic activities of Cleistocalyx operculatus from Hetauda, Nepal. Am. J. Essent. Oils Nat. Prod. 2015, 3, 34–37. [Google Scholar]
- Mitić-Culafić, D.; Zegura, B.; Nikolić, B.; Vuković-Gacić, B.; Knezević-Vukcević, J.; Filipic, M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009, 47, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hossain, C.F.; Al-Amin, M.; Sayem, A.S.M.; Siragee, I.H.; Tunan, A.M.; Hassan, F.; Kabir, M.M.; Sultana, G.N.N. Antinociceptive principle from Curcuma aeruginosa. BMC Complement. Altern. Med. 2015, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Chen, X.; Tan, W.; Xu, Z.; Zhou, K.; Wu, T.; Cui, L.; Wang, Y. Germacrone inhibits the proliferation of breast cancer cell lines by inducing cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2011, 667, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, Y.Q.; Wang, X.M.; Wang, Y.C.; Fu, L.Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014, 10, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Fang, B.; Ma, F.; Zheng, Q.; Deng, P.; He, G. Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2013, 698, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, O.A.A.; Ye, L.J.; Kamarudin, M.N.A.; Hazni, H.; Paydar, M.; Looi, C.Y.; Shilpi, J.A.; Kadir, H.A.; Awang, K. Neuroprotective and antioxidant constituents from Curcuma zedoaria rhizomes. Rec. Nat. Prod. 2015, 9, 349–355. [Google Scholar]
- Diastuti, H.; Syah, Y.M.; Juliawaty, L.D.; Singgih, M. Antibacterial activity of germacrane type sesquiterpenes from Curcuma heyneana rhizomes. Indones. J. Chem. 2014, 14, 32–34. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.O.; Hwang, J.K.; Park, K.K. Xanthorrhizol has a potential to attenuate the high dose cisplatin-induced nephrotoxicity in mice. Food Chem. Toxicol. 2005, 43, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.F.; Cheah, Y.H. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.A.; Kim, S.H.; Chung, W.Y.; Hwang, J.K.; Park, K.K. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem. Biophys. Res. Commun. 2005, 326, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Gao, J.; Xie, J.; Yang, L.; Hu, S. Downregulation effects of beta-elemene on the levels of plasma endotoxin, serum TNF-alpha, and hepatic CD14 expression in rats with liver fibrosis. Front. Med. 2011, 5, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wang, G.; Huang, F.; Banda, M.; Reed, E. Antineoplastic effect of beta-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010, 62, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Zhong, W.; Cai, W. Clinical study on treatment of 40 cases of malignant brain tumor by elemene emulsion injection. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 645–648. [Google Scholar] [PubMed]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Xu, Y.; Yang, W.; Qu, L.; Xiang, Q.; Liu, C.; Li, D. Chemical composition and synergistic antioxidant activities of essential oils from Atractylodes macrocephala and Astragalus membranaceus. Nat. Prod. Commun. 2013, 8, 1321–1324. [Google Scholar] [PubMed]
- Zhang, W.; Wang, Z.; Chen, T. Curcumol induces apoptosis via caspases independent mitochondrial pathway in human lung adenocarcinoma ASTC-a-1 cells. Med. Oncol. 2011, 28, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B. The use of herbs as anticancer agents. Am. J. Chin. Med. 1980, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.R.S.; Carvalho, F.A.A.; Miúra, L.M.C.V. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Maia, J.G.S.; Dosoky, N.S.; Setzer, W.N. Antioxidant, antimicrobial, and cytotoxic properties of Aniba parviflora essential oils from the Amazon. Nat. Prod. Commun. 2016, 11, 1025–1028. [Google Scholar]
- ar-Turmerone. Sigma-Aldrich Online Catalog. Available online: https://www.sigmaaldrich.com/catalog/product/sial/42258?lang=en®ion=US (accessed on 21 August 2018).
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Heal. 2011, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Melis, K.; Janssens, G.; Bochner, A. Accidental nasal eucalyptol and menthol instillation. Acta Clin. Belg. Suppl. 1990, 13, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Al, E. Food flavorings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Kubo, I.; Chaudhuri, S.K.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Ishikawa, H.; Haraguchi, H. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 62, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; Figueiredo, P.M.; Yano, T. Chemotherapeutic potential of the volatile oils from Zanthoxylum rhoifolium Lam leaves. Eur. J. Pharmacol. 2007, 576, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Frosch, P.J.; Johansen, J.D.; Menné, T.; Pirker, C.; Rastogi, S.C.; Andersen, K.E.; Bruze, M.; Goossens, A.; Lepoittevin, J.P.; White, I.R. Further important sensitizers in patients sensitive to fragrances. Contact Dermat. 2002, 47, 279–287. [Google Scholar] [CrossRef]
- Matura, M.; Skold, M.; Borje, A.; Al, E. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermat. 2005, 52, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Evandri, M.G.; Mazzanti, G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. Mutat. Res. 2008, 653, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Paumgartten, F.J.; De-Carvalho, R.R.; Souza, C.A.; Madi, K.; Chahoud, I. Study of the effects of beta-myrcene on rat fertility and general reproductive performance. Braz. J. Med. Biol. Res. 1998, 31, 955–965. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of beta-myrcene (CAS No. 123-35-3) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2010, 557, 1–163. [Google Scholar]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Mattock, H.; Straif, K. Some chemicals that cause tumours of the urinary tract in rodents. Lancet Oncol. 2017, 18, 1003–1004. [Google Scholar] [CrossRef]
- Beta-Myrcene. Sigma-Aldrich On-line Catalog. Available online: https://www.sigmaaldrich.com/catalog/product/sial/64643?lang=en®ion=US (accessed on 21 August 2018).
- Germacrone. Sigma-Aldrich On-line Catalog. Available online: https://www.sigmaaldrich.com/catalog/product/sial/42924?lang=en®ion=US (accessed on 21 August 2018).
- Xanthorrhizol. Cayman Chemical Company On-line Catalog. Available online: https://www.caymanchem.com/product/14668 (accessed on 21 August 2018).
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. 2005, 62, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Liu, W.; Yu, L. Beta-Elemene induces apoptosis of K562 leukemia cells. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001, 23, 196–198. [Google Scholar]
- Yan, B.; Zhou, Y.; Feng, S.; Lv, C.; Xiu, L.; Zhang, Y.; Shi, J.; Li, Y.; Wei, P.; Qin, Z. β-Elemene-attenuated tumor angiogenesis by targeting Notch-1 in gastric cancer stem-like cells. Evid. Based. Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1976, 14, 197–198. [Google Scholar] [CrossRef]
- Hausen, B.M.; Reichling, J.; Harkenthal, M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am. J. Contact Dermat. 1999, 10, 68–77. [Google Scholar] [CrossRef]
- Ahn, B.Z.; Lee, J.H. Cytotoxic and cytotoxicity-potentiating effects of the Curcuma root on L1210 cell. Korean J. Pharmacogn. 1989, 20, 223–226. [Google Scholar]
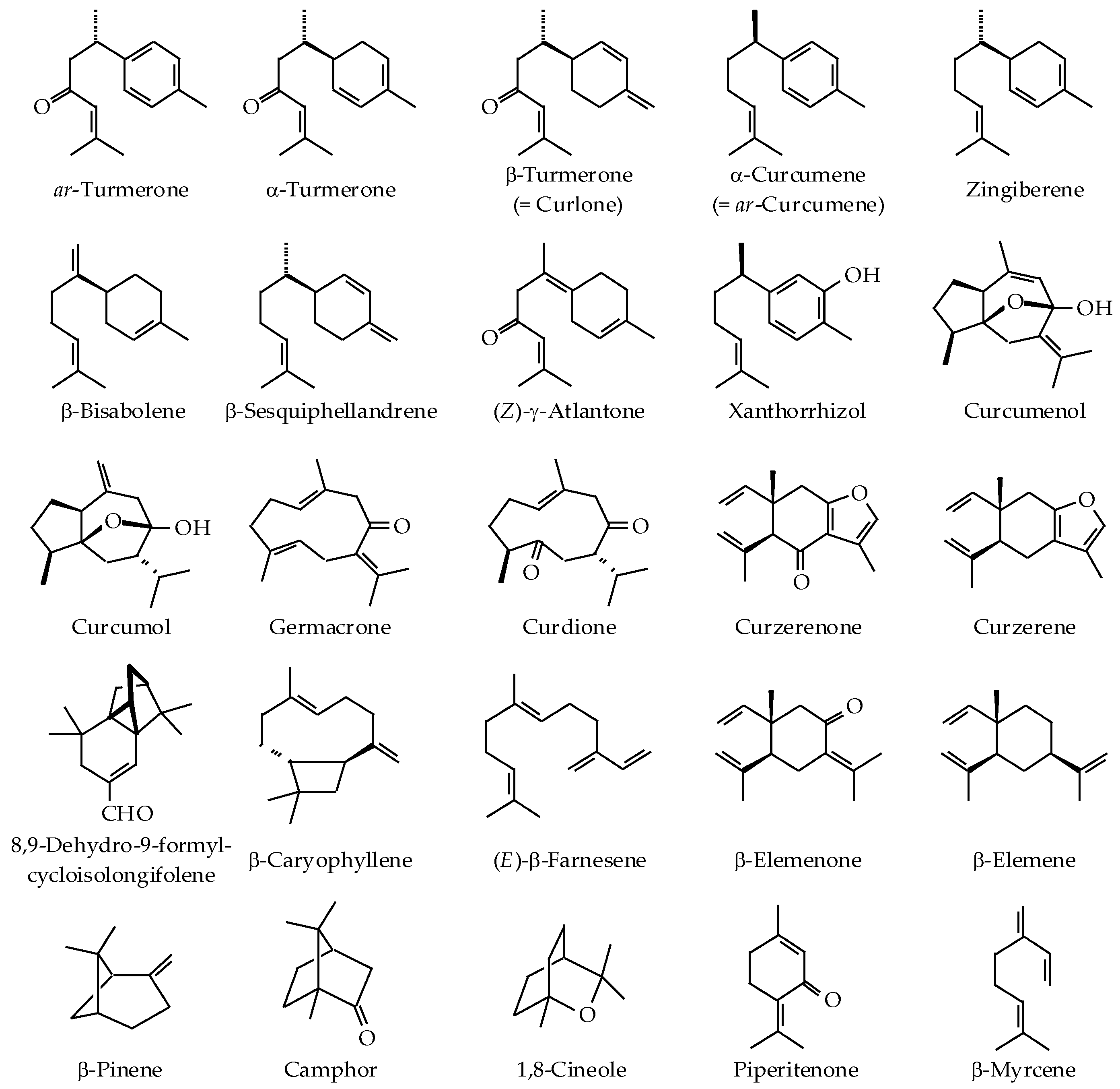
| Curcuma Species | Origin | Part Used (Extraction Method) | Major Components (>5%) | Reference |
|---|---|---|---|---|
| C. aeruginosa Roxb. | Pahang, Malaysia | Rhizome (SD) | 8,9-Dehydro-9-formyl-cycloisolongifolene (35.3%), dihydrocostunolide (22.5%), velleral (10.0%), and germacrone (6.5%) | [28] |
| C. aeruginosa Roxb. | Ratchaburi, Thailand | Fresh rhizome (HD) | Germacrone (23.5%), curzerenone (11.8%) and 1,8-cineole (10.9%) | [29] |
| C. aeruginosa Roxb. | Phetchabun, Thailand | Powdered rhizome (HD) | 1,8-Cineole (22.7%), germacrone (17.7%), furanodiene (11.4%), and β-pinene (8.0%) | [30] |
| C. aeruginosa Roxb. | Malaysia | Rhizome (HD) | 1,8-Cineole (23.2%) and curzerenone (28.4%) | [31] |
| C. aeruginosa Roxb. | Malaysia | Rhizome (HD) | Curzerenone (24.6%), 1,8-cineole (11.0%), camphor (10.6%), zedoarol (6.3%), isocurcumenol (5.8%), curcumenol (5.6%), and furanogermenone (5.5%) | [32] |
| C. aeruginosa Roxb. | Chiang Mai, Thailand | Rhizome (HD) | Camphor (29.4%), germacrone (21.2%), borneol (7.3%), and germacrene B (5.2%) | [2] |
| C. aeruginosa Roxb. | Kerala, India | Rhizome (HD) | Curcumenol (38.7%) and β-pinene (27.5%) | [17] |
| C. aeruginosa Roxb. | Pahang, Malaysia | Rhizome (SE, MTBE) | Methenolone (16.6%), 8,9-dehydro-9-formyl-cycloisolongifolene (15.9%), labd-13-en-15-oic acid,8,12-epoxy-12-hydroxy-γ-lactone (10.8%), propiolic acid, 3-(1-hydroxy)-2 isopropyl-1,5-methylcyclohexyl) (7.8%), and 4-oxo-β-isodamascol (5.2%) | [33] |
| C. aeruginosa Roxb. | Phetchabun, Thailand | Rhizome (SE, hexane) | Dehydrocurdione (27.6%), curcumenol (15.1%), germacrone (10.2%), and gajutsulactone A (6.3%) | [30] |
| C. aeruginosa Roxb. | South India | Leaf (HD) | 1,8-Cineole (17.7%), curzerenone (10.5%), furanogermenone (7.8%), camphor (7.5%), (Z)-3-hexenol (5.8%), and furanodienone (5.1%) | [34] |
| C. aeruginosa Roxb. | Vietnam | Leaf (HD) | Curzerene (16.2%), germacrone (13.6%), 1,8-cineole (13.5%), and camphor (5.7%) | [35] |
| C. albiflora Thwaites | Ratnapura, Sri Lanka | Rhizome (HD) | α-Pinene (14.5%), caryophyllene oxide (9.4%), and alconfor (5.1%) | [13] |
| C. alismatifolia Gagnep. | Prachin Buri, Thailand | Fresh root (HD) | (–)-Xanthorrhizol (52.4%) and ar-curcumene (27.4%) | [36] |
| Prachin Buri, Thailand | Fresh rhizome (HD) | β-Curcumene (42.0%), (-)-xanthorrhixol (36.6%), and ar-curcumene (7.5%) | [36] | |
| C. amada Roxb. | Andhra Pradesh, India | Rhizome (HD) | Myrcene (80.5%) | [37] |
| C. amada Roxb. | Uttarakhand, India | Rhizome (HD) | Myrcene (88.8%) | [38] |
| C. amada Roxb. | Northeastern India | Fresh rhizome (HD) | Myrcene (88.6%) | [39] |
| C. amada Roxb. | New Delhi, India | Rhizome (SD) | (Z)-β-Farnesene (21.9%), guaia-6,9-diene (19.8%), α-longipinene (14.8%), α-guaiene (14.5%), and camphor (5.5%). | [40] |
| C. amada Roxb. | Mysore, India | Fresh rhizome (HD) | (E)-Hydroocimene (15.9%), (Z)-hydroocimene (14.2%), myrcene (14.9%), and linalool (13.4%) | [41] |
| C. amada Roxb. | Lucknow, India | Rhizome (HD) | ar-Curcumene (28.1%), β-curcumene (11.2%), camphor (11.2%), curzerenone (7.1%), and 1,8-cineole (6.0%) | [42] |
| C. amada Roxb. | Uttarakhand, India | Leaf (HD) | Camphor (17.9%), epi-curzerenone (10.8%), curzerenone (9.5%), and isoborneol (7.3%) | [38] |
| C. angustifolia Roxb. | Central India | Rhizome (HD) | Xanthorrhizol isomer (12.7%), methyleugenol (10.5%), and palmitic acid (5.2%) | [43] |
| C. angustifolia Roxb. | Southern India | Rhizome (HD) | Germacrone (12.8%), camphor (12.3%), isoborneol (8.7%), and curdione (8.4%) | [43] |
| C. angustifolia Roxb. | Chiang Mai, Thailand | Root (HD) | β-Elemenone (65.0%) | [44] |
| C. angustifolia Roxb. | Chiang Mai, Thailand | Rhizome (HD) | Camphor (36.9%) and germacrone (31.5%) | [44] |
| C. angustifolia Roxb. | India | Rhizome (HD) | Curzerenone (72.6%) | [45] |
| C. angustifolia Roxb. | India | Leaf (HD) | Curzerenone (33.2%), 14-hydroxy-δ-cadinene (18.6%), and γ-eudesmol acetate (7.3%) | [45] |
| C. aromatica Salisb. | Northeast India | Rhizome (HD) | Camphor (32.3%), curzerenone (11.0%), α-turmerone (6.7%), ar-turmerone (6.3%), and 1,8-cineole (5.5%) | [46] |
| C. aromatica Salisb. | China | Rhizome (SD) | 8,9-Dehydro-9-formyl-cycloisolongifolene (2.7–36.8%), germacrone (4.3–16.5%), ar-turmerone (2.5–17.7%), turmerone (2.6–18.4%), ermanthin (0.8–13.3%), β-sesquiphellandrene (0.3–11.3%), and ar-curcumene (0.3–10.5%). | [47] |
| C. aromatica Salisb. | Assam, India | Rhizome (SD) | Camphor (25.6%), curzerenone (10.9%), germacrone (10.6%), 1,8-cineole (9.3%), isoborneol (8.2%), and camphene (7.4%) | [48] |
| C. aromatica Salisb. | Kerala, India | Rhizome (HD) | Camphor (18.8%), camphene (10.2%), 1,8-cineole (10.1%), borneol (8.2%), and β-elemene (7.5%) | [17] |
| C. aromatica Salisb. | Yulin, China | Fresh rhizome (SD) | Curdione (50.6%) and germacrone (9.5%) | [49] |
| C. aromatica Salisb. | Japan | Dry rhizome (SD) | Curcumol (35.8%), 1,8-cineole (12.2%), ar-turmerone (7.0%), linalool (6.4%), humulene oxide (6.1%), and caryophyllene oxide (5.9%) | [50] |
| C. aromatica Salisb. | Kerala, India | Rhizome (HD) | Xanthorrhizol (26.3%), ar-curcumene (19.5%), and di-epi-α-cedrene (16.5%) | [51] |
| C. aromatica Salisb. | Ratnapura, Sri Lanka | Rhizome (HD) | Camphor (32.3%), curzerenone (11.0%), α-turmerone (6.7%), ar-turmerone (6.3%), and 1,8-cineole (5.5%) | [13] |
| C. aromatica Salisb. | Thailand | Rhizome (HD) | 1H-3a,7-methanoazulene (30.0%), curcumene (25.7%), and xanthorrhizol (13.7%) | [52] |
| C. aromatica Salisb. | Thailand | Rhizome (SE, hexane) | Xanthorrhizol (35.1%), 1H-3a,7-methanoazulene (21.8%), and curcumene (13.8%) | [52] |
| C. aromatica Salisb. | Hebei, China | Dry root (HSME) | β-Elemene (6.3%), germacrone (5.6%), and arzingiberone (5.3%) | [53] |
| C. aromatica Salisb. | Hebei, China | Dry root (SD) | Germacrone (9.1%), curcumenol (8.5%), isocurcumenol (7.5%), and arzingiberone (5.1%) | [53] |
| C. aromatica Salisb. | Hebei, China | Dry root (SPME) | Curcumenol (8.9%), isocurcumenol (8.7%), germacrone (6.7%), 1-methoxy-4-(1-propenyl)-benzene (5.7%), and curzerenone (5.3%) | [53] |
| C. aromatica Salisb. | Assam, India | Leaf (SD) | 1,8-Cineole (20.0%), camphor (18.0%) germacrone (11.8%), camphene (9.4%), limonene (8.6%), and isoborneol (6.4%) | [48] |
| C. aromatica Salisb. | Gorakhpur, India | Leaf (HD) | p-Cymene (25.2%), 1,8-cineole (24.0%), α-terpineol (8.1%), and 2-oxabicyclo (3,2,1) octane-1-,4-dimethyl-8-methylene (8.1%) | [37] |
| C. aromatica Salisb. | Northeast India | Leaf (HD) | Camphor (28.5%), curzerenone (6.2%), and 1,8-cineole (6.1%) | [46] |
| C. aromatica Salisb. | Kushtia, Bangladesh | Leaf (HD) | Camphor (26.3%), borneol (16.5%), vinyldimethylcarbinol (12.2%), caryophyllene oxide (6.3%), cubenol (5.6%), and cucumber alcohol (5.2%) | [54] |
| C. aromatica Salisb. | Assam, India | Petiole (SD) | Camphor (16.8%), 1,8-cineole (8.8%), caryophyllene oxide (8.7%), patchouli alcohol (8.4%), isoborneol (6.8%), and elsholtzia ketone (6.0%) | [48] |
| C. aurantiaca Zijp | Kerala, India | Fresh rhizome (HD) | Piperitenone (65.2%), 1,8-cineole (13.1%), and camphor (5.7%) | [55] |
| C. aurantiaca Zijp | Zhejiang, India | Fresh rhizome (HD) | 1,8-cineole (15.3%), camphor (10.1%), germacrone (6.9%), β-elemene (6.3%), curzerene (6.7%), and β-elemenone (5.2%) | [56] |
| C. caesia Roxb. | Kerala, India | Rhizome (HD) | 1,8-Cineole (30.1%), camphor (15.2%), ar-curcumene (14.8%), and camphene (8.2%) | [17] |
| C. caesia Roxb. | Central India | Rhizome (HD) | Camphor (28.3%), ar-turmerone (12.3%), (Z)-β-ocimene (8.2%), ar-curcumene (6.8%), and 1,8-cineole (5.3%) | [57] |
| C. caesia Roxb. | India | Leaf (HD) | 1,8-Cineole (27.0%) and camphor (16.8%) | [58] |
| C. elata Roxb. | Guangzhou, China | Fresh rhizome (SD) | 8,9-Dehydro-9-formyl-cycloisolongifolene (52.2%) and germacrone (14.0%) | [49] |
| C. glans K. Larsen and Mood | Chiang Mai, Thailand | Rhizome (HD) | Germacrone (15.8%), β-pinene (10.0%), camphor (10.0%), and 2-nonanol (6.9%) | [2] |
| C. haritha Mangaly and M. Sabu | Southern India | Rhizome (HD) | Camphor (36.0%), 1,8-cineole (13.9%), isoborneol (10.6%), curdione (6.9%), and camphene (5.7%) | [59] |
| C. harmandii Gagnep. | Vietnam | Rhizome (SD) | 1,8-Cineole (4.5-12.5%), germacrone (9.0–20.5%), β-pinene (1.2–22.6%), β-elemene (6.5–11.3%), and isocurcumenol (3.7–13.4%) | [60] |
| C. harmandii Gagnep. | Vietnam | Root (SD) | Germacrone (24.4%), isocurcumenol (12.9%), and curcumenol (10.8%) | [60] |
| C. harmandii Gagnep. | Vietnam | Leaf (SD) | 1,8-Cineole (13.5%), germacrone (11.5%), and curdione (36.8%) | [60] |
| C. harmandii Gagnep. | Vietnam | Stem (SD) | 1,8-Cineole (21.8%), germacrone (15.5%), and curdione (25.3%) | [60] |
| C. harmandii Gagnep. | Vietnam | Flower (SD) | Curdione (27.0%) and an unidentified oxygenated sesquiterpene (12.3%) | [60] |
| C. inodora Blatt. | Malaysia | Fresh rhizome (HD) | Curzerenone (20.8%), germacrone (11.1%), curdione (7.5%), and 1,8-cineole (5.3%) | [61] |
| C. inodora Blatt. | Malaysia | Leaf (HD) | Curzerenone (16.9%), germacrone (7.5%), 1,8-cineole (5.3%), and farnesol (5.0%) | [61] |
| C. kwangsiensis S.G. Lee and C.F. Liang | Guangzhou, China | Fresh rhizome (SD) | α-Elemene (12.8%), germacrene D (8.2%), spathulenol (5.8%), curdinone (5.9%), and β-bisabolene (5.4%) | [49] |
| C. kwangsiensis S.G. Lee and C.F. Liang | Guangxi, China | Rhizome (HD) | Germacrone (13.2%), β-elemenone (12.8%), β-elemene (4.5–6.8%), curzerenone (5.6–7.6%), and curdione (3.0–6.0%) | [62] |
| C. kwangsiensis S.G.Lee and C.F.Liang | China | Rhizome (HD) | 8,9-Dehydro-9-formyl-cycloisolongifolene (2.37–42.59%), germacrone (6.53–22.20%), and l-camphor (0.19–6.12%). | [63] |
| C. longa L. | Tamil Nadu, India | Dry rhizome (HD) | ar-Turmerone (53.1%), β-turmerone (6.4%), and α-turmerone (6.2%) | [64] |
| C. longa L. | Mumbai, India | Dry rhizome (HD) | ar-Turmerone + turmerone (68–70%) and curlone (12–15%) | [65] |
| C. longa L. | Kanpur, India | Fresh rhizome (HD) | ar-Turmerone (31.7%), α-turmerone (12.9%), β-turmerone (12.0%), and (Z)-β-ocimene (5.5%) | [66] |
| C. longa L. | Gorakhpur, India | Rhizome (HD) | ar-Turmerone (51.7%), β-bisabolene (10.7%), α-turmerone (11.9%), zingiberene (10.2%), and β-caryophyllene (5.6%) | [37] |
| C. longa L. | Gorakhpur, India | Fresh rhizome (HD) | ar-Turmerone (24.4%), α-turmerone (20.5%), and β-turmerone (11.1%) | [23] |
| C. longa L. | Gorakhpur, India | Dry rhizome (HD) | ar-Turmerone (21.4%), α-santalene (7.2%), ar-curcumene (6.6%), and santalenone (5.6%) | [23] |
| C. longa L. | Gorakhpur, India | Fresh rhizome (SE, ethanol) | α-Turmerone (53.4%), β-turmerone (18.1%), and ar-turmerone (6.2%) | [23] |
| C. longa L. | Gorakhpur, India | Dry rhizome (SE, ethanol) | ar-Turmerone (9.6%), α-santalene (7.8%), β-sesquiphellandrene (6.9%), α-turmerone (6.5%), and α-zingiberene (6.1%) | [23] |
| C. longa L. | Karnataka, India | Fresh rhizome (HD) | α-Turmerone (33.5%), ar-turmerone (21.0%), and β-turmerone (18.9%) | [67] |
| C. longa L. | Karnataka, India | Dry rhizome (HD) | ar-Turmerone (30.3%), α-turmerone (26.5%), and β-turmerone (19.1%) | [67] |
| C. longa L. | Karnataka, India | Cured rhizome (HD) | ar-Turmerone (28.3%), α-turmerone (24.8%), and β-turmerone (21.1%) | [67] |
| C. longa L. | Mysore, India | Rhizome (SE, hexane) | ar-Turmerone (21.4%), zingiberene (15.0%), (Z)-β-farnesene (14.0%), ar-curcumene (10.3%), turmerone (6.2%), and curlone (5.1%) | [22] |
| C. longa L. | Bangalore, India | Rhizome (HD) | Turmerone (44.1%), β-turmerone (18.5%), and ar-turmerone (5.4%) | [68] |
| C. longa L. | Gorakhpur, India | Dried rhizome (HD) | ar-Turmerone (49.1%) and α-turmerone (11.6%) | [69] |
| C. longa L. | Calicut, India | Rhizome (HD) | ar-Turmerone (31.1%), curlone (10.6%), turmerone (10.0%), and ar-curcumene (6.3%) | [70] |
| C. longa L. | Calicut, India | Root (HD) | ar-Turmerone (46.8%) and ar-curcumene (7.0%) | [70] |
| C. longa L. | Kuala Selangor, Malaysia | Fresh rhizome (HD) | ar-Turmerone (45.8%) and curcumenol (18.2%) | [71] |
| C. longa L. | Faisalabad, Pakistan | rhizome (SD) | ar-Turmerone (25.3 %), α-tumerone (18.3 %), and curlone (12.5 %) | [72] |
| C. longa L. | Pakistan | Rhizome (HD) | ar-Turmerone (38.6%), a-turmerone (8.9%), and β-turmerone (12.9%) | [73] |
| C. longa L. | Sichuan, China | Dried rhizomes (SD) | ar-Turmerone (49.0%), humulene oxide (16.6%), β-selinene (10.2%), and caryophyllene oxide (5.6%) | [50] |
| C. longa L. | China | Fresh rhizome (HD) | ar-Turmerone (0.9–42.9%), β-turmerone (5.1–42.5%), α-zingiberene (0.3–25.1%), ar-curcumene (1.2–15.7%), and β-sesquiphellandrene (0.1–14.9%) | [74] |
| C. longa L. | Sichuan, China | Rhizome (SFE) | α-Turmerone (40.8%), zingiberene (16.9%), β-turmerone (14.1%), ar-turmerone (11.0%), and β-sesquiphellandrene (10.0%) | [75] |
| C. longa L. | Mara Rosa, Brazil | Rhizome (HD) | ar-Turmerone (33.2%), α-turmerone (23.5%), and β-turmerone (22.7%) | [76] |
| C. longa L. | Mara Rosa, Brazil | Fresh rhizome (HD) | α-Turmerone (42.6%), β-turmerone (16%), ar-turmerone (12.9%), and α-phellandrene (6.5%) | [77] |
| C. longa L. | Minas Gerais, Brazil | Rhizome (SE) | (Z)-γ-Atlantone (33.4%), ar-turmerone (21.8 %), and (E)-γ-atlantone (18.7%) | [78] |
| C. longa L. | Minas Gerais, Brazil | Rhizome (HD) | (Z)-γ-Atlantone (44.0%), (E)-γ-atlantone (18.3%), and ar-turmerone (18.0%) | [78] |
| C. longa L. | Isfahan, Iran | Dry rhizome (HD) | ar-Turmerone (68.9%) and α-turmerone (20.9%) | [79] |
| C. longa L. | Brazil | Rhizome (SFE) | ar-Turmerone (51.9%) and (E)-γ-atlantone (19.6%) | [80] |
| C. longa L. | Brazil | Rhizome (HD) | ar-Turmerone (49.3%) and (E)-γ-atlantone (19.2%) | [80] |
| C. longa L. | Ondo, Nigeria | Fresh rhizome (HD) | Turmerone (35.9%), α-phyllandrene (15.5%), curlone (12.9%), 1,8-cineole (10.3%), and ar-turmerone (10.0%) | [81] |
| C. longa L. | Cameroon | Rhizome (HD) | α-Turmerone (43.1%), ar-turmerone (17.6%), and curlone (17.5%) | [82] |
| C. longa L. | Bhutan | Rhizome (HD) | α-Turmerone (30.0–32.0%), ar-turmerone (17.0–26.0%), and β-turmerone (15.0–18.4%) | [83] |
| C. longa L. | Reunion, France | Rhizome (SD) | α-Turmerone (21.4%), terpinolene (15.8%), zingiberene (11.8%), β-sesquiphellandrene (8.8%), ar-turmerone (7.7%), β-turmerone (7.1%), and β-caryophyllene (5.7%) | [84] |
| C. longa L. | North Central Nigeria | Fresh rhizome (HD) | β-Bisabolene (13.9%), (E)-β-ocimene (9.8%), myrcene (7.6%), 1,8-cineole (6.9%), α-thujene (6.7%), α-phellandrene (6.4%), limonene (5.3%), zingiberene (5.2%), and β-sesquiphellandrene (5.2%) | [85] |
| C. longa L. | North Indian Plains | Rhizome (HD) | 1,8-Cineole (11.2%), α-turmerone (11.1%), β-caryophyllene (9.8%), ar-turmerone (7.3%), and β-sesquiphellandrene (7.1%) | [86] |
| C. longa L. | Kerala, India | Rhizome (HD) | 1,8-Cineole (28.2%), β-elemene (8.2%), camphor (6.9%), α-farnesene (6.3%), and (Z,Z)-farnesol (5.2%) | [17] |
| C. longa L. | São Tomé and Principe | Rhizome (HD) | α-Phellandrene (15.5–30.4%), α-turmerone (12.2–23.9%), 1,8-cineole (10.2–23.0%), ar-turmerone (4.0–12.8%), β-turmerone (4.3–11.5%), and p-cymene (2.5–5.5%) | [87] |
| C. longa L. | Colombo, Sri Lanka | Rhizome (HD) | α-Phellandrene (18.2%), 1,8-cineole (14.6%), p-cymene (13.3%), and terpinolene (11.6%) | [13] |
| C. longa L. | Malaysia | Rhizome (HD) | Furanogermenone (53.1%), germacrone (9.6%) and β-elemene (8.8%), camphor (6.3%), and isofuranodiene (5.6%) | [88] |
| C. longa L. | Malaysia | Rhizome (HD) | α-Tumerone (45.3%), linalool (14.9%), and β-tumerone (13.5%) | [31] |
| C. longa L. | Calicut, India | Flower (HD) | p-Cymen-8-ol (26.0%) and terpinolene (7.4%) | [70] |
| C. longa L. | Reunion, France | Flower (SD) | Terpinolene (67.4%) | [84] |
| C. longa L. | Reunion, France | Leaf (SD) | Terpinolene (76.8%) | [84] |
| C. longa L. | Kanpur, India | Fresh leaf (HD) | α-Phellandrene (9.1%), terpinolene (8.8%), 1,8-cinceole (7.3%), undecanol (7.1), and p-cymene (5.5%) | [66] |
| C. longa L. | Kerala, India | Leaf (HD) | α-Phellandrene (24.4%), terpinolene (13.1%), p-cymene (11.1%), and 1,8-cineole (7.0%) | [89] |
| C. longa L. | Uttar Pradesh, India | Leaf (HD) | p-Cymene (25.4%), 1,8-cineole (18.0%), cis-sabinol (7.4%), and α-pinene (6.3%) | [90] |
| C. longa L. | Bangalore, India | Leaf (HD) | α-Phellandrene (53.4%), terpinolene (11.5%), and 1,8-cineole (10.5%) | [68] |
| C. longa L. | Calicut, India | Leaf (HD) | α-Phellandrene (32.6%), terpinolene (26%), 1,8-cineole (6.5%), and p-cymene (5.9%) | [70] |
| C. longa L. | Bhutan | Leaf (HD) | α-Phellandrene (18.2%), 1,8-cineole (14.6%), p-cymene (13.3%), terpinolene (11.6%), and β-pinene (7.2%), | [83] |
| C. longa L. | Nigeria | Leaf (HD) | α-Phellandrene (47.7%) and terpinolene (28.9%) | [91] |
| C. longa L. | Kerala, India | Leaf (HD) | β-Sesquiphellandrene (22.8%) and terpinolene (9.5%) | [92] |
| C. longa L. | Nainital, India | Leaf (SD) | Terpinolene (71.2%) and 1,8-cineole (6.2%) | [93] |
| C. longa L. | Southern Nigeria | Leaf (HD) | ar-Turmerone (63.4%), α-turmerone (13.7%), and β-turmerone (12.6%) | [94] |
| C. longa L. | Selangor, Malaysia | Leaf (PLE) | α-Phellandrene (13.8–20.7%), 1,8-cineole (14.4–15.1%), terpinolene (7.7–9.4%), and p-cymene (5.0–6.4%) | [95] |
| C. longa L. | Belem, Brazil | Fresh leaf (HD) | β-Phyllandrene (31.5%), α-terpinolene (22.5%), and 1,8-cineole (15.2) | [96] |
| C. longa L. | Vietnam | Leaf (HD) | α-Phellandrene (24.5%), 1,8-cineole (15.9%), p-cymene (13.2%) and β-pinene (8.9%) | [97] |
| C. longa L. | India | Leaf (HD) | Terpinolene (87.8%) | [58] |
| C. longa L. | India | Leaf (HD) | Myrcene (48.8%) and terpinolene (10.1%) | [58] |
| C. mangga Valeton and Zijp | Pahang, Malaysia | Rhizome (SD) | Caryophyllene oxide (18.7%) and caryophyllene (12.7%) | [28] |
| C. mangga Valeton and Zijp | Malaysia | Rhizome (HD) | Myrcene (46.5%) and β-pinene (14.6%) | [98] |
| C. mangga Valeton and Zijp | Penang, Malaysia | Rhizome (HD) | Myrcene (78.7%) and (E)-β-ocimene (5.1%) | [99] |
| C. mangga Valeton and Zijp | Malaysia | Rhizome (HD) | Myrcene (81.4%) | [31] |
| C. nankunshanensis N. Liu, X.B. Ye and Juan Chen | Huizhou, China | Fresh rhizome (SD) | Curdione (23.7%), germacrone (18.8%), 8,9-dehydro-9-formyl-cycloisolongifolene (10.7%), and velleral (6.1%) | [49] |
| C. oligantha Trimen | Badulla, Sri Lanka | Rhizome (HD) | Caryophyllene (15.1%), phytol (13.4), α-humulene (8.2%), γ-elemene (6.1%), and caryophyllene oxide (5.8%) | [13] |
| C. phaeocaulis Valeton | China | Rhizome (SD) | 8,9-Dehydro-9-formyl-cycloisolongifolene (15.6–46.2%), germacrone (8.9–21.2%), curlone (0.8–20.2%), α-caryophyllene (0.1–11.0%), curzerene (0.6–9.8%), and β-elemene (0.6–5.4%) | [100] |
| C. pierreana Gagnep. | Vietnam | Flower (HD) | Isoborneol (27.3%), camphor (24.1%), isobornyl acetate (7.3%), camphene (6.7%), and α-pinene (5.1%) | [101] |
| C. pseudomontana J. Graham | Tamil Nadu, India | Rhizome (HD) | β-Elemenone (22.1%), pseudocumenol (20.7%), germacrone (15.2%), 2-(4-methoxyphenyl) N, N-trimethyl-1-pyrrolamine (13.1%), and (1,5 dimethyl-4-hexenyl)-4-methylbenzene (7.3%) | [102] |
| C. purpurascens Blume | Yogyakarta, Indonesia | Dried rhizome (HD) | Turmerone (13.5%), germacrone (13.2%), ar-turmerone (9.4%), germacrene-B (8.8%), curlone (6.2%), and curzerene (5.8%) | [103] |
| C. rhabdota Sirirugsa and M.F. Newman | Bangkok, Thailand | Fresh rhizome (HD) | Germacrone (24.4%), butyl butanoate (14.2%), sec-butyl butanoate (8.8%), camphene (7.0%), and germacrene B (6.3%) | [104] |
| C. rubescens Roxb. | Guangzhou, China | Fresh rhizome (SD) | Zerumbone (15.5%), ar-turmerone (13.8%), germacrone (13.5%), camphor (8.7%), and aromadendrene oxide (7.1%) | [49] |
| C. sichuanensis X.X. Chen | Chengdu, China | Fresh rhizome (SD) | Germacrone (28.1%), β-elemenone (10.7%), and isoaromadendrene epoxide (8.4%) | [49] |
| C. sichuanensis X.X. Chen | Sichuan, China | Dried rhizome (SD) | ar-Turmerone (43.5%), β-selinene (13.4%), δ-cadinene (13.2%), humulene oxide (8.0%), and curcumol (6.9%) | [50] |
| C. sichuanensis X.X. Chen | Sichuan, China | Rhizome (SD) | epi-Curzerenone (26.9%), germacrone (12.4%), isocurcumenol (9.7%), β-elemene (6.4%), and curzerene (6.2%) | [105] |
| C. singulris Gagnep. | Gia Lai, Vietnam | Fresh rhizome (SD) | Camphor (25.8%) and germacrone (8.0%) | [106] |
| C. sylvatica Valeton | Kerala, India | Rhizome (HD) | α-Fenchene (70.0%) | [17] |
| C. trichosantha Gagnep | Vietnam | Rhizome (HD) | Curdione (47.4%), curcumol (7.0%), and germacrone (6.1%) | [107] |
| C. yunnanensis N. Liu and S.J. Chen | Guangzhou, China | Fresh rhizome (SD) | Germacrone (13.5%), 8,9-dehydro-9-formyl-cycloisolongifolene (13.1%), dihydrocostunolide (12.3%), β-farnesene (7.5%), and aromadendrene oxide (7.4%) | [49] |
| C. zanthorrhiza Roxb. | Mustika Ratu Jakarta, Indonesia | Dry rhizome (SD) | α-Curcumene (64.8%) and camphor (6.0%) | [108] |
| C. zanthorrhiza Roxb. | Chiang Mai Province, Thailand | Rhizome (HD) | α-Terpinolene (24.9%), p-cymen-7-ol (12.2%), p-cymene (8.1%), and β-pinene (6.8%) | [2] |
| C. zanthorrhiza Roxb. | Kuala Selangor, Malaysia | Fresh rhizome (HD) | Xanthorrhizol (31.9%), β-curcumene (17.1%), ar-curcumene (13.2%), citronellyl pentanoate (5.7%), and camphor (5.4%) | [71] |
| C. zanthorrhiza Roxb. | Malaysia | Rhizome (HD) | Xanthorrhizol (44.5%) | [31] |
| C. zedoaria (Christm.) Roscoe | MaharajGanj, India | Rhizome (HD) | 1,8-Cineole (18.5%), p-cymene (18.4%), and α-phellandrene (14.9%) | [37] |
| C. zedoaria (Christm.) Roscoe | Ruian, China | Rhizome (SD) | Curzerene (29.4%), curdione (19.6%), 1,8-cineole (9.7%), germacrone (9.2%), and β-elemene (8.1%) | [109] |
| C. zedoaria (Christm.) Roscoe | Changhwa, Taiwan | Dry rhizome (SD) | Epicurzerene (24.1%), curzerene (10.4%), and curdione (7.0%) | [7] |
| C. zedoaria (Christm.) Roscoe | China | Dry rhizome (HD) | Epicurzerene (46.6%), curdione (13.7%), and 5-isopropylidene-3,8-dimethyl-1(5H)-azulenone (9.2%) | [110] |
| C. zedoaria (Christm.) Roscoe | Kerala, India | Rhizome (HD) | Epicurzerenone (19.0%), ar-curcumene (12.1%), zingiberene (12.0%), β-sesquiphellandrene (9.8%), curzerene (8.0%), and germacrene B (6.0%). | [17] |
| C. zedoaria (Christm.) Roscoe | Gorakhpur, India | Rhizome (HD) | Curzerenone (31.6%), germacrone (10.8%) and camphor (10.3%) | [111] |
| C. zedoaria (Christm.) Roscoe | Colombo, Sri Lanka | Rhizome (HD) | Debromofiliforminol (31.5%), camphor (11.8%), aromadendrene (11.8%), benzofuran (8.8%), and germacrone (5.2%) | [13] |
| C. zedoaria (Christm.) Roscoe | Gorakhpur, India | Dry rhizome (HD) | Curzerene (31.6%), germacrone (10.8%), and camphor (10.3%) | [111] |
| C. zedoaria (Christm.) Roscoe | Northeast India | Rhizome (HD) | Curzerene (22.3%), 1,8-cineole (15.9%), and germacrone (9.0%) | [112] |
| C. zedoaria (Christm.) Roscoe | Kerala, India | Rhizome (HD) | 1,8-Cineole (40.8%), curcumenene (18.7%), and camphor (10.2%) | [17] |
| C. zedoaria (Christm.) Roscoe | Kerala, India | Rhizome (HD) | 1,8-Cineole (24.6%), β-sesquiphellandrene (21.5%), and elemenone (13.6%) | [17] |
| C. zedoaria (Christm.) Roscoe | Thailand | Rhizome (HD) | 1,8-Cineol (37.6%) and curzerenone (13.7%) | [113] |
| C. zedoaria (Christm.) Roscoe | Shanghai, China | Commercial | Curzerene (26.5%), 1,8-cineole (12.0%), curcumol (9.0%), pyridine (8.0%), germacrone (7.9%), and β-elemene (7.4%) | [114] |
| C. zedoaria (Christm.) Roscoe | Lucknow, India | Leaf (HD) | α-Terpinyl acetate (8.4%), isoborneol (7.0%), dehydrocurdione (9.0%), and selina-4(15),7(11)-dien-8-one (9.4%) | [115] |
| Curcuma Essential Oil | Biological Activity | Reference |
|---|---|---|
| C. longa rhizome EO | Antihyperlipidemic (in vivo, high-fat diet-induced hyperlipidemia rats, and hyperlipidemic golden Syrian hamsters) | [75,163] |
| Antidiabetic and hypoglycemic (in vivo, obese diabetic rats, ≥620 mg/kg/day) | [164] | |
| Antiobesity (in vivo, obese diabetic rats, ≥620 mg/kg/day) | [165] | |
| α-Glucosidase and α-amylase inhibitor | [96,166,167] | |
| Antioxidant (in vitro, DPPH assay, FRAP assay, superoxide anion assay, and metal chelating assay) | [50,74,168,169] | |
| Neuroprotective (in vivo, postmyocardial ischemia/reperfusion in rats) | [166,170,171,172] | |
| Antiplatelet and antithrombosis (in vivo, myocardial ischemia-reperfusion and thrombosis rat models, 500 mg/kg, p.o.) | [172,173,174] | |
| Cytotoxic (in vitro, KB, P388, PANC-1, B16, LNCaP and HeLa cells) | [23,74,175,176,177,178] | |
| Anti-inflammatory (in vitro) | [176,178,179,180,181] | |
| Antiarthritic and joint-protective (in vivo, i.p., animal model of rheumatoid arthritis) | [23,182] | |
| Hepatoprotective and antihepatotoxic (in vivo, acute ethanol-induced fatty liver in rats, 200 mg/kg) | [23,183] | |
| Antiatherosclerotic | [96] [184] | |
| Hypothermic | [81] | |
| Anxiolytic | [81] | |
| Anticonvulsant | [81] | |
| Spasmolytic | [185] | |
| Antifatty liver (in vivo, acute ethanol-induced fatty liver in rats, 200 mg/kg) | [186] | |
| Antimutagenic (in vitro) | [178,187] | |
| Sedative and anesthetic (in vivo, mouse model and fish) | [81,96] | |
| Antivenom (in vivo, mouse model, Bothrops jararaca and Crotalus durissus venom) | [188] | |
| Antibacterial (Helicobacter pylori, Bacillus cereus, B. coagulans, B. subtilis, Staphylococcus aureus, Escherichia coli, Vibrio parahaemolyticus, Proteus mirabilis, and Pseudomonas aeruginosa) | [189,190] | |
| Antifungal (Aspergillus flavus, A. niger, A. parasiticum, Rhizoctonia solani, Helminthosporium oryzae, Trichoconis padwickii, Curvularia lunata, C. pallescens, C. trifolii, Fusarium verticillioides, F. moniliforme, F. oxysporum, Penicillium digitatum, Alternaria dianthi, Trichophyton longifusus and Colletotrichum falcatum) | [23,77,189,191,192] | |
| Antiaflatoxigenic | [76] | |
| Insecticidal (Odontotermes obesus) | [37,193,194] | |
| Insect repellent | [194,195] | |
| Mosquitocidal (Aedes aegypti and Anopheles quadrimaculatus) | [194] | |
| Phytotoxic (Avena fatua, Echinochloa crus-galli, Allium cepa and Phalaris minor) | [189] | |
| C. longa leaf EO | Cytotoxic (in vitro, Hs578T and PC-3 cells) | [94] |
| Antibacterial | [89,94,194] | |
| Antifungal and antiaflatoxigenic | [89,94,194] | |
| Mosquitocidal | [89,94,194] | |
| C. zedoaria rhizome EO | Antioxidant (in vitro, DPPH assay) | [7,23,111,196,197] |
| Cytotoxic (in vitro, SiHa, SNU-1, HepG2, AGS, B16BL6, SMMC-7721, SKOV3, H1299 and HL-60 cells) | [7,110,114,198,199] | |
| Antiangiogenic (in vitro and in vivo) | [200] | |
| Antitumor (in vivo, hepatoma-transplanted rats) | [201,202,203] | |
| Hypoglycemic (in vivo, streptozotocin-induced hyperglycemic Wistar rats) | [204] | |
| Anti-gingivitis (in vivo, streptozotocin-induced hyperglycemic Wistar rats) | [14,204] | |
| Anti-inflammatory | [14] | |
| Antimicrobial (Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus cereus, Salmonella typhimurium and Pseudomonas aeruginosa) | [110] | |
| Antifungal (Colletotrichum falcatum) | [37] | |
| Insecticidal (Odontotermes obesus) | [37] | |
| Larvicidal (Anopheles dirus, LC50= 29.69 ppm; Aedes aegypti, LC50= 31.87 ppm) | [129] | |
| C. aeruginosa rhizome EO | Antiandrogenic (in vivo, patients with androgenic alopecia, 5% w/w) | [30] |
| Antinociceptive | [15] | |
| Antipyretic | [15] | |
| Anti-inflammatory | [15] | |
| Hair regrowth stimulant (in vivo, bald males) | [205] | |
| Skin penetration enhancer (in vivo, androgenic alopecia patients) | [30] | |
| Axillary hair-growth suppressant (in vivo, randomized double-blinded trial, 1 and 5% w/w EO) | [206] | |
| Axillary skin-brightness enhancer (in vivo, randomized double-blinded trial, 1 and 5% w/w EO) | [206] | |
| Antibacterial (Enterococcus faecalis, MIC = 6.25 µg/mL; Streptococcus mutans, MIC= 15.63 µg/mL; Staphylococcus aureus, MIC= 125 µg/mL; Bacillus cereus, MIC = 125 µg/mL) | [29,207] | |
| Antifungal (Candida albicans, MIC= 250 µg/mL) | [2] | |
| Antioxidant (in vitro, DPPH assay, EC50 = 24.32 µg/mL) | [29] | |
| C. aromatica rhizome EO | Anti-inflammatory (in vitro) | [47,49] |
| Cytotoxic (in vitro, LNCaP, HepG2, NSCLC and B16 cells) | [47,49,201,208,209] | |
| Antiproliferative (in vitro, Hep-2 cells; in vivo, mouse model with hepatoma) | [210] | |
| Antitumor (in vivo, patients with primary liver cancer; rats with transplanted hepatoma; and mouse model) | [211,212,213] | |
| Chemoprotective and antifibrosis (in vivo, renal interstitial fibrosis rats, 100, 200 and 300 mg/kg BW, i.p.) | [214,215] | |
| Antioxidant (in vitro, DPPH assay, ABTS assay and β-carotene bleaching tests) | [47,50,54,147] | |
| Antiplatelet aggregation and antithrombotic (in vitro and in vivo) | [216] | |
| Antibacterial (Staphylococcus aureus, Listeria monocytogenes, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) | [47,54,217] | |
| Antifungal (Candida albicans, Saccharomyces cerevisiae) | [47] | |
| Cardioprotective (in vivo, isoproterenol-induced acute myocardial ischemia rats) | [218] | |
| Antidiabetic | [51] | |
| Insecticidal (Liposcelis bostrychophila) | [56] | |
| Antimosquito (Aedes aegypti) | [52] | |
| C. aromatica leaf EO | Antifungal (Colletotrichum falcatum) | [37] |
| Insecticidal (Odontotermes obesus) | [37] | |
| C. phaeocaulis rhizome EO | Antimicrobial (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus) | [100,219] |
| Antifungal (Candida albicans; Saccharomyces cerevisiae) | [100,219] | |
| Antioxidant (in vitro, DPPH assay, IC50 = 2.17–22.36 µg/mL) | [100] | |
| Anti-inflammatory (in vivo, TPA-induced skin inflammation model) | [100] | |
| Cytotoxic (in vitro, LNCaP and B16 cells, IC50 = 20.36–79.44 µg/mL) | [100] | |
| C. zanthorrhiza rhizome EO | Antiproliferative | [220] |
| Anti-inflammatory (in vitro) | [141,221] | |
| Antidiuretic | [141] | |
| Hypotensive | [141] | |
| Antihepatotoxic | [141] | |
| Antioxidant | [141,146] | |
| Antibacterial (Staphylococcus aureus, ZOI = 11.53 ± 0.27 mm) | [2,141,146] | |
| Antifungal (Candida albicans, ZOI = 7.29 ± 0.17 mm) | [2,141] | |
| Analgesic (in vivo, mouse model) | [222] | |
| Antihyperlipidemic (in vivo, rats, 0.2% or 0.5%) | [108] | |
| Antiobesogenic (in vivo, obese rats) | [108] | |
| Hypoglycemic and hypotriglyceridemic (in vivo, diabetic rats) | [223,224] | |
| Larvicidal | [146] | |
| C. amada rhizome EO | Analgesic | [157] |
| Anti-inflammatory | [157] | |
| Antiplatelet | [157] | |
| Cytotoxic (U-87MG, IC50 = 4.92 µg/mL; SJRH30, IC50 = 7.13 µg/mL); RD, IC50 = 7.50 µg/mL) | [157,225,226] | |
| Antitumor (human glioblastoma multiforme cells both in vitro and in nude mice xenografts) | [227] | |
| Hypotriglyceridemic | [157] | |
| Antifungal (Physalospora tucumanensis, Sclerotium rolfsii, Helminthosporium sacchari, Cephalosporium sacchari) | [157,228] | |
| Hepatoprotective (in vivo, carbon tetrachloride-induced hepatotoxicity in male Wister rats) | [156] | |
| Antioxidant (in vitro, DPPH assay, FRAP assay and nitric oxide scavenging assay) | [156,229] | |
| Antibacterial (Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella paratyphi, Vibrio cholera, Enterobacter aerogenes, Streptococcus pneumoniae, Bacillus subtilis, Bacillus cereus, Proteus mirabilis, Proteus vulgaris, Serratia marcescens) | [156,229] | |
| Insect repellent and insecticidal (Musca domestica) | [230] | |
| C. mangga rhizome EO | Antibacterial (Staphylococcus aureus, MIC= 1.2 µL/mL; Bacillus cereus, MIC= 11.1 µL/mL; P. aeruginosa, ZOI = 9.0 mm; E. coli, ZOI= 7.0 mm) | [28] |
| Antifungal (Candida albicans, MIC= 3.7 µL/mL; Cryptococcus neoformans, MIC= 0.1 µL/mL) | [28] | |
| C. glans rhizome EO | Antibacterial (Staphylococcus aureus, ZOI= 17.24 ± 0.07 mm) | [2] |
| Antifungal (C. albicans, ZOI= 7.27 ± 0.17 mm) | [2] | |
| C. singularis rhizome EO | Antibacterial (Bacillus subtillis, MIC= 100 µg/mL; E. coli, MIC= 200 µg/mL) | [106] |
| C. alismatifolia rhizome EO | Antioxidant (in vitro, DPPH and FRAP assays) | [36] |
| C. angustifolia rhizome EO | Antioxidant | [45] |
| C. elata rhizome EO | Antioxidant (in vitro, DPPH assay) | [49] |
| Cytotoxic (in vitro, LNCaP, IC50 = 18.4 μg/mL; HepG2, IC50 = 167.75 μg/mL) | [49] | |
| Anti-inflammatory (in vivo, TPA-induced edema model) | [49] | |
| C. kwangsiensis rhizome EO | Cytotoxic (in vitro, LNCaP, B16 and HepG2) | [49,63] |
| Antitumor | [62,63] | |
| Antioxidant | [62,63] | |
| Anti-inflammatory | [62,63] | |
| Bactericidal | [62,63] | |
| Antifungal | [62,63] | |
| Antiviral | [62,63] | |
| C. yunnanensis rhizome EO | Cytotoxic (in vitro, LNCaP, B16 and HepG2) | [49] |
| C. nankunshanensis rhizome EO | Cytotoxic (in vitro, LNCaP, B16 and HepG2) | [49] |
| Anti-inflammatory (in vivo, TPA-induced edema model) | [49] | |
| C. sichuanensis rhizome EO | Cytotoxic (in vitro, LNCaP, B16 and HepG2) | [49] |
| Antioxidant (in vitro, DPPH assay, IC50= 4.52 μg/mL) | [49,50] | |
| Anti-inflammatory (in vivo, TPA-induced edema model) | [49] | |
| C. rubescens rhizome EO | Cytotoxic (in vitro, LNCaP, B16 and HepG2) | [49] |
| Antioxidant (in vitro, DPPH assay, IC50 = 22.32 μg/mL) | [49] | |
| C. purpurascens rhizome EO | Cytotoxic (in vitro, HT-29, IC50 = 4.9 ± 0.4 μg/mL) | [103] |
| Compound | Biological Activity | Reference |
|---|---|---|
| ar-Turmerone | Antiplatelet Aggregation | [174] |
| Antimutagenic | [178] | |
| Hypoglycemic | [167] | |
| Anti-inflammatory | [71,242,243] | |
| Neuroprotective | [244] | |
| Cytotoxic and antiproliferative | [220,245,246,247,248] | |
| Chemopreventive | [249] | |
| Insect repellent | [120] | |
| Antivenom | [188] | |
| Antibacterial | [250] | |
| Antifungal | [251] | |
| Curdione | Anticancer | [252] |
| Anti-inflammatory | [253] | |
| Antibacterial | [72] | |
| Antifungal | [72] | |
| 1,8-Cineole | Antioxidant | [254,255] |
| Anticarcinogenic | [256] | |
| β-Caryophyllene | Antitumor | [125,257,258,259] |
| Antileishmanial | [260] | |
| Antitrypanosomal | [261] | |
| Myrcene | Antimutagenic | [262] |
| Chemopreventive | [263] | |
| Antiproliferative | [264,265] | |
| Antioxidant | [266] | |
| Germacrone | Anti-inflammatory | [131,267] |
| Antiandrogenic | [137] | |
| Skin-penetration enhancer | [30] | |
| Antiproliferative | [268,269,270] | |
| Antitumor | [270] | |
| Antioxidant | [271] | |
| Antibacterial | [28,272] | |
| Xanthorrhizol | Antioxidant | [273,274] |
| Nephroprotective | [273] | |
| Neuroprotective | [273,274] | |
| Chemopreventive | [249] | |
| Hepatoprotective | [273,274] | |
| Estrogenic | [273,274] | |
| Antiproliferative | [274] | |
| Antitumor | [275] | |
| Anti-inflammatory | [71] | |
| Antibacterial | [273,274] | |
| β-Elemene | Antiproliferative | [210,237] |
| Antiangionenic | [276] | |
| Hepatoprotective | [277] | |
| Antitumor | [278,279] | |
| Terpinolene | Antioxidant | [280] |
| Anti-inflammatory | [125] | |
| Chemoprotective | [263] | |
| 8,9-Dehydro-9-formylcycloiso-longifolene | Antioxidant | [281] |
| Anti-inflammatory | [199] | |
| Curcumol | Anticancer | [282] |
| Curzerene | Antioxidant | [168] |
| Anticancer | [283] | |
| β-Sesquiphellandrene | Antioxidant | [168] |
| Anticancer | [284] | |
| ar-Curcumene | Antitumor | [248] |
| α-Phellandrene | Antioxidant | [285,286] |
| Antinociceptive | [285,286] | |
| Anti-inflammatory | [285,286] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. https://doi.org/10.3390/nu10091196
Dosoky NS, Setzer WN. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients. 2018; 10(9):1196. https://doi.org/10.3390/nu10091196
Chicago/Turabian StyleDosoky, Noura S., and William N. Setzer. 2018. "Chemical Composition and Biological Activities of Essential Oils of Curcuma Species" Nutrients 10, no. 9: 1196. https://doi.org/10.3390/nu10091196
APA StyleDosoky, N. S., & Setzer, W. N. (2018). Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients, 10(9), 1196. https://doi.org/10.3390/nu10091196







