Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification
Abstract
:1. Introduction
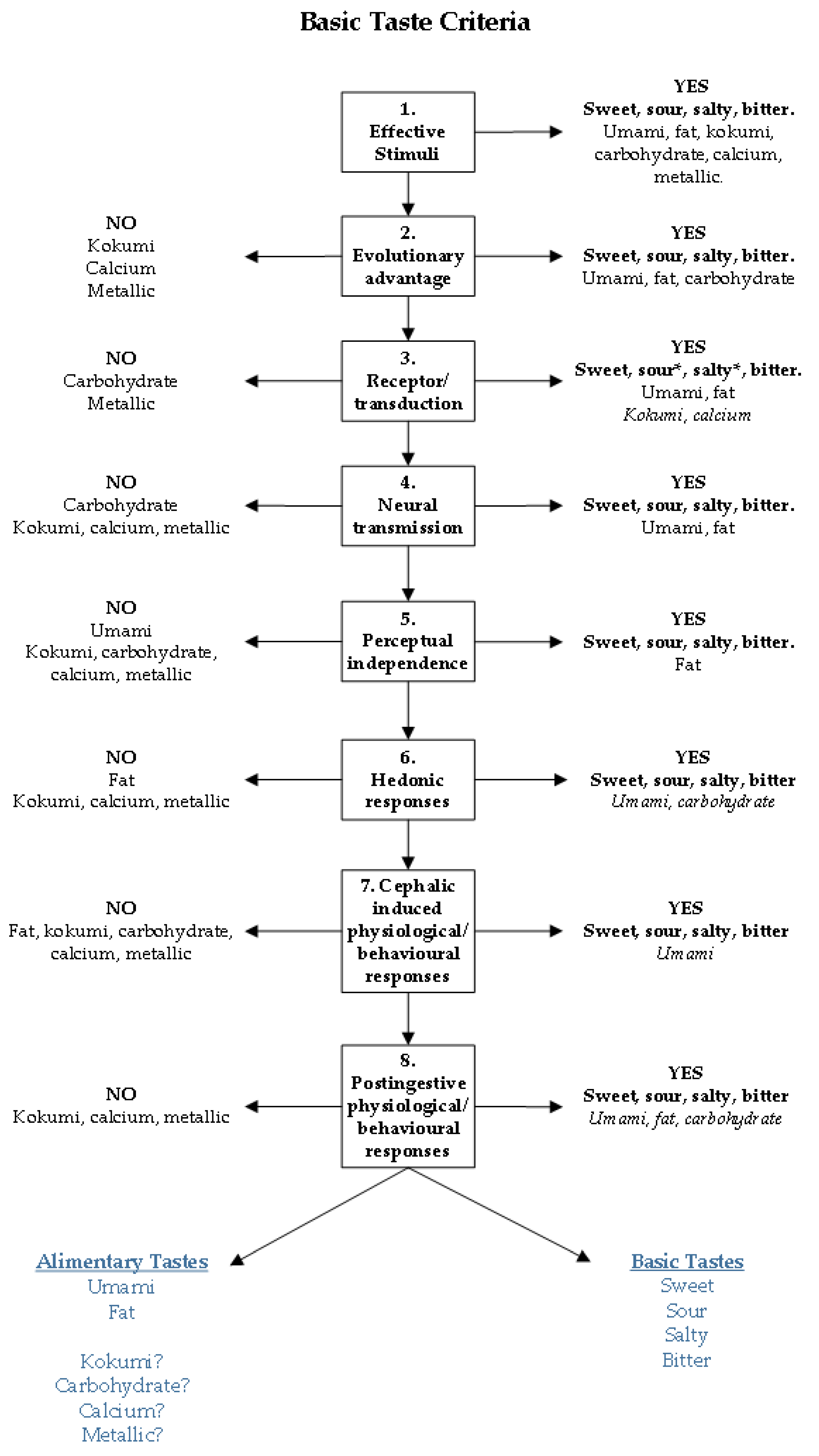
2. Basic Taste Criteria
- A distinct class of effective stimuli must exist.
- Detection of effective stimuli must have an evolutionary benefit.
- Transduction mechanisms that can convert the chemical code of the effective stimuli into an electrical signal, including receptors, must exist.
- Neurotransmission of this electrical signal to taste processing regions of the brain must occur.
- Perceptual quality arising from this processing must be independent from other taste qualities.
- Hedonic responses occur from taste perception.
- Physiological and/or behavioural responses must occur following the activation of taste bud cells by the effective stimuli.
3. Umami Taste and Unique Class of Umami Effective Stimuli
4. Umami Taste from an Evolutionary Perspective
5. Unique Receptor and Neural Transmission of Umami Effective Stimuli
5.1. Unique Receptors for l-glutamate
5.2. Neural Responses to Umami Stimuli
6. Perceptual Independence of Umami Taste
6.1. Umami and Salty
6.2. Umami and Sweet
7. Umami and Hedonics
8. Relationship between Receptor, Perception, and Behavioural Responses of Umami and Sweet Taste
9. Behavioural and Physiological Responses to Umami Effective Stimuli
10. Summary—Is Umami A Basic Taste?
- Having an evolutionary or adaptive advantage: Yes. Umami taste appears to have a biphasic effect due to its involvement in appetite stimulation and then digestion regulation. This occurs through both increasing satiety [43], and the presence of glutamate receptors in the gastrointestinal tract stimulating the release of digestive hormones [40,41,42], providing evidence for umami taste perception existing for evolutionary purposes.
- A distinct class of effective stimuli must exist: Yes. Unique umami effective stimuli found in food includes free l-glutamate, and 5’ribonucleotides, and the prototypical umami taste stimuli are the salts of glutamic acid, MSG or MPG, and disodium salts of IMP and GMP [14,23]. There are a number of foods high in free l-glutamate that would not commonly be described as umami, raising the question of whether high concentrations of naturally occurring l-glutamate elicits an umami like taste in all foods [14,64,65]. Although, common food processing such as curing, and ageing, can increase free l-glutamate and IMP in certain foods, enhancing the umami taste through the glutamate and IMP synergism [26]. Finally, there is similarity between kokumi and umami stimuli, predominately due to the involvement of glutamic acid derivatives [30].
- Transduction mechanisms that can convert the chemical code of the stimulus into an electrical signal is required, including receptors: Yes. Glutamate taste receptors have been identified (T1R1/T1R3, mGluR1, and mGluR4), and these respond to umami stimuli [17,46,47]. This glutamate taste receptor heterodimer (T1R1/T1R3), shares a receptor subunit with the sweet taste receptor (T1R2/T1R3) which has been hypothesised to relate to the perceptual associations that has been found between sweet and umami taste [69,78].
- Neurotransmission of this electrical signal to processing regions of the brain must occur: Yes. Neurotransmission of signals transduced from glutamate receptors occurs, interestingly evidence suggests that different stimuli (MSG, MPG, MSG+IMP) are transduced by different gustatory afferent nerves (CT, GL) for umami taste.
- Perceptual experience arising from this processing must be independent from other taste qualities: No. Studies using multidimensional scaling have found that umami lies perceptually outside of the four basic tastes (sweet, sour, salty, and bitter) (cited in [25]), and individual variation in taste perception across multiple taste dimensions has been established. Nevertheless, prototypical umami stimuli (l-glutamate or IMP/GMP) require cations to produce an umami taste, regardless of whether this cation is sodium or potassium, the additional taste that is imparted is difficult to negate in psychophysical testing. Studies have found perceptual associations with umami and salty taste, specifically in participants considered umami hypotasters at DT [60], and increased saltiness perception of MSG at suprathreshold concentrations [67]. For umami and sweet taste, associations have similarly been found at DT [67] and RT [69], possibly owing to the shared receptor subunit T1R3. Considering current research finds perceptual associations between umami, other basic tastes (salty and sweet) and putative tastes (kokumi), it is relevant to question umami’s classification as a basic taste. Perhaps umami taste fits into a taste classification with other basics (fat) or putative tastes including carbohydrate, kokumi, metallic, and calcium tastes that do elicit a taste perception when presented at high enough concentrations in the oral cavity but this is not necessarily a unique or perceptually salient taste experience.
- Hedonic response from tasting umami stimuli: Yes. Although in aqueous solution MSG is not pleasant in taste, when mixed to certain foods it enhances palatability. The combination of l-glutamate, IMP, sodium, and often kokumi peptides is important in enhancing palatability of certain foods and is found across many cuisines globally.
- Physiological effects must occur following activation of taste bud cells: Yes. Free l-glutamate is not only detected in the oral cavity, but also in the gastrointestinal tract where glutamate taste receptors (T1R1/T1R3) are present [40,42,83]. Glutamate taste receptor heterodimers have been suggested to affect nutrient absorption through regulation of a peptide transporter and glucose transporter through the activation of T1R1/T1R3 by l-glutamate (reviewed in [42]), which also results in CCK secretion in vitro [41]. Although the findings in the literature is mixed, behavioural studies have shown that consumption of MSG and particularly MSG+IMP influences satiety, satiation, and food intake, possibly owing to the secretion of digestive peptides upon stimulation of glutamate receptors in the gastrointestinal tract.
11. A New Class for New Tastes: Alimentary Taste
Author Contributions
Funding
Conflicts of Interest
References
- Kurihara, K. Glutamate: From discovery as a food flavor to role as a basic taste (umami). Am. J. Clin. Nutr. 2009, 90, 719S–722S. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Keast, R. The Test–Retest Reliability of Fatty Acid Taste Thresholds. Chemosens. Percept. 2013, 6, 70–77. [Google Scholar] [CrossRef]
- Dunkel, A.; Köster, J.; Hofmann, T. Molecular and Sensory Characterization of γ-Glutamyl Peptides as Key Contributors to the Kokumi Taste of Edible Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 6712–6719. [Google Scholar] [CrossRef]
- Ueda, Y.; Yonemitsu, M.; Tsubuku, T.; Sakaguchi, M.; Miyajima, R. Flavor Characteristics of Glutathione in Raw and Cooked Foodstuffs. Biosci. Biotechnol. Biochem. 1997, 61, 1977–1980. [Google Scholar] [CrossRef] [Green Version]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Evidence supporting oral sensitivity to complex carbohydrates independent of sweet taste sensitivity in humans. PLOS ONE 2017, 12, e0188784. [Google Scholar] [CrossRef] [PubMed]
- Tordoff, M.G. Calcium: Taste, intake, and appetite. Physiol. Rev. 2001, 81, 1567–1597. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Stevens, D.A.; Chapman, K.W.; Kurtz, A. Metallic taste from electrical and chemical stimulation. Chem. Senses 2005, 30, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.; Costanzo, A. Is fat the sixth taste primary? Evidence and implications. Flavour 2015, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Breslin, P.A.S. An Evolutionary Perspective on Food Review and Human Taste. Curr. Biol. 2013, 23, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Accumulating Evidence Supports a Taste Component for Free Fatty Acids in Humans. Physiol. Behav. 2011, 104, 624–631. [Google Scholar] [CrossRef]
- Kurihara, K.; Kashiwayanagi, M. Physiological Studies on Umami Taste. J. Nutr. 2000, 130, 931S–934S. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef]
- Bartoshuk, L.M. History of Taste Research; Carterette, E.C., Friedman, M.P., Eds.; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- McBurney, D.H.; Gent, J.F. On the nature of taste qualities. Psychol. Bull. 1979, 86, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199. [Google Scholar] [CrossRef]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of fat taste perception: Association with diet and obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the Calcium-sensing Receptor in Human Taste Perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Carbohydrate Taste Sensitivity Is Associated with Starch Intake and Waist Circumference in Adults. J. Nutr. 2017, 147, 2235–2242. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K. New Seasonings. Chem. Senses 2002, 27, 847–849. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S. The Synergistic Taste Effect of Monosodium Glutamate and Disodium 5′-Inosinate. J. Food Sci. 1967, 32, 473–478. [Google Scholar] [CrossRef]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef]
- Shigemura, N.; Shirosaki, S.; Sanematsu, K.; Yoshida, R.; Ninomiya, Y. Genetic and molecular basis of individual differences in human umami taste perception. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. Umami and Food Palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K. Natural occurrence. Food Rev. Int. 1998, 14, 177–211. [Google Scholar] [CrossRef]
- Agostoni, C.; Carratu, B.; Boniglia, C.; Lammardo, A.M.; Riva, E.; Sanzini, E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 508–512. [Google Scholar] [CrossRef]
- Toelstede, S.; Hofmann, T. Kokumi-Active Glutamyl Peptides in Cheeses and Their Biogeneration by Penicillium roquefortii. J. Agric. Food Chem. 2009, 57, 3738–3748. [Google Scholar] [CrossRef]
- Kuroda, M.; Kato, Y.; Yamazaki, J.; Kai, Y.; Mizukoshi, T.; Miyano, H.; Eto, Y. Determination and Quantification of γ-Glutamyl-valyl-glycine in Commercial Fish Sauces. J. Agric. Food Chem. 2012, 60, 7291–7296. [Google Scholar] [CrossRef]
- Paolella, S.; Prandi, B.; Falavigna, C.; Buhler, S.; Dossena, A.; Sforza, S.; Galaverna, G. Occurrence of non-proteolytic amino acyl derivatives in dry-cured ham. Food Res. Int. 2018, 114, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.E.; Glaser, D.; Hawilo, M.E.; Berridge, K.C. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001, 25, 53–74. [Google Scholar] [CrossRef]
- Peng, Y.; Gillis-Smith, S.; Jin, H.; Tränkner, D.; Ryba, N.J.; Zuker, C.S. Sweet and bitter taste in the brain of awake behaving animals. Nature 2015, 527, 512. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Why do we like sweet taste: A bitter tale? Physiol. Behav. 2016, 164, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.; Haryono, R.; Keast, R. Functionality of Fatty Acid Chemoreception: A Potential Factor in the Development of Obesity? Nutrients 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K. Sensory and receptor responses to umami: An overview of pioneering work. Am. J. Clin. Nutr. 2009, 90, 723S–727S. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K. Umami: A universal taste. Food Rev. Int. 2002, 18, 23–38. [Google Scholar] [CrossRef]
- Vazquez, M.; Pearson, P.B.; Beauchamp, G.K. Flavor preferences in malnourished Mexican infants. Physiol. Behav. 1982, 28, 513–519. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Pearson, P. Human development and umami taste. Physiol. Behav. 1991, 49, 1009–1012. [Google Scholar] [CrossRef]
- Kondoh, T.; Mallick, H.N.; Torii, K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am. J. Clin. Nutr. 2009, 90, 832S–837S. [Google Scholar] [CrossRef]
- San Gabriel, A.; Uneyama, H. Amino acid sensing in the gastrointestinal tract. Amino Acids 2013, 45, 451–461. [Google Scholar] [CrossRef]
- Daly, K.; Al-Rammahi, M.; Moran, A.; Marcello, M.; Ninomiya, Y.; Shirazi-Beechey, S.P. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am. J. Physiol. 2013, 304, G271–G282. [Google Scholar] [CrossRef]
- Wauson, E.M.; Lorente-Rodríguez, A.; Cobb, M.H. Minireview: Nutrient Sensing by G Protein-Coupled Receptors. Mol. Endocrinol. 2013, 27, 1188–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masic, U.; Yeomans, M.R. Umami flavor enhances appetite but also increases satiety. Am. J. Clin. Nutr. 2014, 100, 532–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmanov, A.A.; Beauchamp, G.K. Taste Receptor Genes. Annu. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.P.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Delay, E.R.; Hernandez, N.P.; Bromley, K.; Margolskee, R.F. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem. Senses 2006, 31, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Pereira, E.; Margolskee, R.F.; Chaudhari, N.; Roper, S.D. Umami Responses in Mouse Taste Cells Indicate More than One Receptor. J. Neurosci. 2006, 26, 2227–2234. [Google Scholar] [CrossRef] [Green Version]
- Pal Choudhuri, S.; Delay, R.J.; Delay, E.R. Metabotropic glutamate receptors are involved in the detection of IMP and l-amino acids by mouse taste sensory cells. Neuroscience 2016, 316, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, Y.; Yoshida, R.; Ohkuri, T.; Yasumatsu, K.; Voigt, A.; Hübner, S.; Maeda, K.; Boehm, U.; Meyerhof, W.; Ninomiya, Y. Taste responses in mice lacking taste receptor subunit T1R1. J. Physiol. 2013, 591, 1967–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blonde, G.D.; Travers, S.P.; Spector, A.C. Taste sensitivity to a mixture of monosodium glutamate and inosine 5′-monophosphate by mice lacking both subunits of the T1R1+T1R3 amino acid receptor. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R802–R810. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Manabe, T.; Yoshida, R.; Iwatsuki, K.; Uneyama, H.; Takahashi, I.; Ninomiya, Y. Involvement of multiple taste receptors in umami taste: Analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J. Physiol. 2014, 593, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Alarcon, S.; Tharp, A.; Ahmed, O.M.; Estrella, N.L.; Greene, T.A.; Rucker, J.; Breslin, P.A.S. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am. J. Clin. Nutr. 2009, 90, 770S–779S. [Google Scholar] [CrossRef]
- Raliou, M.; Grauso, M.; Hoffmann, B.; Schlegel–Le-Poupon, C.; Nespoulous, C.; Débat, H.; Belloir, C.; Wiencis, A.; Sigoillot, M.; Preet Bano, S.; et al. Human Genetic Polymorphisms in T1R1 and T1R3 Taste Receptor Subunits Affect Their Function. Chem. Senses 2011, 36, 527–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araujo, I.E.T.; Kringelbach, M.L.; Rolls, E.T.; Hobden, P. Representation of umami taste in the human brain. J. Neurophysiol. 2003, 90, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Yasumatsu, K.; Ogiwara, Y.; Takai, S.; Yoshida, R.; Iwatsuki, K.; Torii, K.; Margolskee, R.F.; Ninomiya, Y. Umami taste in mice uses multiple receptors and transduction pathways. J. Physiol. 2012, 590, 1155–1170. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.; Pereira, E.; Roper, S.D. Taste receptors for umami: The case for multiple receptors. Am. J. Clin. Nutr. 2009, 90, 738S–742S. [Google Scholar] [CrossRef]
- Lugaz, O.; Pillias, A.M.; Faurion, A. A new specific ageusia: Some humans cannot taste L-glutamate. Chem. Senses 2002, 27, 105–115. [Google Scholar] [CrossRef]
- Mojet, J.; Christ-Hazelhof, E.; Heidema, J. Taste perception with age: Generic or specific losses in threshold sensitivity to the five basic tastes? Chem. Senses 2001, 26, 845–860. [Google Scholar] [CrossRef]
- Prescott, J. Effects of added glutamate on liking for novel food flavors. Appetite 2004, 42, 143–150. [Google Scholar] [CrossRef]
- Yamamoto, T.; Watanabe, U.; Fujimoto, M.; Sako, N. Taste preference and nerve response to 5′-inosine monophosphate are enhanced by glutathione in mice. Chem. Senses 2009, 34, 809–818. [Google Scholar] [CrossRef]
- Löliger, J.R. Function and Importance of Glutamate for Savory Foods. J. Nutr. 2000, 130, 915S–920S. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, T. Free and Bound Glutamate in Natural Products. In Glutamic Acid; Raven Press: New York, NY, USA, 1979; pp. 25–34. [Google Scholar]
- Jinap, S.; Hajeb, P. Glutamate. Its applications in food and contribution to health. Appetite 2010, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese Women Have Lower Monosodium Glutamate Taste Sensitivity and Prefer Higher Concentrations Than Do Normal-weight Women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child. 2012, 97, 1048. [Google Scholar] [CrossRef]
- Kubota, M.; Toda, C.; Nagai-Moriyama, A. Relationship between umami taste acuity with sweet or bitter taste acuity and food selection in Japanese women university students. Asia Pac. J. Clin. Nutr. 2018, 27, 107–112. [Google Scholar] [CrossRef]
- Singh, P.B.; Schuster, B.; Seo, H.S. Variation in umami taste perception in the German and Norwegian population. Eur. J. Clin. Nutr. 2010, 64, 1248–1250. [Google Scholar] [CrossRef] [Green Version]
- Sinopoli, D.A.; Lawless, H.T. Taste Properties of Potassium Chloride Alone and in Mixtures with Sodium Chloride Using a Check-All-That-Apply Method. J. Food Sci. 2012, 77, S319–S322. [Google Scholar] [CrossRef]
- Sforza, S.; Cavatorta, V.; Galaverna, G.; Dossena, A.; Marchelli, R. Accumulation of non-proteolytic aminoacyl derivatives in Parmigiano-Reggiano cheese during ripening. Int. Dairy J. 2009, 19, 582–587. [Google Scholar] [CrossRef]
- Kuroda, M.; Kato, Y.; Yamazaki, J.; Kai, Y.; Mizukoshi, T.; Miyano, H.; Eto, Y. Determination and quantification of the kokumi peptide, gamma-glutamyl-valyl-glycine, in commercial soy sauces. Food Chem. 2013, 141, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef]
- Jinap, S.; Hajeb, P.; Karim, R.; Norliana, S.; Yibadatihan, S.; Abdul-Kadir, R. Reduction of sodium content in spicy soups using monosodium glutamate. Food Nutr. Res. 2016, 60, 30463. [Google Scholar] [CrossRef] [Green Version]
- Leong, J.; Kasamatsu, C.; Ong, E.; Hoi, J.T.; Loong, M.N. A study on sensory properties of sodium reduction and replacement in Asian food using difference-from—Control test. Food Sci. Nutr. 2016, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F. Experimental studies of food choices and palatability responses in European subjects exposed to the Umami taste. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 376–379. [Google Scholar]
- Noel, C.A.; Finlayson, G.; Dando, R. Prolonged Exposure to Monosodium Glutamate in Healthy Young Adults Decreases Perceived Umami Taste and Diminishes Appetite for Savory Foods. J. Nutr. 2018, 148, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Son, H.J.; Kim, Y.; Kim, K.H.; Kim, J.T.; Moon, H.; Kim, M.J.; Misaka, T.; Rhyu, M.R. Modulation of sweet taste by umami compounds via sweet taste receptor. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.; Nowson, C.; Orellana, L.; Bolhuis, D.; Duesing, K.; Keast, R. Effect of dietary fat intake and genetics on fat taste sensitivity: A co-twin randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 683–694. [Google Scholar] [CrossRef]
- Kitamura, A.; Tsurugizawa, T.; Torii, K. Biological Significance of Glutamate Signaling during Digestion of Food through the Gut-Brain Axis. Digestion 2011, 83 (Suppl. 1), 37–43. [Google Scholar] [CrossRef]
- Hodson, N.A.; Linden, R.W.A. The effect of monosodium glutamate on parotid salivary flow in comparison to the response to representatives of the other four basic tastes. Physiol. Behav. 2006, 89, 711–717. [Google Scholar] [CrossRef]
- Norton, M.; Murphy, K.G. Targeting gastrointestinal nutrient sensing mechanisms to treat obesity. Curr. Opin. Pharmacol. 2017, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H. Gut peptides in the control of food intake. Int. J. Obes. 2009, 33, S7. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiol. Behav. 2013, 116–117, 23–29. [Google Scholar] [CrossRef]
- Anderson, G.H.; Fabek, H.; Akilen, R.; Chatterjee, D.; Kubant, R. Acute effects of monosodium glutamate addition to whey protein on appetite, food intake, blood glucose, insulin and gut hormones in healthy young men. Appetite 2018, 120, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Beauchamp, G.K.; Mennella, J.A. Infant regulation of intake: The effect of free glutamate content in infant formulas. Am. J. Clin. Nutr. 2012, 95, 875–881. [Google Scholar] [CrossRef]
- Masic, U.; Yeomans, M.R. Monosodium glutamate delivered in a protein-rich soup improves subsequent energy compensation. J. Nutr. Sci. 2014, 3, e15. [Google Scholar] [CrossRef]
- van Avesaat, M.; Troost, F.J.; Ripken, D.; Peters, J.; Hendriks, H.F.J.; Masclee, A.A.M. Intraduodenal infusion of a combination of tastants decreases food intake in humans. Am. J. Clin. Nutr. 2015, 102, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.; Zuker, C.S. The cells and peripheral representation of sodium taste in mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. https://doi.org/10.3390/nu11010182
Hartley IE, Liem DG, Keast R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients. 2019; 11(1):182. https://doi.org/10.3390/nu11010182
Chicago/Turabian StyleHartley, Isabella E, Djin Gie Liem, and Russell Keast. 2019. "Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification" Nutrients 11, no. 1: 182. https://doi.org/10.3390/nu11010182
APA StyleHartley, I. E., Liem, D. G., & Keast, R. (2019). Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients, 11(1), 182. https://doi.org/10.3390/nu11010182





