A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota
Abstract
:1. Introduction
2. Effect of HMs, Pesticides and ABs on the Composition and Function of GM
2.1. HMs
2.2. Pesticides
2.3. ABs
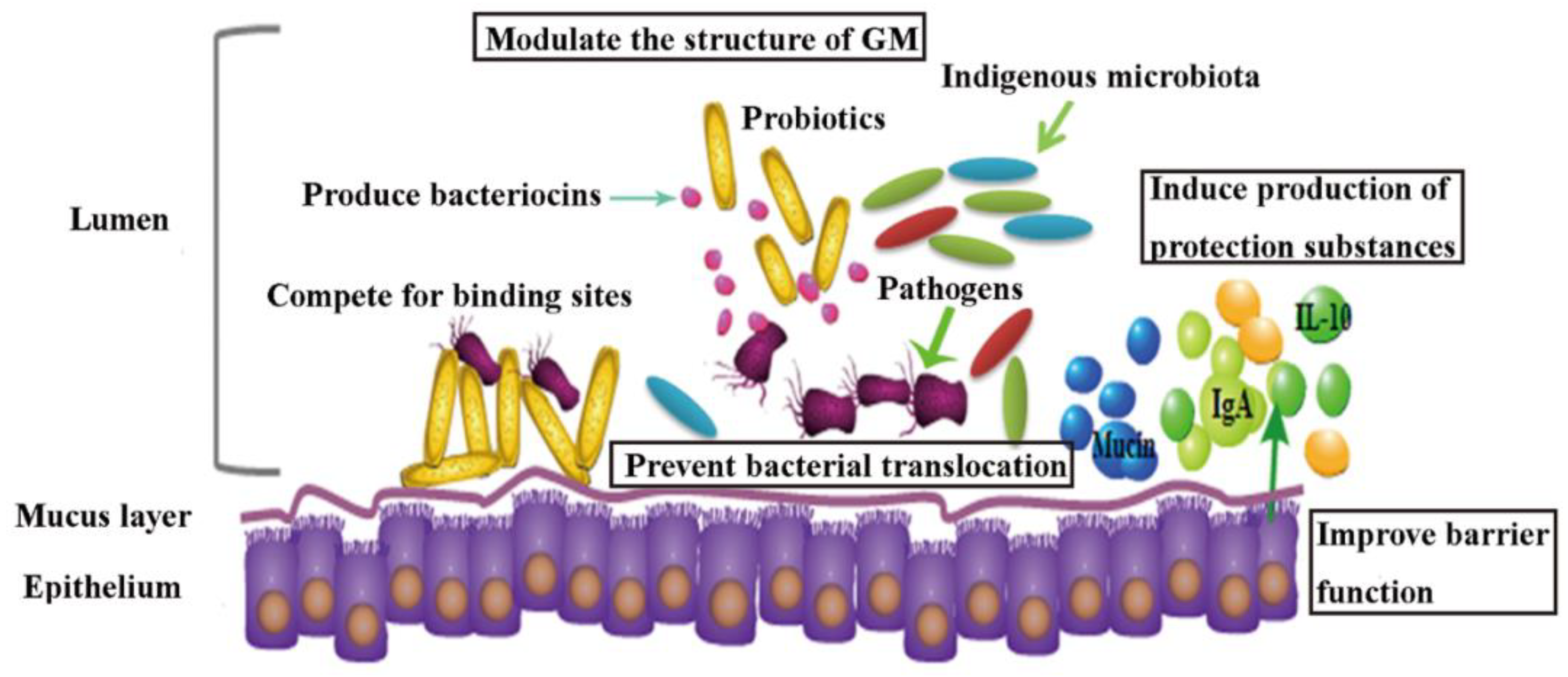
3. Probiotics as a Potential Tool in Contaminants Remediation
3.1. Role of Probiotics in HMs Remediation In Vivo
3.2. Probiotics’ Role in Pesticides Remediation In Vivo
3.3. Probiotic Intervention in AAD Patients and Animal Models
4. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. Int. J. Environ. Res. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Calvelo Pereira, R.; Camps-Arbestain, M.; Rodríguez Garrido, B.; Macías, F.; Monterroso, C. Behaviour of α-, β-, γ-, and δ-hexachlorocyclohexane in the soil–plant system of a contaminated site. Environ. Pollut. 2006, 144, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sridhara Chary, N.; Kamala, C.T.; Samuel Suman Raj, D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.Z.; Hafida, M.; Merzouk, S.A.; Loukidi, B.; Taouli, K.; Narce, M. Hemostatic, inflammatory, and oxidative markers in pesticide user farmers. Biomarkers 2016, 21, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016, 65, 1100–1109. [Google Scholar] [CrossRef]
- Narayana, K. An aminoglycoside antibiotic gentamycin induces oxidative stress, reduces antioxidant reserve and impairs spermatogenesis in rats. Toxicol. Sci. 2008, 33, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Metal-induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Koner, B.C.; Banerjee, B.D.; Ray, A. Organochlorine pesticide-induced oxidative stress and immune suppression in rats. Indian J. Exp. Biol. 1998, 36, 395–398. [Google Scholar] [PubMed]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-Induced Perturbations of the Intestinal Microbiota Alter Host Susceptibility to Enteric Infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef] [Green Version]
- Poet, T.S.; Wu, H.; Kousba, A.A.; Timchalk, C. In Vitro Rat Hepatic and Intestinal Metabolism of the Organophosphate Pesticides Chlorpyrifos and Diazinon. Toxicol. Sci. 2003, 72, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, J.; Massart, S.; Vandamme, P.; De Brandt, E.; Pot, B.; Foligné, B. Ecotoxicology inside the gut: Impact of heavy metals on the mouse microbiome. BMC Pharmacol. Toxicol. 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol. Res. 2010, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl. Environ. Microbiol. 2013, 79, 1508–1515. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective Effects of Lactobacillus plantarum CCFM8610 against Chronic Cadmium Toxicity in Mice Indicate Routes of Protection besides Intestinal Sequestration. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef]
- Kamaladevi, A.; Ganguli, A.; Balamurugan, K. Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in Caenorhabditis elegans. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2016, 179, 19–28. [Google Scholar] [CrossRef]
- Trinder, M.; McDowell, T.W.; Daisley, B.A.; Ali, S.N.; Leong, H.S.; Sumarah, M.W.; Reid, G. Probiotic Lactobacillus rhamnosus reduces organophosphate pesticide absorption and toxicity to Drosophila melanogaster. Appl. Environ. Microbiol. 2016, 82, 6204–6213. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Breton, J.; Daniel, C.; Dewulf, J.; Pothion, S.; Froux, N.; Sauty, M.; Thomas, P.; Pot, B.; Foligné, B. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 2013, 222, 132–138. [Google Scholar] [CrossRef]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? npj Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Wu, G.; Xiao, X.; Feng, P.; Xie, F.; Yu, Z.; Yuan, W.; Liu, P.; Li, X. Gut remediation: A potential approach to reducing chromium accumulation using Lactobacillus plantarum TW1-1. Sci. Rep. 2017, 7, 15000. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Zeng, Z.; Liu, Z.; Fu, Z. Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem. Res. Toxicol. 2015, 28, 2000–2009. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, Z.; Wu, Y.; Zhang, S.; Fu, Z. Oral Exposure of Mice to Carbendazim Induces Hepatic Lipid Metabolism Disorder and Gut Microbiota Dysbiosis. Toxicol. Sci. 2015, 147, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, J.L.; Kang, H.K.; Spivey, G. Arsenic levels in human blood, urine, and hair in response to exposure via drinking water. Environ. Res. 1979, 20, 24–32. [Google Scholar] [CrossRef]
- Wester, R.C.; Maibach, H.I.; Sedik, L.; Melendres, J.; Dizio, S.; Wade, M. In Vitro Percutaneous Absorption of Cadmium from Water and Soil into Human Skin. Toxicol. Sci. 1992, 19, 1–5. [Google Scholar] [CrossRef]
- Loubières, Y.; Lassence, A.D.; Bernier, M.; Vieillard-Baron, A.; Schmitt, J.-M.; Page, B.; Jardin, F. Acute, Fatal, Oral Chromic Acid Poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lu, L.; Jin, C.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie van Leeuwenhoek 2017, 110, 501–513. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Liu, K.; Shen, J. Exposing to Cadmium Stress Cause Profound Toxic Effect on Microbiota of the Mice Intestinal Tract. PLoS ONE 2014, 9, e85323. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Hassanzadeh, P.; Alaei, S. Cadmium chloride exhibits a profound toxic effect on bacterial microflora of the mice gastrointestinal tract. Hum. Exp. Toxicol. 2010, 30, 152–159. [Google Scholar] [CrossRef]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef]
- Wu, J.; Wen, X.W.; Faulk, C.; Boehnke, K.; Zhang, H.; Dolinoy, D.C.; Xi, C. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-specific Bodyweight Increases in Adult Mice. Toxicol. Sci. 2016, 151, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, B.M.; Becker, A.; Ayiseh, R.B.; Hildebrand, F.; Raes, J.; Huys, G.; Vandenabeele, P. Gut microbiota affects sensitivity to acute DSS-induced colitis independently of host genotype. Inflamm. Bowel Dis. 2013, 19, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Vich Vila, A.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction Between Obesity and the Gut Microbiota: Relevance in Nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Abdou, R.M.; Zhu, L.; Baker, R.D.; Baker, S.S. Gut Microbiota of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral administration of probiotics inhibits heavy metal cadmium absorption by protecting intestinal barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef]
- Sarwar, M. The dangers of pesticides associated with public health and preventing of the risks. Int. J. Bioinform. Biomed. Eng. 2015, 1, 130–136. [Google Scholar]
- El-Gendy, K.S.; Aly, N.M.; Mahmoud, F.H.; Kenawy, A.; El-Sebae, A.K.H. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem. Toxicol. 2010, 48, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Caldini, G.; Moretti, M.; Trotta, F.; Pasquini, R.; Cenci, G. Modulatory activity of a Lactobacillus casei strain on 1,2-dimethylhydrazine-induced genotoxicity in rats. Environ. Mol. Mutagen. 2008, 49, 192–199. [Google Scholar] [CrossRef]
- Daisley, B.A.; Trinder, M.; McDowell, T.W.; Collins, S.L.; Sumarah, M.W.; Reid, G. Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent lactobacilli interactions in a Drosophila melanogaster insect model. Appl. Environ. Microbiol. 2018, 84, e02820-17. [Google Scholar] [CrossRef] [PubMed]
- Harishankar, M.K.; Sasikala, C.; Ramya, M. Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos. 3 Biotech 2013, 3, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Racke, K.D.; Bollag, J. Isolation and characterization of a chlorinated-pyridinol-degrading bacterium. Appl. Environ. Microbiol. 1997, 63, 4096–4098. [Google Scholar]
- Fang, B.; Li, J.W.; Zhang, M.; Ren, F.Z.; Pang, G.F. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem. Toxicol. 2018, 111, 144–152. [Google Scholar] [CrossRef]
- Joly Condette, C.; Bach, V.; Mayeur, C.; Gay-Quéheillard, J.; Khorsi-Cauet, H. Chlorpyrifos Exposure During Perinatal Period Affects Intestinal Microbiota Associated With Delay of Maturation of Digestive Tract in Rats. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 30–40. [Google Scholar] [CrossRef]
- Joly, C.; Gay-Quéheillard, J.; Léké, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and in the rat. Environ. Sci. Pollut. Res. 2013, 20, 2726–2734. [Google Scholar] [CrossRef]
- Reygner, J.; Joly Condette, C.; Bruneau, A.; Delanaud, S.; Rhazi, L.; Depeint, F.; Abdennebi-Najar, L.; Bach, V.; Mayeur, C.; Khorsi-Cauet, H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. Int. J. Environ. Res. Public Health 2016, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Chi, L.; Tu, P.; Bian, X.; Thomas, J.; Ru, H.; Lu, K. The organophosphate malathion disturbs gut microbiome development and the quorum-Sensing system. Toxicol. Lett. 2018, 283, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehata, A.A.; Schrödl, W.; Aldin, A.A.; Hafez, H.M.; Krüger, M. The Effect of Glyphosate on Potential Pathogens and Beneficial Members of Poultry Microbiota In Vitro. Curr. Microbiol. 2013, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shao, W.; Zhang, C.; Xu, C.; Wang, Q.; Liu, H.; Sun, H.; Jiang, Z.; Gu, A. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 2017, 226, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Coman, M.M.; Olek, R.A.; Fiorini, D.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Fedeli, D.; Gabbianelli, R. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ. Sci. Pollut. Res. 2016, 23, 10930–10937. [Google Scholar] [CrossRef]
- Kan, H.; Zhao, F.; Zhang, X.-X.; Ren, H.; Gao, S. Correlations of Gut Microbial Community Shift with Hepatic Damage and Growth Inhibition of Carassius auratus Induced by Pentachlorophenol Exposure. Environ. Sci. Technol. 2015, 49, 11894–11902. [Google Scholar] [CrossRef]
- Jin, C.; Zeng, Z.; Fu, Z.; Jin, Y. Oral imazalil exposure induces gut microbiota dysbiosis and colonic inflammation in mice. Chemosphere 2016, 160, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Luo, T.; Zhu, Z.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z.; Jin, Y. Imazalil exposure induces gut microbiota dysbiosis and hepatic metabolism disorder in zebrafish. Comp. Biochem. Phys. C 2017, 202, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Growth promotion and gut microbiota: Insights from antibiotic use. Environ. Microbiol. 2015, 17, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Dong, H.; Yuan, X.; Wang, W.; Qiang, Z. Occurrence and removal of antibiotics in ecological and conventional wastewater treatment processes: A field study. J. Environ. Manag. 2016, 178, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Wu, H.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; Lin, H.; Ma, J. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef]
- Demoly, P.; Benahmed, S.; Valembois, M.; Sahla, H.; Messaad, D.; Godard, P.; Michel, F.B.; Bousquet, J. Allergy to macrolide antibiotics. Review of the literature. Presse Med. 2000, 29, 321–326. [Google Scholar]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-induced alterations of the murine gut microbiota and subsequent effects on colonization resistance against Clostridium difficile. mBio 2015, 6, e00974-15. [Google Scholar] [CrossRef]
- Buffie, C.G.; Jarchum, I.; Equinda, M.; Lipuma, L.; Gobourne, A.; Viale, A.; Ubeda, C.; Xavier, J.; Pamer, E.G. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 2012, 80, 62–73. [Google Scholar] [CrossRef]
- Grazul, H.; Kanda, L.L.; Gondek, D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes 2016, 7, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2014, 517, 205–208. [Google Scholar] [CrossRef]
- Fouhy, F.; Guinane, C.M.; Hussey, S.; Wall, R.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; et al. High-Throughput Sequencing Reveals the Incomplete, Short-Term Recovery of Infant Gut Microbiota following Parenteral Antibiotic Treatment with Ampicillin and Gentamicin. Antimicrob. Agents Chemother. 2012, 56, 5811–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; Forslund, K.; Bork, P.; De Vos, W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 2016, 7, 10410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, M.C.; Dobson, A.; O’Sullivan, O.; Crispie, F.; Fouhy, F.; Cotter, P.D.; Shanahan, F.; Kiely, B.; Hill, C.; Ross, R.P. Effect of broad-and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA 2011, 108, 4639–4644. [Google Scholar] [CrossRef] [PubMed]
- Pien, F.D.; Shrum, S.; Swenson, J.M.; Hill, B.C.; Thornsberry, C.; Farmer, J.J. Colonization of human wounds by Escherichia vulneris and Escherichia hermannii. J. Clin. Microbiol. 1985, 22, 283–285. [Google Scholar]
- Chaudhury, A.; Nath, G.; Tikoo, A.; Sanyal, S.C. Enteropathogenicity and Antimicrobial Susceptibility of New Escherichia Spp. J. Diarrhoeal Dis. Res. 1999, 17, 85–87. [Google Scholar]
- Jantsch, J.; Chikkaballi, D.; Hensel, M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 2011, 240, 185–195. [Google Scholar] [CrossRef]
- Reeves, A.E.; Theriot, C.M.; Bergin, I.L.; Huffnagle, G.B.; Schloss, P.D.; Young, V.B. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes 2011, 2, 145–158. [Google Scholar] [CrossRef]
- Schubert, A.M.; Rogers, M.A.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 2014, 5, e01021-14. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Stephens, D.A.; Loo, V.G.; Edens, T.J.; Behr, M.A.; Dewar, K.; Manges, A.R. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome 2013, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobel, Y.R.; Cox, L.M.; Kirigin, F.F.; Bokulich, N.A.; Yamanishi, S.; Teitler, I.; Chung, J.; Sohn, J.; Barber, C.M.; Goldfarb, D.S.; et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015, 6, 7486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Pi, Y.; Mu, C.-L.; Peng, Y.; Huang, Z.; Zhu, W.-Y. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Q.; Huan, F.; Qu, J.; Liu, W.; Gu, A.; Wang, Y.; Jiang, Z. Changes in Gut Microbiota May Be Early Signs of Liver Toxicity Induced by Epoxiconazole in Rats. Chemotherapy 2014, 60, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tuomola, E.; Crittenden, R.; Playne, M.; Isolauri, E.; Salminen, S. Quality assurance criteria for probiotic bacteria. Am. J. Clin. Nutr. 2001, 73, 393s–398s. [Google Scholar] [CrossRef] [PubMed]
- Chiocchetti, G.M.; Jadán-Piedra, C.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2018, 1–12. [Google Scholar] [CrossRef]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Chaudhari, A.; Kumar, G.N. Amelioration of cadmium- and mercury-induced liver and kidney damage in rats by genetically engineered probiotic Escherichia coli Nissle 1917 producing pyrroloquinoline quinone with oral supplementation of citric acid. Nutrition 2016, 32, 1285–1294. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Khatun, S.; Maity, M.; Jana, S.; Perveen, H.; Dash, M.; Dey, A.; Jana, L.R.; Maity, P.P. Association of Vitamin B12, Lactate Dehydrogenase, and Regulation of NF-κB in the Mitigation of Sodium Arsenite-Induced ROS Generation in Uterine Tissue by Commercially Available Probiotics. Probiotics Antimicrob. Proteins 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ojekunle, O.; Banwo, K.; Sanni, A.I. In vitro and in vivo evaluation of Weissella cibaria and Lactobacillus plantarum for their protective effect against cadmium and lead toxicities. Lett. Appl. Microbiol. 2017, 64, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Toxicological aspects of metallothionein. Cell. Mol. Biol. (Noisy-le-Grand France) 2000, 46, 451–463. [Google Scholar]
- Randazzo, C.L.; Pino, A.; Ricciardi, L.; Romano, C.; Comito, D.; Arena, E.; Saitta, S.; Caggia, C. Probiotic supplementation in systemic nickel allergy syndrome patients: Study of its effects on lactic acid bacteria population and on clinical symptoms. J. Appl. Microbiol. 2014, 118, 202–211. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.; Burton, J.P.; Gloor, G.B.; Reid, G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014, 5, e01580-14. [Google Scholar] [CrossRef] [PubMed]
- Masson, P. Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicol. Lett. 2011, 206, 5–13. [Google Scholar] [CrossRef]
- Bouhafs, L.; Moudilou, E.N.; Exbrayat, J.M.; Lahouel, M.; Idoui, T. Protective effects of probiotic Lactobacillus plantarum BJ0021 on liver and kidney oxidative stress and apoptosis induced by endosulfan in pregnant rats. Ren. Fail. 2015, 37, 1370–1378. [Google Scholar] [CrossRef]
- Cao, L.; Yang, X.J.; Li, Z.J.; Sun, F.F.; Wu, X.H.; Yao, J.H. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.202912. Poult. Sci. 2012, 91, 3065–3071. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Russell, R.J.; Scott, C.; Jackson, C.J.; Pandey, R.; Pandey, G.; Taylor, M.C.; Coppin, C.W.; Liu, J.-W.; Oakeshott, J.G. The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol. Appl. 2011, 4, 225–248. [Google Scholar] [CrossRef]
- Daisley, B.A.; Trinder, M.; McDowell, T.W.; Welle, H.; Dube, J.S.; Ali, S.N.; Leong, H.S.; Sumarah, M.W.; Reid, G. Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model. Sci. Rep. 2017, 7, 2703. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, I.; Sybesma, W.; Phothirath, P.; Ananta, E.; Mercenier, A. Application of probiotics in food products—Challenges and new approaches. Curr. Opin Biotechnol. 2010, 21, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Bengmark, S.; Enck, P.; Haller, D.; Herz, U.; Kalliomaki, M.; Kudo, S.; Lenoir-Wijnkoop, I.; Mercenier, A.; Myllyluoma, E.; et al. Guidance for Substantiating the Evidence for Beneficial Effects of Probiotics: Current Status and Recommendations for Future Research. J. Nutr. 2010, 140, 671S–676S. [Google Scholar] [CrossRef] [Green Version]
- Conway, S.; Hart, A.; Clark, A.; Harvey, I. Does eating yogurt prevent antibiotic-associated diarrhoea?: A placebo-controlled randomised controlled trial in general practice. Br. J. Gen. Pract. 2007, 57, 953–959. [Google Scholar] [CrossRef]
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; Gravenor, M.B. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013, 382, 1249–1257. [Google Scholar] [CrossRef]
- Kotowska, M.; Albrecht, P.; Szajewska, H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: A randomized double-blind placebo-controlled trial. Aliment. Pharmacol. Ther. 2005, 21, 583–590. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015, 42, 1149–1157. [Google Scholar] [CrossRef]
- Beniwal, R.S.; Arena, V.C.; Thomas, L.; Narla, S.; Imperiale, T.F.; Chaudhry, R.A.; Ahmad, U.A. A Randomized Trial of Yogurt for Prevention of Antibiotic-Associated Diarrhea. Dig. Dis. Sci. 2003, 48, 2077–2082. [Google Scholar] [CrossRef]
- McFarland, L.V. Meta-Analysis of Probiotics for the Prevention of Antibiotic Associated Diarrhea and the Treatment of Clostridium difficile Disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef]
- Schmidt, V.; Gomez-Chiarri, M.; Roy, C.; Smith, K.; Amaral-Zettler, L. Subtle Microbiome Manipulation Using Probiotics Reduces Antibiotic-Associated Mortality in Fish. mSystems 2017, 2, e00133-17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Narbad, A.; Chen, W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J. Appl. Microbiol. 2018, 124, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial Population Differentials between Mucosal and Submucosal Intestinal Tissues in Advanced Crohn’s Disease of the Ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Vaneechoutte, M.; Doerffel, Y. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel Dis. 2008, 14, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Day, A.S.; Huinao, K.D.; Leach, S.T.; Lemberg, D.A.; Dowd, S.E.; Mitchell, H.M. Microbial dysbiosis in pediatric patients with Crohn’s disease. J. Clin. Microbiol. 2012, 50, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Theissig, F.; Rückert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kühn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef]
- Schmidt, V.T.; Reveillaud, J.; Zettler, E.; Mincer, T.J.; Murphy, L.; Amaral-Zettler, L.A. Oligotyping reveals community level habitat selection within the genus Vibrio. Front. Microbiol. 2014, 5, 563. [Google Scholar] [CrossRef]
- De Schryver, P.; Vadstein, O. Ecological theory as a foundation to control pathogenic invasion in aquaculture. ISME J. 2014, 8, 2360–2368. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ai, C.; Wang, G.; Liu, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Oral application of lactic acid bacteria following treatment with antibiotics inhibits allergic airway inflammation. J. Appl. Microbiol. 2015, 119, 809–817. [Google Scholar] [CrossRef]
- Ekmekciu, I.; von Klitzing, E.; Fiebiger, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Bereswill, S.; Heimesaat, M.M. The probiotic compound VSL#3 modulates mucosal, peripheral, and systemic immunity following murine broad-spectrum antibiotic treatment. Front. Cell. Infect. Microbiol. 2017, 7, 167. [Google Scholar] [CrossRef]
- Jijon, H.; Backer, J.; Diaz, H.; Yeung, H.; Thiel, D.; McKaigney, C.; De Simone, C.; Madsen, K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology 2004, 126, 1358–1373. [Google Scholar] [CrossRef]
- Shi, Y.; Zhai, Q.; Li, D.; Mao, B.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Restoration of cefixime-induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol. Res. 2017, 200, 14–24. [Google Scholar] [CrossRef]
- Buts, J.-P.; Keyser, N.D.; Raedemaeker, L.D. Saccharomyces boulardii Enhances Rat Intestinal Enzyme Expression by Endoluminal Release of Polyamines. Pediatr. Res. 1994, 36, 522–527. [Google Scholar] [CrossRef]
- Qamar, A.; Aboudola, S.; Warny, M.; Michetti, P.; Pothoulakis, C.; LaMont, J.T.; Kelly, C.P. Saccharomyces boulardii stimulates intestinal immunoglobulin a immune response to clostridium difficiletoxin a in mice. Infect. Immun. 2001, 69, 2762–2765. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [Green Version]
- Gopal, P.K.; Prasad, J.; Smart, J.; Gill, H.S. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food. Microbiol. 2001, 67, 207–216. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [Green Version]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Gironés, O.; Múzquiz, J.L. In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 2007, 122, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jama, A.M.; Mitić-Ćulafić, D.; Kolarević, S.; Đurašević, S.F.; Knežević-Vukčević, J. Protective effect of probiotic bacteria against cadmium-induced genotoxicity in rat hepatocytes in vivo and in vitro. Arch. Biol. Sci. 2012, 64, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The protective effects of probiotic bacteria on cadmium toxicity in rats. J. Med. Food 2016, 20, 189–196. [Google Scholar] [CrossRef]
- Majlesi, M.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of probiotic Bacillus coagulans and Lactobacillus plantarum on alleviation of mercury toxicity in rat. Probiotics Antimicrob. Proteins 2017, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jin, D.; Yu, S.; Etareri Evivie, S.; Muhammad, Z.; Huo, G.; Liu, F. In Vitro and In Vivo Evaluation of Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207 for the Alleviative Effect on Lead Toxicity. Nutrients 2017, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388.e21–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chow, K.-H.; Fleming, E.; Oh, J. Selective colonization ability of human fecal microbes in different mouse gut environments. ISME J. 2018. [Google Scholar] [CrossRef]

| Type | References | Models | Pollutants and Dosage | Outcomes | Main Conclusion on GM |
|---|---|---|---|---|---|
| HMs | [30] | Mice | Cd at 10 mg/L for 10 weeks | Hepatic inflammation, energy metabolism dysregulation | Firmicutes↓, Bacteroidetes↑, γ- Proteobacteria↓ |
| [41] | Mice | Pb at 32 ppm for 2 weeks | Bodyweight ↑ | Firmicutes/Bacteroidetes↑, Desulfovibrionaceae↑, Barnesiella↑, Clostridium XIVb↑, Lactococcus↓, Enterorhabdus↓, Caulobacterales↓ | |
| [40] | Mice | As at 10 ppm for 4 weeks | Perturbed lipid metabolites, indole-containing metabolites, isoflavone metabolites, and bile acid metabolites | Firmicutes↓, Bacteroidetes↑ | |
| [29] | Mice | Cr (VI) at 2 mM for 7 weeks | Oxidative stress↑, liver damage, GM disturbance | Bacteroidetes↑, Tenericutes↑, Firmicutes↓, Paraprevotellaceae↑, S24-7↑, Lachnospiraceae↓ | |
| Pesticides | [58,60] | Rats | Chlorpyrifos at 0.3 or 3.0 mg/kg bodyweight/day for 9 weeks | Obese and diabetic phenotypes↑, bacterial translocation↑ | Sutterella↑, Candidatus arthromitus↓, Olsenella↑ Clostridium sensu stricto 1↑, Amphibacillus↑, Enterorhabdus↑, Alloprevotella↑ |
| [65] | Mice | Malathion at 2 mg/L in drinking water (∼0.6 mg/kg bodyweight/ day) for 13 weeks | Motility and pathogenicity↑ | Corynebacterium↑, S24-7↑, Planococcaceae↓, Christenseneellaceae↓, Clostridium↑, Lachnospiraceae_Other↓, Anaerostipes↓, Blautia↓, Dorea↓, Roseburia↓, Mogibacteriaceae↑, Akkermansia↓, | |
| [66] | Mice | Diazinon at 4 mg/L for 13 weeks | Taurine level↑, glycine acetyltransferase and threonine dehydrogenase↓ in male mice | Bacteroidaceae_Bacteroides↑, Burkholderiales_Other↑, Clostridiaceae_Other↑, Erysipelotrichaceae_Coprobacillus↑, Lachnospiraceae_Butyrivibrio↓, Lachnospiraceae_Shuttleworthia↓, Staphylococcaceae_Staphylococcus ↓ | |
| [68] | Mice | p,p’-dichlorodiphenyldichloroethylene and β-hexachlorocyclohexane at 1 and 10 mg/kg body weight/day, for 8 weeks, respectively | Bile acid reabsorption in the terminal ileum and compensatory↓, bile acid and hydrophobicity↑, the genes expression on synthesis of bile acids in the liver↑ | Firmicutes↑, Proteobacteria↑, Bacteroidetes↓, Verrucomicrobia↓, Actinobacteria↓ | |
| [70] | Gold Fish | Pentachlorophenol at 0, 10, 50, and 100 μg/L for 28 days | Body weight and liver weight↓, oxidative stress↑, liver damage↑ | Bacteroidetes↑, Firmicutes↓, Bacteroides↑, Chryseobacterium↓, Microbacterium↓, Arthrobacter↓, Legionella↓ | |
| ABs | [72] | Zebrafish | Imazalil at 100 and 1000 μg/L for 1, 7 and 21 days | Glucokinase↓, hexokinase 1↓, pyruvate kinase↓, cytosolic Phosphoenol pyruvate carboxykinase (Pepckc) in liver ↓ | Bacteroidetes↓, Firmicutes↑ |
| [71] | Mice | Imazalil at 25, 50 or 100 mg/kg body weight daily for 4 weeks | Genes related to glycolysis and lipid metabolism↓ | Lactobacillus↓, Bifidobacterium↓ Deltaproteobacteria↑, Desulfovibrio↑ | |
| [96] | Rats | Epoxiconazole at 4 and 100 mg/kg body weight/day for 90 days | Weight of the liver and kidney↑, total bilirubin and cholinesterase in serum↓, blood glucose↑ | Firmicutes↓, Bacteroidetes↑, Proteobacteria↑, Lactobacillaceae↓, Bacteroidaceae↑, Enterobacteriaceae↑, Lachnospiraceae↑ | |
| [81] | Mice | The mixture of ampicillin, streptomycin, and clindamycin at 1 mg/mL for 2–4 week | The ceca size↑, a deeper shade of brown in ceca | Microbial diversity↓, Bacteroidetes↓, Stenotrophomonas↑, Xanthamonas↑ | |
| [95] | Piglets | The mixture of ampicillin, gentamicin, and metronidazole at 150, 4, and 30 mg/kg/day, respectively, for 25 days | Neurotransmitters in blood and hypothalamus↓, amino acids in feces, blood and hypothalamus↓ | Microbial diversity in feces↓, Firmicutes↑, Actinobacteria↑, Streptococcus↑, Lactobacillus↑, Bifidobacterium↑, Blautia↑, Klebsiella↑, Euryarchaeota↓, Spirochaetes↓, Tenericutes↓, Ruminococcus↓, Clostridium↓, unclassified Clostridiales↓, Christensenella↓, Methanobrevibacter↓, Prevotella↓ |
| Type | References | Models | Contaminants Dosage | Supplementation Dosage | Main Conclusion |
|---|---|---|---|---|---|
| HMs | [142,143] | Rats | Cd | CdCl2 at 70 ppm, the mixture of L. acidophilus Rosell-52, L. rhamnosus Rosell-11 and B. longum Rosell-175 (5 × 108 CFU/g food) for 5 weeks | Marked decrease genotoxicity and the toxicity to lactobacilli, promoted Cd excretion in feces; decreased Cd in body; relieved liver and kidney damage, increased the number of L. acidophilus in feces |
| [144] | Rats | Hg | A total of 0.5 mL HgCl2 at 20 μg/mL and 1ml B. coagulans and L. plantarum CNR273 (109 CFU/mL) daily for 48 days | Marked increase Hg excretion in feces; reduce Hg levels in liver and kidney; prevent oxidative stress; reduce liver and kidney damage; increase the number of fecal LAB and the total bacteria counts | |
| [145] | Mice | Pb | A total of 2 mg (CH3COO)2Pb·3H2O in 0.4 mL plain water, L. bulgaricus KLDS1.0207 1 × 1010 (high dose), 1 × 109 (medial dose) and 1 × 108 (low dose) CFU/mL in 0.4 mL skim milk | Lower mortality rates, increased Pb excretion in feces, decreased tissue Pb enrichment, improved the antioxidant in the liver and kidney, and relieved renal pathological damage | |
| [101] | Rats | As | NaAsO2 at 1.0 mg/100 g body weight, the mixture of L. acidophilus, L. rhamnosus, B. longum, and S. boulardii at 0.25 mg/100 g body weight for 16 days | Reduction of oxidative stress, inflammation in uterine, protection against mutagenic uterine DNA-breakage, necrosis, ovarian-uterine tissue damages | |
| [29] | Mice | Cr (VI) | A total of 1mM K2Cr2O7 in drinking water, L. plantarum TW1-1 (1 × 109 CFU/once every other day) for 7 weeks | Promoted Cr excretion in feces, reduced Cr accumulation in tissues; decreased oxidative stress and damage in liver; partially restored the GM community | |
| Pesticides | [107] | Rats | Endosulfan | Endosulfan at 4 mg/kg bodyweight from the 6th to 20th day of gestation, L. plantarum BJ0021 0.1 mL per os and one hour before the administration endosulfan | Significantly reduced the cholesterol level and marked depletion of hepatic enzymes, decreased the number of apoptotic nuclei in kidney |
| [20] | Caenorhabditis elegans | Malathion | Exposure to malathion at 300 mM for 4 h at 20 °C after administration L. casei liquid cultures of 0.1 OD at 600 nm for 4 h | Reproduction protection with increase of rate of egg laying and brood size, and rescued locomotion of C. elegans | |
| [21] | Drosophila melanogaster | Chloropyrifos parathion | Co-exposure 10 μM chloropyrifos parathion and 100 μL L. rhamnosus GG (109 CFU) for 12 days | Prolonged overall survival and decreased early deaths | |
| ABs | [81] | Mice | Different ABs | Ampicillin, Streptomycin, and Clindamycin at 1 mg/mL, A cocktails of L. rhamnosus A191, L. acidophilus, B. breve, B. longum (4 × 109/mL) at 0.1 mL/mouse for 2 weeks | Lead a rise in microbial diversity; small increase in Firmicutes, increase in Enterobacteriaceae, and a bloom of Anaerotruncus, decrease in Xanthamonas |
| [121] | Fish | Streptomycin sulfate | A total of 200 g/mL of streptomycin sulfate daily for 13 days, 1 × 105 CFU/mL P. inhibens S4Sm and B. pumilus RI06-95Sm daily for 5 days following ABs treatment | Probiotics can colonize fish microbiome, decrease mortality in fish with subtle GM changes | |
| [122] | Mice | Ampicillin | Ampicillin (500 mg/kg) twice-daily for 14 days, a cocktail of L. plantarum, L. casei, L. rhamnosus and L. helveticus (2 × 109 CFU/0.2 mL/dose) for 4 weeks | Restore diversity of GM, decrease Firmicutes, reduce Desulfovibrionales, Dorea, Ruminococcus, Clostridia and Helicobacter, enrich Akkermansia, Alistipes and Porphyromonadaceae |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients 2019, 11, 22. https://doi.org/10.3390/nu11010022
Feng P, Ye Z, Kakade A, Virk AK, Li X, Liu P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients. 2019; 11(1):22. https://doi.org/10.3390/nu11010022
Chicago/Turabian StyleFeng, Pengya, Ze Ye, Apurva Kakade, Amanpreet Kaur Virk, Xiangkai Li, and Pu Liu. 2019. "A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota" Nutrients 11, no. 1: 22. https://doi.org/10.3390/nu11010022
APA StyleFeng, P., Ye, Z., Kakade, A., Virk, A. K., Li, X., & Liu, P. (2019). A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients, 11(1), 22. https://doi.org/10.3390/nu11010022






