Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cooked Navy Bean Powder for Dietary Intervention and Metabolomics
2.3. Stool Sample Collection and Preparation for Metabolomics
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) (Navy Bean and Stool)
2.5. Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS/MS) (Navy Bean and Stool)
2.6. Visualization of Metabolic Pathway Networks, Including Pathway Enrichment Score (PES)
2.7. Statistical Analyses
3. Results
3.1. Navy Bean (Food) Metabolome
3.2. Dietary Modulation of Stool Metabolite Composition with One Month Exposure
3.3. Stool Metabolic Pathways Impacted by Control and Navy Bean Groups after One Month
3.4. Stool Metabolite Distinctions between Navy Bean and Control Group at End of Study
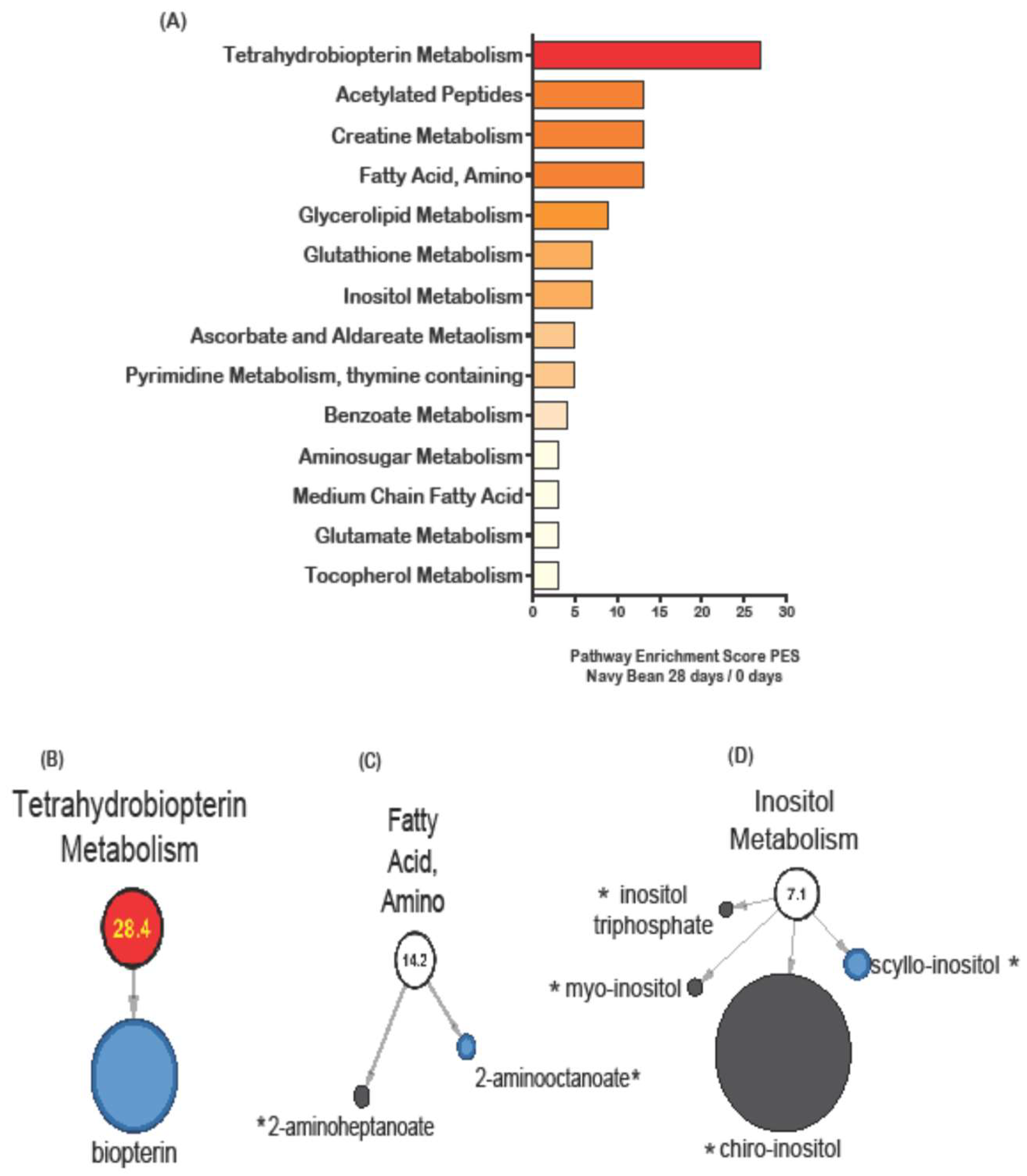
3.5. Network of Metabolic Pathway Differences Between Control and Navy Bean Groups after the One Month Feeding Period
3.6. Overlap between Navy Bean (Food) Metabolome and Stool Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary guidance for pulses: The challenge and opportunity to be part of both the vegetable and protein food groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet. Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; McGinley, J.; Neil, E.; Brick, M. Beneficial effects of common bean on adiposity and lipid metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Fulgoni, V.L. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: Results from the national health and nutrition examination survey 1999–2002. J. Am. Coll. Nutr. 2008, 27, 569–576. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Hamaker, B.R.; Lovejoy, J.C.; Eichelsdoerfer, P.E. Pulse consumption, satiety, and weight management. Adv. Nutr. 2010, 1, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.; Gillespie, C.; Seymour, J.; Serdula, M.; Blanck, H.M. Fruit and vegetable intake among adolescents and adults in the United States: Percentage meeting individualized recommendations. Medscape J. Med. 2009, 11, 26. [Google Scholar] [PubMed]
- Palmer, S.M.; Winham, D.M.; Oberhauser, A.M.; Litchfield, R.E. Socio-ecological barriers to dry grain pulse consumption among low-income women: A mixed methods approach. Nutrients 2018, 10, 1108. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 437S–442S. [Google Scholar] [CrossRef]
- Kisuse, J.; La-Ongkham, O.; Nakphaichit, M.; Therdtatha, P.; Momoda, R.; Tanaka, M.; Fukuda, S.; Popluechai, S.; Kespechara, K.; Sonomoto, K.; et al. Urban diets linked to gut microbiome and metabolome alterations in children: A comparative cross-sectional study in Thailand. Front. Microbiol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Martin, F.-P.; Collino, S.; Rezzi, S.; Kochhar, S. Metabolomic applications to decipher gut microbial metabolic influence in health and disease. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- Playdon, M.C.; Sampson, J.N.; Cross, A.J.; Sinha, R.; Guertin, K.A.; Moy, K.A.; Rothman, N.; Irwin, M.L.; Mayne, S.T.; Stolzenberg-Solomon, R.; et al. Comparing metabolite profiles of habitual diet in serum and urine. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: Ms-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2012, 31, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Chino, X.M.S.; Martínez, C.J.; Garzón, V.R.V.; González, I.Á.; Treviño, S.V.; Bujaidar, E.M.; Ortiz, G.D.; Hoyos, R.B. Cooked chickpea consumption inhibits colon carcinogenesis in mice induced with azoxymethane and dextran sulfate sodium. J. Am. Coll. Nutr. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905. [Google Scholar] [CrossRef] [PubMed]
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Bazan, M.; Rao, S.; Bailey, S.M.; Wdowik, M.; et al. A randomized controlled trial to increase navy bean or rice bran consumption in colorectal cancer survivors. Nutr. Cancer 2016, 68, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Bobe, G.; Barrett, K.G.; Mentor-Marcel, R.A.; Saffiotti, U.; Young, M.R.; Colburn, N.H.; Albert, P.S.; Bennink, M.R.; Lanza, E. Dietary cooked navy beans and their fractions attenuate colon carcinogenesis in azoxymethane-induced ob/ob mice. Nutr. Cancer 2008, 60, 373–381. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant activity in common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Schneider, A.V.C. Overview of the market and consumption of pulses in Europe. Br. J. Nutr. 2007, 88, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Leterme, P. Recommendations by health organizations for pulse consumption. Br. J. Nutr. 2007, 88, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Lanza, E.; Freedman, L.S.; Tangrea, J.; Cooper, M.R.; Marshall, J.R.; Murphy, P.A.; Selby, J.V.; Shike, M.; Schade, R.R.; et al. The polyp prevention trial i: Rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiol. Biomark. Prev. 1996, 5, 375–383. [Google Scholar]
- Daniel, C.R.; Park, Y.; Chow, W.-H.; Graubard, B.I.; Hollenbeck, A.R.; Sinha, R. Intake of fiber and fiber-rich plant foods is associated with a lower risk of renal cell carcinoma in a large us cohort. Am. J. Clin. Nutr. 2013, 97, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Winham, D.M.; Hutchins, A.M.; Johnston, C.S. Pinto bean consumption reduces biomarkers for heart disease risk. J. Am. Coll. Nutr. 2007, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Borresen, E.C.; Gundlach, K.A.; Wdowik, M.; Rao, S.; Brown, R.J.; Ryan, E.P. Feasibility of increased navy bean powder consumption for primary and secondary colorectal cancer prevention. Curr. Nutr. Food Sci. 2014, 10, 112–119. [Google Scholar] [CrossRef]
- Feregrino-Perez, A.A.; Piñol-Felis, C.; Gomez-Arbones, X.; Guevara-González, R.G.; Campos-Vega, R.; Acosta-Gallegos, J.; Loarca-Piña, G. A non-digestible fraction of the common bean (Phaseolus vulgaris L.) induces cell cycle arrest and apoptosis during early carcinogenesis. Plant Foods Hum. Nutr. 2014, 69, 248–254. [Google Scholar] [CrossRef]
- Borresen, E.C.; Jenkins-Puccetti, N.; Schmitz, K.; Brown, D.G.; Pollack, A.; Fairbanks, A.; Wdowik, M.; Rao, S.; Nelson, T.L.; Luckasen, G.; et al. A pilot randomized controlled clinical trial to assess tolerance and efficacy of navy bean and rice bran supplementation for lowering cholesterol in children. Glob. Pediatr. Health 2017, 4. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J. Nutr. Biochem. 2017, 49, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Rao, B.; Deng, L. Gut flora profiling and fecal metabolite composition of colorectal cancer patients and healthy individuals. Exp. Ther. Med. 2017, 13, 2848–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.J.; Borresen, E.C.; Jenkins-Puccetti, N.; Luckasen, G.; Ryan, E.P. Navy bean and rice bran intake alters the plasma metabolome of children at risk for cardiovascular disease. Front. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Company, A.D.M. Cooked Ground Bean Ingredients. Available online: https://www.adm.com/ (accessed on 30 October 2018).
- Brown, D.G.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Heat-stabilized rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: A randomized controlled trial. Br. J. Nutr. 2017, 117, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Rao, S.; Weir, T.L.; O’Malia, J.; Bazan, M.; Brown, R.J.; Ryan, E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Wang, M.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018, 4, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661. [Google Scholar] [CrossRef]
- Raicht, R.F.; Cohen, B.I.; Fuzzing, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403–405. [Google Scholar]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef]
- Heinemann, T.; Axtmann, G.; Bergmann, K.V. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Investig. 1993, 23, 827–831. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Huang, J.; Xu, M.; Fang, Y.-J.; Lu, M.-S.; Pan, Z.-Z.; Huang, W.-Q.; Chen, Y.-M.; Zhang, C.-X. Association between phytosterol intake and colorectal cancer risk: A case–control study. Br. J. Nutr. 2017, 117, 839–850. [Google Scholar] [CrossRef]
- Baskar, A.A.; Numair, K.S.A.; Paulraj, M.G.; Alsaif, M.A.; Muamar, M.A.; Ignacimuthu, S. B-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef]
- Rao, C.V.; Newmark, H.L.; Reddy, B.S. Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect. Prev. 2002, 26, 419–425. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- De Godínez, C.M.; Bressani, R.; Melgar, M. Apparent digestibility of bean protein evaluated in humans, rats and in vitro assays. Nutr. Res. 1992, 12, 235–246. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Dello, S.A.; Neis, E.P.; de Jong, M.C.; van Eijk, H.M.; Kicken, C.H.; Olde Damink, S.W.; Dejong, C.H. Systematic review of ophthalmate as a novel biomarker of hepatic glutathione depletion. Clin. Nutr. 2013, 32, 325–330. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Liu, Z.; Zeng, X.; Li, T.; Yin, Y. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Funct. 2018, 9, 4153–4163. [Google Scholar] [CrossRef]
- Zhu, J.; Djukovic, D.; Deng, L.; Gu, H.; Himmati, F.; Chiorean, E.G.; Raftery, D. Colorectal Cancer Detection Using Targeted Serum Metabolic Profiling. J. Proteome Res. 2014, 13, 4120–4130. [Google Scholar] [CrossRef]
- Zhu, J.; Djukovic, D.; Deng, L.; Gu, H.; Himmati, F.; Abu Zaid, M.; Chiorean, E.G.; Raftery, D. Targeted serum metabolite profiling and sequential metabolite ratio analysis for colorectal cancer progression monitoring. Anal. Bioanal. Chem. 2015, 407, 7857. [Google Scholar] [CrossRef]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef]
- Park, J.-M.; Kwon, S.-H.; Han, Y.-M.; Hahm, K.-B.; Kim, E.-H. Omega-3 polyunsaturated fatty acids as potential chemopreventive agent for gastrointestinal cancer. J. Cancer Prev. 2013, 18, 201–208. [Google Scholar] [CrossRef]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef]
- Jiang, Q.; Ames, B.N. Γ-tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003, 17, 816–822. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Wang, J.; Briggs, S.S.; O’Neill, E.; Li, J.; Leek, R.; Kerr, D.J.; Harris, A.L.; Cai, S. Roles of tetrahydrobiopterin in promoting tumor angiogenesis. Am. J. Pathol. 2010, 177, 2671–2680. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Caltavuturo, L.; Longo, V.; Pucci, L. Antimutagenic and antioxidant activity of a selected lectin-free common bean (Phaseolus vulgaris L.) in two cell-based models. Plant Foods Hum. Nutr. 2015, 70, 35–41. [Google Scholar] [CrossRef]
- Bennink, M.R. Consumption of black beans and navy beans (phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65. [Google Scholar] [CrossRef]
- Rivero-Cruz, B.E.; Esturau, N.; Sanchez-Nieto, S.; Romero, I.; Castillo-Juarez, I.; Rivero-Cruz, J.F. Isolation of the new anacardic acid 6-(16’z-nonadecenyl)-salicylic acid and evaluation of its antimicrobial activity against streptococcus mutans and porphyromonas gingivalis. Nat. Prod. Res. 2011, 25, 1282–1287. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via g1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinogenes. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- Drygalski, K.; Berk, K.; Charytoniuk, T.; Ilowska, N.; Lukaszuk, B.; Chabowski, A.; Konstantynowicz-Nowicka, K. Does the enterolactone (enl) affect fatty acid transporters and lipid metabolism in liver? Nutr. Metab. (Lond.) 2017, 14, 69. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Hu, X.-J.; Li, Y.; Liu, C.-M. Inhibitory effects of enterolactone on growth and metastasis in human breast cancer. Nutr. Cancer 2015, 67, 1326–1334. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Cardiometabolic risk factors are associated with high urinary enterolactone concentration, independent of urinary enterodiol concentration and dietary fiber intake in adults. J. Nutr. 2014, 144, 1445–1453. [Google Scholar] [CrossRef]
- Pathi, S.; Jutooru, I.; Chadalapaka, G.; Nair, V.; Lee, S.-O.; Safe, S. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (sp) transcription factors. PLoS ONE 2012, 7, e48208. [Google Scholar] [CrossRef]



| Control (n = 10) | Navy Bean (n = 8) | |
|---|---|---|
| Age (years) | 65.50 ± 3.07 | 60.9 ± 11.0 |
| Sex | 4 (40%) | 2 (22%) |
| Males (%) Females (%) | 6 (60%) | 7 (78%) |
| BMI (kg/m2) | 27.26 ± 3.29 | 25.9 ± 5.0 |
| Fiber Intake | 25.07 ± 11.2 | 19.94 ± 10.0 |
| Metabolic Pathway | Biochemical Name | Control (day 28/day 0) | Navy Beans (day 28/day 0) | ||
|---|---|---|---|---|---|
| Fold Change | p-Value | Fold Change | p-Value | ||
| Amino Acid | |||||
| Alanine and Aspartate | N-acetylasparagine | 2.37 | 0.0498 | 0.88 | 0.8519 |
| N-propionylalanine | 1.05 | 0.0062 | 0.74 | 0.8716 | |
| Glutamate | glutamate | 1.14 | 0.2647 | 0.82 | 0.0372 |
| Lysine | 2-aminoadipate | 0.74 | 0.0331 | 1.01 | 0.5629 |
| cadaverine | 1.20 | 0.961 | 3.20 | 0.0009 | |
| Phenylalanine and Tyrosine | 4-hydroxyphenylacetate | 1.03 | 0.7962 | 4.51 | 0.0181 |
| 3-hydroxyphenylacetate | 2.43 | 0.0020 | 0.77 | 0.7575 | |
| phenylacetylglutamine | 1.68 | 0.8156 | 0.09 | 0.0494 | |
| 2-(4-hydroxyphenyl)propionate | 1.22 | 0.2846 | 0.03 | 0.0110 | |
| Tryptophan | tryptamine | 1.20 | 0.0032 | 0.29 | 0.1752 |
| Methionine, Cysteine, S-adenosylmethione (SAM) and Taurine | N-acetylmethionine sulfoxide | 1.17 | 0.6650 | 0.43 | 0.0428 |
| homocysteine | 1.67 | 0.003 | 1.11 | 0.5865 | |
| Creatine | creatine | 0.78 | 0.9114 | 0.10 | 0.0074 |
| guanidinoacetate | 3.21 | 0.4745 | 0.07 | 0.0497 | |
| Glutathione | ophthalmate | 1.13 | 0.8969 | 5.25 | 0.0116 |
| Carbohydrate | |||||
| Pentose | ribose | 1.38 | 0.0261 | 0.92 | 0.7411 |
| ribonate | 22.89 | 0.0159 | 0.91 | 0.9095 | |
| xylonate | 12.63 | 0.0029 | 1.57 | 0.3878 | |
| 2-deoxyribose | 1.42 | 0.1133 | 1.62 | 0.0269 | |
| Aminosugar | diacetylchitobiose | 1.89 | 0.6057 | 3.42 | 0.0066 |
| Advanced Glycation | N6-carboxymethyllysine | 1.14 | 0.0076 | 0.95 | 0.6244 |
| Lipid | |||||
| Medium Chain Fatty Acid | caprylate (8:0) | 3.94 | 0.1863 | 0.31 | 0.0441 |
| laurate (12:0) | 8.15 | 0.0175 | 0.14 | 0.4344 | |
| Long Chain Fatty Acid | myristate (14:0) | 3.43 | 0.0186 | 0.37 | 0.4247 |
| cis-vaccenate (18:1n7) | 1.99 | 0.0335 | 0.94 | 0.7617 | |
| Polyunsaturated Fatty Acid (n3 and n6) | docosahexaenoate (DHA; 22:6n3) | 21.69 | 0.0099 | 1.13 | 0.6900 |
| docosatrienoate (22:3n3) | 3.96 | 0.0094 | 1.36 | 0.8702 | |
| linoleate (18:2n6) | 1.58 | 0.0499 | 0.84 | 0.6734 | |
| arachidonate (20:4n6) | 9.64 | 0.0128 | 1.05 | 0.9502 | |
| docosadienoate (22:2n6) | 4.83 | 0.0414 | 1.79 | 0.8219 | |
| Fatty Acid, Amino | 2-aminooctanoate | 0.99 | 0.154 | 0.68 | 0.0285 |
| Fatty Acid, Monohydroxy | 3-hydroxysebacate | 3.20 | 0.0242 | 0.54 | 0.7037 |
| Inositol | scyllo-inositol | 0.74 | 0.2135 | 0.52 | 0.0473 |
| Glycerolipid | glycerol 3-phosphate (G3P) | 1.00 | 1.0000 | 0.63 | 0.0454 |
| Monoacylglycerol | 1-myristoylglycerol (1-monomyristin) | 2.54 | 0.0384 | 0.29 | 0.0132 |
| Sphingolipid | N-acetylsphingosine | 1.87 | 0.0159 | 0.50 | 0.4079 |
| Steroid | 5alpha-androstan-3alpha,17beta-diol disulfate | 3.24 | 0.0453 | 0.63 | 0.5735 |
| Secondary Bile Acid | 7,12-diketolithocholate | 0.24 | 0.0251 | 23.33 | 0.5602 |
| 6-oxolithocholate | 1.02 | 0.9860 | 0.49 | 0.0210 | |
| glycocholenate sulfate * | 1.10 | 0.3844 | 0.08 | 0.0118 | |
| Nucleotide | |||||
| Purine, (Hypo)Xanthine/Inosine containing | hypoxanthine | 1.91 | 0.0258 | 1.46 | 0.8596 |
| Purine, Adenine containing | adenosine-2′,3′-cyclic monophosphate | 1.00 | 1.0000 | 1.31 | 0.0413 |
| Pyrimidine, Thymine containing | 5,6-dihydrothymine | 1.15 | 0.4695 | 1.69 | 0.0209 |
| Cofactors and Vitamins | |||||
| Ascorbate and Aldarate | ascorbate (Vitamin C) | 0.95 | 0.8606 | 0.46 | 0.0130 |
| Tocopherol | gamma-CEHC | 0.62 | 0.6196 | 0.40 | 0.0248 |
| Tetrahydrobiopterin | biopterin | 1.06 | 0.8275 | 0.21 | 0.0444 |
| Benzoate | 3-hydroxybenzoate | 2.96 | 0.0112 | 0.51 | 0.3146 |
| catechol sulfate | 0.48 | 0.3614 | 0.22 | 0.0376 | |
| 3-(2-hydroxyphenyl)propionate | 2.99 | 0.0005 | 2.74 | 0.0059 | |
| Xanthine | 1-methylurate | 0.73 | 0.0151 | 0.46 | 0.5649 |
| 7-methylurate | 0.94 | 0.0275 | 0.27 | 0.1830 | |
| 1,3-dimethylurate | 1.07 | 0.6615 | 0.72 | 0.0112 | |
| 1-methylxanthine | 1.90 | 0.0466 | 1.12 | 0.9862 | |
| 7-methylxanthine | 2.25 | 0.0202 | 0.31 | 0.1809 | |
| Other Phytochemicals | piperidine | 0.48 | 0.3284 | 2.59 | 0.0176 |
| 2-piperidinone | 0.71 | 0.0190 | 1.16 | 0.8974 | |
| (15:0)-anacardic acid | 1.65 | 0.8377 | 9.38 | 0.0448 | |
| apigenin | 21.86 | 0.0001 | 0.46 | 0.5761 | |
| luteolin | 25.1 | 0.0299 | 0.35 | 0.7539 | |
| abscisate | 0.31 | 0.0138 | 0.62 | 0.8696 | |
| enterolactone | 2.00 | 0.0278 | 2.81 | 0.0017 | |
| indolin-2-one | 0.80 | 0.0013 | 1.13 | 0.6222 | |
| sitostanol | 0.59 | 0.0060 | 0.79 | 0.0767 | |
| Diphenhydramine (drug) | 0.62 | 0.0429 | 1.00 | 1.0000 | |
| loperamide | 0.65 | 0.0324 | 1.00 | 1.0000 | |
| salicylate | 1.83 | 0.0179 | 1.08 | 0.3910 | |
| N-methylpipecolate | 0.83 | 0.3601 | 1.57 | 0.0038 | |
| Metabolic Pathway | Metabolite | HMDB | Navy Bean day 28 | Control day 28 | Fold Difference (NB/Control) | p-Value |
|---|---|---|---|---|---|---|
| Amino Acid | ||||||
| Histidine | formiminoglutamate | 0.564 | 5.328 | 0.11 | 0.019 | |
| hydantoin-5-propionic acid | HMDB01212 | 0.829 | 0.249 | 3.71 | 0.016 | |
| Lysine | 2-aminoadipate | HMDB00510 | 1.741 | 0.695 | 2.69 | 0.000 |
| N2,N6-diacetyllysine | 1.553 | 0.512 | 3.17 | 0.032 | ||
| Leucine, Isoleucine and Valine | 4-methyl-2-oxopentanoate | HMDB00695 | 0.9896 | 2.5642 | 0.41 | 0.024 |
| N-acetylisoleucine | 0.722 | 0.189 | 0.15 | 0.039 | ||
| 3-methyl-2-oxovalerate | HMDB03736 | 0.856 | 3.084 | 0.37 | 0.034 | |
| 3-methyl-2-oxobutyrate | HMDB00019 | 0.937 | 2.831 | 0.41 | 0.048 | |
| Glutathione | 5-oxoproline | HMDB00267 | 1.202 | 3.316 | 0.23 | 0.031 |
| ophthalmate | HMDB05765 | 1.480 | 0.282 | 3.49 | 0.031 | |
| Peptide | ||||||
| Gamma-glutamyl Amino Acid | gamma-glutamylglutamine | HMDB11738 | 2.798 | 1.211 | 2.60 | 0.01 |
| Energy | ||||||
| TCA Cycle | malate | HMDB00156 | 0.690 | 1.464 | 0.39 | 0.029 |
| Lipid | ||||||
| Medium Chain Fatty Acid | caprylate (8:0) | HMDB00482 | 0.682 | 4.610 | 0.1 | 0.020 |
| undecanoate (11:0) | HMDB00947 | 0.853 | 1.627 | 0.50 | 0.015 | |
| Polyunsaturated Fatty Acid (n3 and n6) | eicosapentaenoate (EPA; 20:5n3) | HMDB01999 | 4.014 | 25.09 | 0.05 | 0.031 |
| Fatty Acid (Acyl Glycine) | valerylglycine | HMDB00927 | 0.775 | 6.876 | 0.13 | 0.048 |
| Monoacylglycerol | 1-myristoylglycerol (1-monomyristin) | HMDB11561 | 0.875 | 2.246 | 0.31 | 0.039 |
| Secondary Bile Acid | glycolithocholate sulfate * | HMDB02639 | 0.802 | 1.487 | 0.39 | 0.035 |
| glycocholenate sulfate * | 0.171 | 1.750 | 0.10 | 0.026 | ||
| Nucleotide | ||||||
| Purine | allantoin | HMDB00462 | 0.173 | 6.796 | 0.11 | 0.049 |
| Pyrimidine | 4-ureidobutyrate | 0.642 | 1.921 | 0.31 | 0.038 | |
| 5,6-dihydrothymine | HMDB1 2308 | 0.626 | 0.416 | 1.56 | 0.047 | |
| Food Derived | ||||||
| Other Phytochemicals | vanillin | HMDB12308 | 1.228 | 1.515 | 0.51 | 0.036 |
| nobiletin | HMDB29540 | 0.528 | 0.371 | 1.48 | 0.048 | |
| salicylate | HMDB01895 | 2.142 | 3.094 | 0.77 | 0.043 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baxter, B.A.; Oppel, R.C.; Ryan, E.P. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients 2019, 11, 28. https://doi.org/10.3390/nu11010028
Baxter BA, Oppel RC, Ryan EP. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients. 2019; 11(1):28. https://doi.org/10.3390/nu11010028
Chicago/Turabian StyleBaxter, Bridget A., Renee C. Oppel, and Elizabeth P. Ryan. 2019. "Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors" Nutrients 11, no. 1: 28. https://doi.org/10.3390/nu11010028
APA StyleBaxter, B. A., Oppel, R. C., & Ryan, E. P. (2019). Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients, 11(1), 28. https://doi.org/10.3390/nu11010028






