The Winning Weaning Food (WWF): The Development of a Complementary Food for Food-Insecure Infants and Young Children in Malawi
Abstract
1. Introduction
2. Methodology
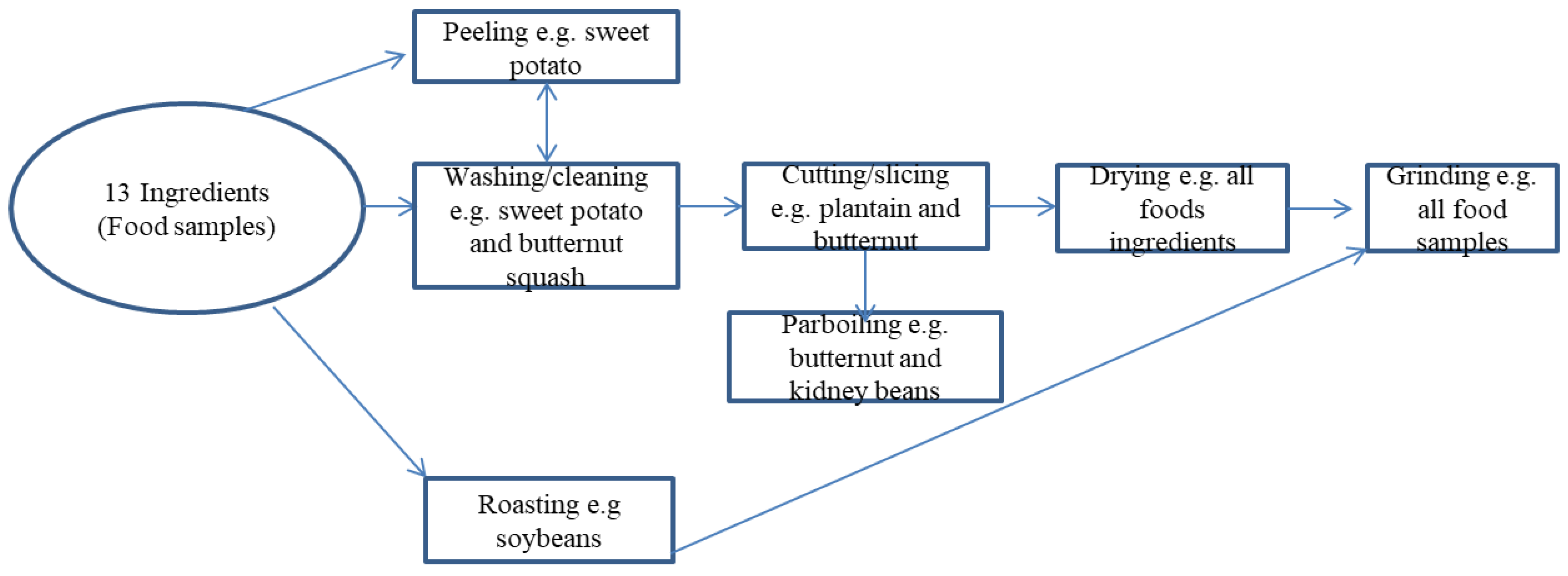
2.1. Stage 1
2.2. Stage 2
The Development of the Complementary Food
3. Results and Discussion
- For each component used in the recipe, a nutrient value per 1 g was sampled from a normal distribution with mean and standard deviation as empirically estimated.
- That sampled value was multiplied by the amount of the component used in the recipe.
- An estimate of the total amount of the nutrient in a fully prepared dish was simulated by summing its components independently.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Org.: Rome, Italy, 2018. [Google Scholar]
- Jones, A.D.; Ngure, F.M.; Pelto, G.; Young, S.L. What are we assessing when we measure food security? A compendium and review of current metrics. Adv. Nutr. 2013, 4, 481–505. [Google Scholar] [CrossRef] [PubMed]
- FAO; ECA. Regional Overview of Food Security and Nutrition: Addressing the Threat from Climate Variability and Extremes for Food Security and Nutrition; FAO: Accra, Ghana, 2018; 116p. [Google Scholar]
- USDA: Agriculture and Food Security. Available online: https://www.usaid.gov/malawi/agriculture-and-food-security (accessed on 3 February 2019).
- Stevens, T.; Madani, K. Future climate impacts on maize farming and food security in Malawi. Sci. Rep. 2016, 6, 36241. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization (FAO). Country Profile: Malawi. Available online: http://www.fao.org/countryprofiles/index/en/?iso3=mwi (accessed on 3 February 2019).
- Food Insecurity Response Plan (FIRP); The Republic of Malawi office of the Vice President Department of Disaster Management Affairs: Lilongwe, Malawi, 2018.
- Riley, L.; Chilanga, E. Food Security in Africa’s Secondary Cities: No. 1. Mzuzu, Malawi; Southern African Migration Programme; African Food Security Urban Network: Cape Town, South Africa, 2018. [Google Scholar]
- Jash, A. China’s Quest for Food Security Challenges and Policies; IndraStra Global: New York, NY, USA, 2015. [Google Scholar]
- Shamah-Levy, T.; Mundo-Rosas, V.; Rivera-Dommarco, J.A. Magnitude of food insecurity in Mexico: Its relationship with nutritional status and socioeconomic factors. Salud Publica Mex. 2014, 56, 79–85. [Google Scholar] [CrossRef]
- Ghattas, H. Food Security and Nutrition in the Context of the Global Nutrition Transition; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Betebo, B.; Ejajo, T.; Alemseged, F.; Massa, D. Household food insecurity and its association with nutritional status of children 6–59 months of age in east Badawacho District, south Ethiopia. J. Environ. Public Health 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2013: A Wealth of Information on Global Public Health; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Raymond, J.; Kassim, N.; Rose, J.W.; Agaba, M. Optimal dietary patterns designed from local foods to achieve maternal nutritional goals. BMC Public Health 2018, 18, 451. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Office (NSO); ICF. Malawi Demographic and Health Survey 2015–2016; NSO: Zomba, Malawi; ICF: Rockville, MD, USA, 2017.
- World Health Organisation Staff; World Health Organization; UNICEF. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Bryce, J.; Boschi-Pinto, C.; Shibuya, K.; Black, R.E.; WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet 2005, 365, 1147–1152. [Google Scholar] [CrossRef]
- Wubante, A.A. Determinants of infant nutritional status in Dabat district, North Gondar, Ethiopia: A case control study. PLoS ONE 2017, 12, e0174624. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Verma, S.; Faridi, M.M. Complementary feeding—Reasons for inappropriateness in timing, quantity and consistency. Indian J. Pediatr. 2008, 75, 49. [Google Scholar] [CrossRef]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- World Food Program: Cost of Hunger in Malawi. Available online: https://www.wfp.org/content/cost-hunger-malawi (accessed on 10 January 2018).
- Zotor, F.B.; Ellahi, B.; Amuna, P. Applying the food multimix concept for sustainable and nutritious diets. Proc. Nutr. Soc. 2015, 74, 505–516. [Google Scholar] [CrossRef]
- Fahmida, U.; Santika, O.; Kolopaking, R.; Ferguson, E. Complementary feeding recommendations based on locally available foods in Indonesia. Food Nutr. Bull. 2014, 35 (Suppl. 4), S174–S179. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.H. Design of animal protein drying techniques: Moving local drying knowledge from fish to goat meat, Malawi. 2019; unpublished; manuscript in preparation. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Luque-Garcia, J.L.; Sanchez-Díaz, R.; Lopez-Heras, I.; Camara, C.; Martin, P. Bioanalytical strategies for in-vitro and in-vivo evaluation of the toxicity induced by metallic nanoparticles. TrAC Trends Anal. Chem. 2013, 43, 254–268. [Google Scholar] [CrossRef]
- USDA. Food Composition Databases. 2019. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 8 January 2018).
- Codex Alimentarius Commission. Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for Its Application; Annex to CAC/RCP 1-1969, Rev. 3.; Food and Agriculture Organization: Rome, Italy, 1997. [Google Scholar]
- Society of Sensory Professional (SSP). The 9-point Hedonic Scale. Available online: https://www.sensorysociety.org/knowledge/sspwiki/Pages/The%209point%20Hedonic%20Scale.aspx (accessed on 12 February 2018).
- Misselhorn, A.; Hendriks, S.L. A systematic review of sub-national food insecurity research in South Africa: Missed opportunities for policy insights. PLoS ONE 2017, 12, e0182399. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Hossain, M.; Sanin, K.I. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann. Nutr. Metab. 2012, 61 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Mahan, L.K.; Escott-Stump, S. Medical Nutrition Therapy for Anemia: Krause’s Food, Nutrition, and Diet Therapy; WB Saunders: Philadelphia, PA, USA, 2000; p. 469. [Google Scholar]
- Campoy, C.; Campos, D.; Cerdó, T.; Diéguez, E.; García-Santos, J.A. Complementary Feeding in Developed Countries: The 3 Ws (When, What, and Why?). Ann. Nutr. Metab. 2018, 73, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Underwood, B.A. The Role of Vitamin A in Child Growth, Development and Survival. In Nutrient Regulation During Pregnancy, Lactation, and Infant Growth; Springer: Boston, MA, USA, 1994; pp. 201–208. [Google Scholar]
- Gibbs, M.; Bailey, K.B.; Lander, R.D.; Fahmida, U.; Perlas, L.; Hess, S.Y.; Loechl, C.U.; Winichagoon, P.; Gibson, R.S. The adequacy of micronutrient concentrations in manufactured complementary foods from low-income countries. J. Food Compos. Anal. 2011, 24, 418–426. [Google Scholar] [CrossRef]
- De Vries, J. Goats for the poor: Some keys to successful promotion of goat production among the poor. Small Rumin. Res. 2008, 77, 221–224. [Google Scholar] [CrossRef]
- García, R.R.; Celaya, R.; García, U.; Osoro, K. Goat grazing, its interactions with other herbivores and biodiversity conservation issues. Small Rumin. Res. 2012, 107, 49–64. [Google Scholar] [CrossRef]
- Devi, S.M.; Balachandar, V.; Lee, S.I.; Kim, I.H. An outline of meat consumption in the Indian population—A pilot review. Korean J. Food Sci. Anim. Resour. 2014, 34, 507. [Google Scholar] [CrossRef]
- Banda, J.W. Revolutionising the Livestock Industry in Malawi; The 12th University of Malawi Inaugural Lecture; Bunda College: Lilongwe, Malawi, 2008. [Google Scholar]
- Maganga, A.; Chigwa, F.; Mapemba, L. Goat and goat meat markets in selected districts of Malawi: Value chain, structure and efficiency. Livest. Res. Rural Dev. 2015, 27, 28560. [Google Scholar]
- Casey, N.H.; Van Niekerk, W.A.; Webb, E.C. Goats Meat. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglass, P., Eds.; Academic Press: London, UK, 2003; pp. 2937–2944. [Google Scholar]
- Webb, E.C. Goat meat production, composition, and quality. Anim. Front. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- ALIMENTARIUS, Codex. Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended 2010; Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission, FAO: Rome, Italy, 2010. [Google Scholar]
- Del Campo, B.G.; Brumm, T.J.; Bern, C.J.; Nyendu, G.C. Corn cob dry matter loss in storage as affected by temperature and moisture content. Trans. ASABE 2014, 57, 573. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Indicators of Food Microbial Quality and Safety. In Modern Food Microbiology; Springer: Boston, MA, USA, 2005; pp. 473–495. [Google Scholar]
- Mishra, B.; Mishra, J.; Pati, P.; Rath, P. Dehydrated Meat Products—A Review. Int. J. Livest. Res. 2017, 7, 10–22. [Google Scholar] [CrossRef]
- Arabhosseini, A.; Padhye, S.; Huisman, W.; van Boxtel, A.; Müller, J. Effect of drying on the color of tarragon (Artemisia dracunculus L.) leaves. Food Bioprocess Technol. 2011, 4, 1281–1287. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of drying technologies to ensure microbial safety of dried fruits and vegetables. Comp. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V.L., III. Nutrient density: Principles and evaluation tools. Am. J. Clin. Nutr. 2014, 99, 1223S–1228S. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; pp. 1–185. [Google Scholar]
- Weinstein, M.; Babyn, P.; Zlotkin, S. An orange a day keeps the doctor away: Scurvy in the year 2000. Pediatrics 2001, 108, e55. [Google Scholar] [CrossRef] [PubMed]
- El-Ishaq, A.; Obirinakem, S. Effect of temperature and storage on vitamin C content in fruits juice. Int. J. Chem. Biomol. Sci. 2015, 1, 17–21. [Google Scholar]
- Elmadfa, I.; Meyer, A.L. Importance of food composition data to nutrition and public health. Eur. J. Clin. Nutr. 2010, 64, S4. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; La Malfa, G.; Fogliano, V.; Maiani, G.; Quaglia, G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). J. Food Compos. Anal. 2006, 19, 11–19. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, acid-and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.; Chege, P.; Kimiywe, J.; Wiesmann, D.; Hotz, C. Zinc, iron and calcium are major limiting nutrients in the complementary diets of rural Kenyan children. Matern. Child Nutr. 2015, 11, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.; Broersen, B.; van Liere, M.J.; De-Regil, L.M.; Bahirathan, L.; Klassen, E.; Neufeld, L.M. Complementary feeding diets made of local foods can be optimized, but additional interventions will be needed to meet iron and zinc requirements in 6- to 23-month-old children in low-and middle-income countries. Food Nutr. Bull. 2016, 37, 544–570. [Google Scholar] [CrossRef] [PubMed]
- Kathryn, D. Guiding Principles for Complementary Feeding of the Breastfed Child. Pan American Health Organization (PAHO)/World Health Organization (WHO). Available online: https://www.who.int/nutrition/publications/guiding_principles_compfeeding_breastfed.pdf (accessed on 16 August 2018).
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; İbanoğlu, Ş. The advantage of using extrusion processing for increasing dietary fibre level in gluten-free products. Food Chem. 2010, 121, 156–164. [Google Scholar] [CrossRef]


| Food Sample | Percentage Moisture Loss in Dried Foods |
| Goat meat (dry) | 2.64 |
| Kidney beans (dry) | 9.05 |
| Millet (dry) | 3.009 |
| Soybeans (dry) | 5.11 |
| Food Sample | Percentage Moisture Loss in Raw Foods |
| Plantains (raw) | 62.98 |
| Sweet potatoes (raw) | 82.11 |
| Butternut squash (raw) | 90.7 |
| Kale (raw) | 91.2 |
| Spinach (raw) | 92.4 |
| Onions (raw) | 88 |
| Carrots (raw) | 87 |
| Mushrooms (raw) | 90.5 |
| Food Ingredients | Color before Drying | Color after Drying |
|---|---|---|
| Goat meat | Dark red | Grayish brown |
| Butternut Squash | Yellow | Pale brown |
| Sweet potatoes | Red | Reddish brown |
| Spinach | Green | Dark green |
| Plantains | Yellow | Pale yellow |
| Red Onions | White flesh tinged with red | Dark brown |
| Carrots | Bright orange | Pale orange |
| Mushrooms | Grayish white | Dark brown |
| Kale | Green | Dark green |
| Food Sample (100 g) | Energy (Kcal) | Carbohydrate (g) | Protein (g) | Fat (g) | Iron (mg) | Zinc (mg) | Calcium (mg) | * Vitamin A (mcg) | * Vitamin D (mcg) |
|---|---|---|---|---|---|---|---|---|---|
| Goat meat | 393.00 | 4.62 | 83.50 | 4.34 | 10.76 | 4.13 | 14.64 | 0 | 0 |
| Butternut squash | 385 | 87 | 6.42 | 1.19 | 0.28 | 0.82 | 7.68 | 532 | 0 |
| Kidney beans | 361.90 | 63.69 | 23.74 | 1.20 | 10.38 | 8.45 | 42.27 | 0 | 0 |
| Cornmeal | 343.35 | 68.32 | 7.07 | 4.50 | 5.10 | 6.04 | 42.45 | 121 | 0 |
| Sweet potatoes | 375.9 | 81.3 | 10.79 | 0.66 | 0.52 | 0.81 | 9.10 | 8512.2 | 0 |
| Spinach | 329 | 28.48 | 38.87 | 6.59 | 12.72 | 2.07 | 78.47 | 500 | 0 |
| Millet | 405.17 | 77.73 | 13.05 | 4.50 | 35.35 | 6.54 | 5.60 | 0 | 0 |
| Soybeans | 455.32 | 33.52 | 35.80 | 19.59 | 0.29 | 10.48 | 62.19 | 3.85 | 0 |
| Plantains | 377.2 | 89.1 | 3.67 | 0.53 | 0.39 | 0.82 | 0.72 | 45 | 0 |
| Onions | 473.35 | 63.29 | 13.67 | 18.19 | 2.17 | 4.04 | 18.17 | 1 | 0 |
| Carrots | 366.47 | 84.56 | 3.76 | 1.31 | 3.55 | 1.20 | 40.57 | 3120 | 0 |
| Mushrooms | 362.55 | 52.69 | 37.04 | 0.25 | 2.03 | 8.70 | 17.77 | 0 | 7 |
| Kale | 379.17 | 63.06 | 27.68 | 1.64 | 13.86 | 2.07 | 42.45 | 146 | 0 |
| Nutrient (Per 100 g Dried Goat Meat) | Own (Lubbock) | West Africa | Kenya | USDA |
|---|---|---|---|---|
| Energy (Kcal) kJ | (393) 1644 | (216) 900 | (192) 804 | (143) 599 |
| Protein (g) | 83.50 | 25.40 | 26.90 | 27.10 |
| Fat (g) | 4.340 | 12.20 | 9.30 | 3.03 |
| Carbohydrates (g) | 4.62 | 0.00 | 0.00 | 0.00 |
| Vitamin A (mcg) | 0.00 | 0.00 | 21.00 | 0.00 |
| Vitamin D (mcg) | 0.00 | 0.00 | 0.00 | 0.00 |
| Zinc (mg) | 4.13 | 5.00 | 4.48 | 5.27 |
| Calcium (mg) | 14.64 | 16.00 | 14.00 | 17.00 |
| Iron (mg) | 10.76 | 3.30 | 2.60 | 3.73 |
| Fiber | - | - | - | - |
| Ash | 4.91 | 1.60 | 1.50 | 1.46 |
| water (g) | 2.64 | 56.80 | 57.10 | 68.21 |
| Nutrient (Per 100 g of Cornmeal) | Own (Lubbock) | West Africa | Kenya | USDA |
|---|---|---|---|---|
| Energy (Kcal) Kj | (343.35) 1436.57 | (351) 1480 | (345) 1450 | (365) 1527 |
| Protein (g) | 7.07 | 9.70 | 7.94 | 9.42 |
| Fat (g) | 4.50 | 4.00 | 4.50 | 4.74 |
| Carbohydrates (g) | 68.32 | 64.5 | 63.40 | 74.26 |
| Vitamin A (mcg) | 121.00 | 0.00 | 0.00 | 0.00 |
| Vitamin D (mcg) | 0.00 | 0.00 | 0.00 | 0.00 |
| Zinc (mg) | 6.04 | 1.73 | 1.88 | 2.21 |
| Calcium (mg) | 42.45 | 18.00 | 24.00 | 7.00 |
| Iron (mg) | 5.10 | 3.80 | 2.60 | 2.67 |
| Fiber | - | 9.00 | 9.40 | - |
| Ash | 2.09 | 1.40 | 1.20 | 1.20 |
| water (g) | 18.02 | 11.50 | 13.60 | 10.37 |
| Recipe 1 | Recipe 2 | Recipe 3 | Recipe 4 | Recipe 5 |
|---|---|---|---|---|
| * Goat meat | * Goat meat | * Goat meat | * Goat meat | * Goat meat |
| Sweet potatoes | Cornmeal | Plantains | Kidney beans | Mushrooms |
| Kale | Butternut squash | Onions | Spinach | Cornmeal |
| Sunflower oil | Sunflower oil | Soybeans | Millet | Carrots |
| Sunflower oil | Sunflower oil | Sunflower oil |
| Recipe | Serving Size/Container (g) | Total fat (g) (% TE) | Protein (g) (% TE) | Carbohydrate (g) (% TE) | Calories | Vitamin D (mcg) | Calcium (mg) | Iron (mg) | Zinc (mg) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 16 (68) | 8 (15) | 8 (15) | 210 (4.2 kcal/g) | 0 | 7 | 2 | 0 |
| 2 | 48 | 15 (67) | 7 (14) | 9 (18) | 200 (4.2 kcal/g) | 0 | 7 | 1 | 1 |
| 3 | 50 | 13 (55) | 8 (15) | 15 (28) | 210 (4.2 kcal/g) | 0 | 11 | 1 | 2 |
| 4 | 44 | 10 (67) | 7 (14) | 9 (18) | 210 (4.8 kcal/g) | 0 | 11 | 7 | 2 |
| 5 | 44 | 6 (30) | 7 (15) | 24 (53) | 180 (4.1 kcal/g) | 0 | 14 | 2 | 2 |
| Codex | 100 | ≥ 20 | 6 to 15 | - | 400 (4 kcal/g) | 5 | 500 | 5.8–10% bioavailability | 4.1–10% bioavailability |
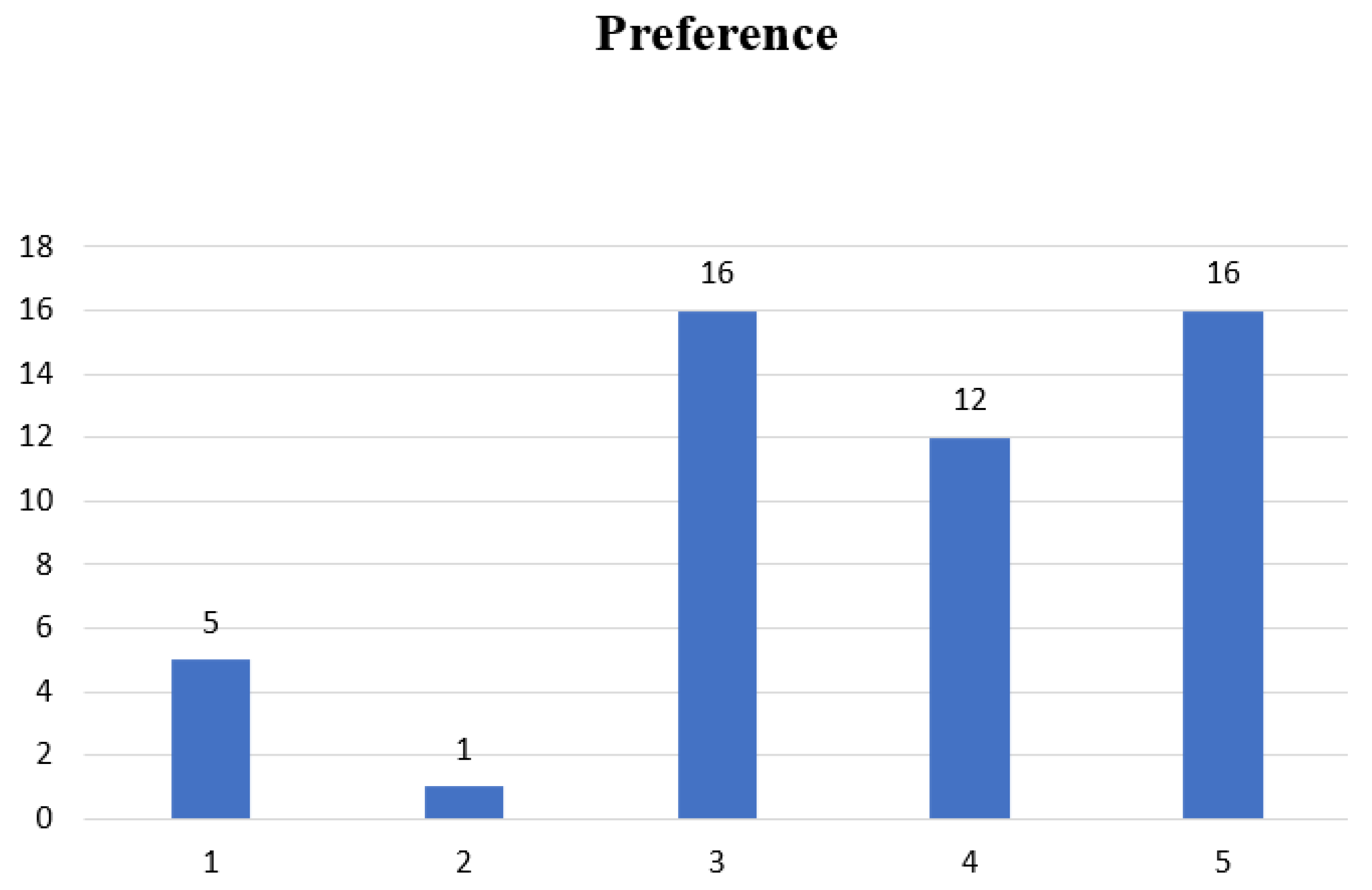
| Mean Scores (± SD) | Significance Difference between Recipes’ | |||||
|---|---|---|---|---|---|---|
| Parameters | Recipe 1 | Recipe 2 | Recipe 3 | Recipe 4 | Recipe 5 | p-Value |
| Appearance | 4.42 ± 0.54 | 5.22 ± 0.54 | 5.64 ± 0.54 | 4.72 ± 0.54 | 5.62 ± 0.54 | 3.00 × 10−4 |
| Taste/Flavor | 4.64 ± 1.0 | 3.14 ± 1.0 | 5.7 ± 1.0 | 4.9 ± 1.0 | 5.48 ± 1.0 | 1.10 × 10−10 |
| Texture/Consistency | 5.3 ± 0.51 | 5 ± 0.51 | 6.04 ± 0.51 | 5.46 ± 0.51 | 6.22 ± 0.51 | 9.60 × 10−6 |
| Aroma | 5.52 ± 0.92 | 3.44 ± 0.92 | 5.1 ± 0.92 | 4.94 ± 0.92 | 5.8 ± 0.92 | 1.80 × 10−9 |
| Overall Acceptability | 4.96 ± 0.87 | 3.44 ± 0.87 | 5.52 ± 0.87 | 5.14 ± 0.87 | 5.82 ± 0.87 | 2.60 × 10−13 |
| Nutrient/ 100 g | Codex A | Recipe 1 | Recipe 2 | Recipe 3 | Recipe 4 | Recipe 5 |
|---|---|---|---|---|---|---|
| Protein | 6–15% | 16.30 p = 1.00 95% CI: (16.2, 16.4) | 16.54 p = 1.00 95% CI: (16.3, 16.8) | 15.07 p = 0.52 95% CI: (12.7, 17.4) | 19.91 p = 1.00 95% CI: (19.5, 20.3) | 16.55 p = 1.00 95% CI: (16.5, 16.6) |
| Fat | 20% minimum | 33.85 p = 0.0001 95% CI: (33.7, 34.1) | 31.44 p = 0.0001 95% CI: (30.9, 32.0) | 26.37 p = 0.0001 95% CI: (26.4, 27.2) | 24.06 p = 0.0001 95% CI: (23.4, 24.8) | 14.25 p = 1.00 95% CI: (13.0, 15.5) |
| Carbohydrate | N | 15.01 95% CI: (11.7, 18.3) | 17.95 95% CI: (17.2, 18.7) | 28.77 95% CI: (26.9, 30.6) | 47.55 95% CI: (46.0, 49.1) | 53.99 95% CI: (52.0, 55.9) |
| Zinc (mg) | (a) 8.3 (b) 4.1 (c) 2.4 | 0.53 p = 1.00 95% CI: (0.52, 0.55) | 1.16 p = 1.00 95% CI: (1.1, 1.2) | 1.98 p = 1.00 95% CI: (1.7, 2.2) | 2.30 p = 1.00 95% CI: (2.2, 2.4) | 2.32 p = 1.00 95% CI: (2.1, 2.5) |
| Calcium (mg) | 500 | 6.66 p = 1.00 95% CI: (5.7, 7.6) | 7.35 p = 1.00 95% CI: (6.6, 8.1) | 10.53 p = 1.00 95% CI: (9.2, 11.9) | 11.33 p = 1.00 95% CI: (11.1, 11.6) | 14.18 p = 1.00 95% CI: (13.7, 14.7) |
| Iron (mg) | (a) 11.6 (b) 5.8 (c) 3.9 | 1.87 p = 1.00 95% CI: (1.3, 2.4) | 5.71 p = 0.69 95% CI: (5.4, 6.1) | 0.73 p = 1.00 95% CI: (0.65, 0.81) | 6.97 p = 0.0001 95% CI: (6.7, 7.3) | 12.13 p = 0.0001 95% CI: (11.9, 12.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theophilus, R.J.; Miller, M.; Oldewage-Theron, W.H.; Dawson, J. The Winning Weaning Food (WWF): The Development of a Complementary Food for Food-Insecure Infants and Young Children in Malawi. Nutrients 2019, 11, 2292. https://doi.org/10.3390/nu11102292
Theophilus RJ, Miller M, Oldewage-Theron WH, Dawson J. The Winning Weaning Food (WWF): The Development of a Complementary Food for Food-Insecure Infants and Young Children in Malawi. Nutrients. 2019; 11(10):2292. https://doi.org/10.3390/nu11102292
Chicago/Turabian StyleTheophilus, Rufus J., Markus Miller, Wilna H. Oldewage-Theron, and John Dawson. 2019. "The Winning Weaning Food (WWF): The Development of a Complementary Food for Food-Insecure Infants and Young Children in Malawi" Nutrients 11, no. 10: 2292. https://doi.org/10.3390/nu11102292
APA StyleTheophilus, R. J., Miller, M., Oldewage-Theron, W. H., & Dawson, J. (2019). The Winning Weaning Food (WWF): The Development of a Complementary Food for Food-Insecure Infants and Young Children in Malawi. Nutrients, 11(10), 2292. https://doi.org/10.3390/nu11102292






