25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis
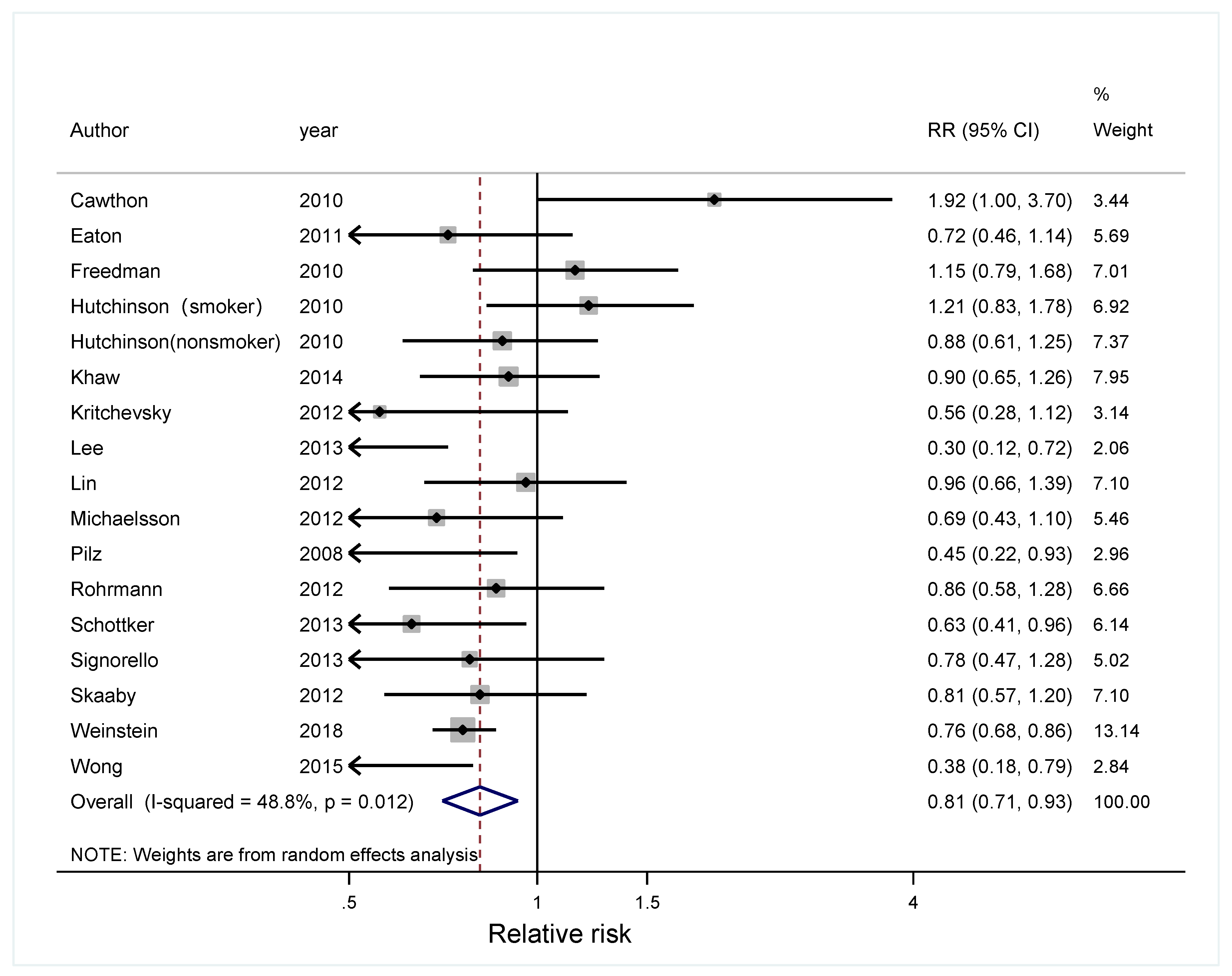
3. Results
3.1. Literature Search and Study Characteristics
3.2. Subgroup Analysis
3.3. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (concord-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Coleman, M.P.; Gatta, G.; Verdecchia, A.; Estève, J.; Berrino, F. Eurocare-3 summary: Cancer survival in europe at the end of the 20th century. Ann. Oncol. 2003, 14 (Suppl. 5) (Suppl. 5), v128–v149. [Google Scholar] [CrossRef]
- Edwards, B.K.; Anne-Michelle, N.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Jane, H.S.; Ahmedin, J.; Hyunsoon, C.; Anderson, R.N.; Kohler, B.A. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef] [PubMed]
- Kitson, M.T.; Roberts, S.K. D-livering the message: The importance of vitamin d status in chronic liver disease. J. Hepatol. 2012, 57, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; Deluca, H.F. Where is the vitamin d receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Mandolini, C.; Conti, P. Immune modulation by vitamin d: Special emphasis on its role in prevention and treatment of cancer. Clin. Ther. 2017, 39, 884–893. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin d reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Fedirko, V.; Duarte-Salles, T.; Bamia, C.; Trichopoulou, A.; Aleksandrova, K.; Trichopoulos, D.; Trepo, E.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Prediagnostic circulating vitamin d levels and risk of hepatocellular carcinoma in european populations: A nested case-control study. Hepatology 2014, 60, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum vitamin D and risk of breast cancer within five years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low plasma 25-hydroxyvitamin d and risk of tobacco-related cancer. Clin. Chem. 2013, 59, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Marco, G.; Danila, D.M.; Maurizio, L.G.; Stefania, A.; Marilena, C.; Carla, F.; Tumminello, F.M.; Gaetano, L. Vitamin d in cancer chemoprevention. Pharm. Biol. 2015, 53, 1399–1434. [Google Scholar]
- Elzbieta, G.; Studzinski, G.P. Vitamin D and differentiation in cancer. Crit. Rev. Clin. Lab. Sci. 2009, 46, 190. [Google Scholar]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin d action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Sam, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2010, 66, S116–S124. [Google Scholar]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brozyna, A.A.; Zmijewski, M.A.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; Tuckey, R.C.; Elmets, C.A. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab. Investig. 2017, 97, 706–724. [Google Scholar] [CrossRef]
- David, F.; Krishnan, A.V.; Srilatha, S.; Edward, G.; Feldman, B.J. The role of vitamin d in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar]
- Krishnan, A.V.; David, F. Molecular pathways mediating the anti-inflammatory effects of calcitriol: Implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer 2010, 17, R19–R38. [Google Scholar] [CrossRef] [PubMed]
- Carlien, L.; Lieve, V.; Annemieke, V. Antineoplastic effects of 1,25(oh)2d3 and its analogs in breast, prostate and colorectal cancer. Endocr. Relat. Cancer 2013, 20, R31–R47. [Google Scholar]
- Swami, S.; Krishnan, A.V.; Feldman, D. Vitamin D metabolism and action in the prostate: Implications for health and disease. Mol. Cell. Endocrinol. 2011, 347, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Zinser, G.M.; Sundberg, J.P.; Welsh, J.E. Vitamin d3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 2002, 23, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Mena, J.M.; Chen, T.; Schottker, B.; Arndt, V.; Brenner, H. Circulating 25-hydroxyvitamin d serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev. Med. 2013, 57, 753–764. [Google Scholar]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.L.; März, W. Vitamin d and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin d in 26916 individuals from a european consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef]
- Schöttker, B.; Jorde, R.; Peasey, A.; Thorand, B.; Jansen, E.H.; Ld, G.; Streppel, M.; Gardiner, J.; Ordóñez-Mena, J.M.; Perna, L. Vitamin d and mortality: Meta-analysis of individual participant data from a large consortium of cohort studies from europe and the united states. BMJ 2014, 348, g3656. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin d and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef]
- Budhathoki, S.; Hidaka, A.; Yamaji, T.; Sawada, N.; Tanaka-Mizuno, S.; Kuchiba, A.; Charvat, H.; Goto, A.; Kojima, S.; Sudo, N.; et al. Plasma 25-hydroxyvitamin d concentration and subsequent risk of total and site specific cancers in japanese population: Large case-cohort study within japan public health center-based prospective study cohort. BMJ 2018, 360, k671. [Google Scholar] [CrossRef]
- Lin, T.; Song, Y.; Zhang, X.; Guo, H.; Liu, L.; Zhou, Z.; Wang, B.; Tang, G.; Liu, C.; Yang, Y.; et al. Plasma 25-hydroxyvitamin D concentrations and risk of incident cancer in adults with hypertension: A nested case-control study. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cheney, C.P.; Thorand, B.; Huth, C.; Berger, K.; Peters, A.; Seifert-Klauss, V.; Kiechle, M.; Strauch, K.; Quante, A.S. The association between serum 25-hydroxyvitamin d and cancer risk: Results from the prospective kora f4 study. Oncol. Res. Treat. 2018, 41, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Mondul, A.M.; Yu, K.; Layne, T.M.; Abnet, C.C.; Freedman, N.D.; Stolzenberg-Solomon, R.Z.; Lim, U.; Gail, M.H.; Albanes, D. Circulating 25-hydroxyvitamin d up to 3 decades prior to diagnosis in relation to overall and organ-specific cancer survival. Eur. J. Epidemiol. 2018, 33, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Guo, P.; Zhong, G.C.; Zhong, S.L. Transforming the reference group of discrete correlated datain original study of dose response meta analysis. Evid. Basedmed. 2016, 16, 60–64. [Google Scholar]
- Stang, A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cook, N.R.; Bergström, A.; Hsieh, C.C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Comput. Stat. Data Anal. 2009, 53, 4157–4167. [Google Scholar] [CrossRef]
- Orsini, N.; Greenland, S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011, 11, 1–29. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Pollock, B.G. Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 1988, 80, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef]
- Seagroatt, V.; Stratton, I. Bias in meta-analysis detected by a simple, graphical test. Test had 10% false positive rate. BMJ Br. Med. J. 1997, 316, 469–471. [Google Scholar]
- Skaaby, T.; Husemoen, L.L.; Thuesen, B.H.; Pisinger, C.; Jorgensen, T.; Roswall, N.; Larsen, S.C.; Linneberg, A. Prospective population-based study of the association between serum 25-hydroxyvitamin-d levels and the incidence of specific types of cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Mena, J.M.; Schottker, B.; Haug, U.; Muller, H.; Kohrle, J.; Schomburg, L.; Holleczek, B.; Brenner, H. Serum 25-hydroxyvitamin d and cancer risk in older adults: Results from a large german prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, K.; Baron, J.A.; Snellman, G.; Gedeborg, R.; Byberg, L.; Sundstrom, J.; Berglund, L.; Arnlov, J.; Hellman, P.; Blomhoff, R.; et al. Plasma vitamin d and mortality in older men: A community-based prospective cohort study. Am. J. Clin. Nutr. 2010, 92, 841–848. [Google Scholar] [CrossRef] [PubMed]
- De Bore, L.H.; Levin, G.; Roblnson-Cohen, C.; Biggs, M.L.; Hoofnagle, A.; Siscovick, D.S.; Kestenbaum, B. Serum 25-hydroxyvitamin d concentration and risk for major clinical disease events in a community-based population of older adults a cohort study. Ann. Intern. Med. 2012, 156, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Vanderschueren, D.; Boonen, S.; O’Neill, T.W.; Pendleton, N.; Pye, S.R.; Ravindrarajah, R.; Gielen, E.; Claessens, F.; Bartfai, G.; et al. Association of 25-hydroxyvitamin d, 1,25-dihydroxyvitamin d and parathyroid hormone with mortality among middle-aged and older european men. Age Ageing 2014, 43, 528–535. [Google Scholar] [CrossRef]
- Lin, S.W.; Chen, W.; Fan, J.H.; Dawsey, S.M.; Taylor, P.R.; Qiao, Y.L.; Abnet, C.C. Prospective study of serum 25-hydroxyvitamin d concentration and mortality in a chinese population. Am. J. Epidemiol. 2012, 176, 1043–1050. [Google Scholar] [CrossRef]
- Wong, G.; Lim, W.H.; Lewis, J.; Craig, J.C.; Turner, R.; Zhu, K.; Lim, E.M.; Prince, R. Vitamin d and cancer mortality in elderly women. BMC Cancer 2015, 15, 106. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Parimi, N.; Barrett-Connor, E.; Laughlin, G.A.; Ensrud, K.E.; Hoffman, A.R.; Shikany, J.M.; Cauley, J.A.; Lane, N.E.; Bauer, D.C.; et al. Serum 25-hydroxyvitamin d, parathyroid hormone, and mortality in older men. J. Clin. Endocrinol. Metab. 2010, 95, 4625–4634. [Google Scholar] [CrossRef]
- Hutchinson, M.S.; Grimnes, G.; Joakimsen, R.M.; Figenschau, Y.; Jorde, R. Low serum 25-hydroxyvitamin d levels are associated with increased all-cause mortality risk in a general population: The tromso study. Eur. J. Endocrinol. 2010, 162, 935–942. [Google Scholar] [CrossRef]
- Freedman, D.M.; Looker, A.C.; Abnet, C.C.; Linet, M.S.; Graubard, B.I. Serum 25-hydroxyvitamin d and cancer mortality in the nhanes iii study (1988–2006). Cancer Res. 2010, 70, 8587–8597. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.; Riedmuller, G.; Fischer, J.E.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; Marz, W. Low serum levels of 25-hydroxyvitamin d predict fatal cancer in patients referred to coronary angiography. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Eaton, C.B.; Young, A.; Allison, M.A.; Robinson, J.; Martin, L.W.; Kuller, L.H.; Johnson, K.C.; Curb, J.D.; Van Horn, L.; McTiernan, A.; et al. Prospective association of vitamin d concentrations with mortality in postmenopausal women: Results from the women’s health initiative (whi). Am. J. Clin. Nutr. 2011, 94, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jorgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin d status and cause-specific mortality: A general population study. PLoS ONE 2012, 7, e52423. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Haug, U.; Schomburg, L.; Kohrle, J.; Perna, L.; Muller, H.; Holleczek, B.; Brenner, H. Strong associations of 25-hydroxyvitamin d concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am. J. Clin. Nutr. 2013, 97, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Han, X.; Cai, Q.; Cohen, S.S.; Cope, E.L.; Zheng, W.; Blot, W.J. A prospective study of serum 25-hydroxyvitamin d levels and mortality among african americans and non-african americans. Am. J. Epidemiol. 2013, 177, 171–179. [Google Scholar] [CrossRef]
- Khaw, K.T.; Luben, R.; Wareham, N. Serum 25-hydroxyvitamin d, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: A 13-y prospective population study. Am. J. Clin. Nutr. 2014, 100, 1361–1370. [Google Scholar] [CrossRef]
- Kritchevsky, S.B.; Tooze, J.A.; Neiberg, R.H.; Schwartz, G.G.; Hausman, D.B.; Johnson, M.A.; Bauer, D.C.; Cauley, J.A.; Shea, M.K.; Cawthon, P.M.; et al. 25-hydroxyvitamin d, parathyroid hormone, and mortality in black and white older adults: The health abc study. J. Clin. Endocrinol. Metab. 2012, 97, 4156–4165. [Google Scholar] [CrossRef]
- Rohrmann, S.; Braun, J.; Bopp, M.; Faeh, D.; Swiss National, C. Inverse association between circulating vitamin d and mortality—Dependent on sex and cause of death? Nutr. Metab. Cardiovasc. Dis. 2013, 23, 960–966. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin d status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef]
- Krause, R.; Schober-Halstenberg, H.J.; Edenharter, G.; Haas, K.; Roth, H.J.; Frei, U. Vitamin D status and mortality of german hemodialysis patients. Anticancer Res. 2012, 32, 391–396. [Google Scholar]
- Goulao, B.; Stewart, F.; Ford, J.A.; MacLennan, G.; Avenell, A. Cancer and vitamin d supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Vitamin D supplements and total cancer incidence and mortality a meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]




| First Author and Cohort | Publication Year and Region | Mean Age (Gender) | Subjects (Cases) | Follow-Up Period | Exposure Measure | Outcome Ascertainment | Covariates Adjusted |
|---|---|---|---|---|---|---|---|
| Afzal [13], CCHS | 2013, Denmark | 57.7 (M) | 9791 (1081) | 28 y | radioimmunoassay | obtained from the Danish Cancer Registry | age, sex, pack-years, BMI, alcohol consumption, leisure time and work-related physical activity, duration of education and month of blood sample |
| Budhathoki [31], JPHC | 2018, Japan | 53.7 (Both) | 33,736 (3301) | 19 y | chemiluminescent enzyme immunoassay | obtained from local hospitals and population-based cancer registries | age, sex, body mass index, smoking, alcohol use, physical activity, family history of cancer, and reported history of diabetes. |
| Lin [32], CSPPT | 2018, China | 61.8 (Both) | 462 (231) | 4.5 y | liquid chromatography tandem mass spectrometry | diagnosed based on either positive pathologic findings or specific clinical manifestations | age, sex, treatment group, and study site, and adjusted for BMI, smoking status, alcohol consumption, baseline SBP and DBP, time-averaged SBP and DBP during treatment, baseline fasting blood glucose, total cholesterol, triglycerides, season of blood collection, plasma calcium levels, folate, vitamin B12, vitamin A, vitamin E and vitamin K |
| Skaaby [42], Monica10, Inter99, and Health2006 | 2014, Denmark | 60.5 (Both) | 12,204 (1248) | 11.3 y | chemiluminescent enzyme immunoassay | obtained from the Danish Central Personal Register | study group (no intervention (participants from Monica10), lifestyle counseling (group B from Inter99), lifestyle and group counseling (group A from Inter99)), gender, education, season, physical activity, smoking habits, alcohol intake, intake of fish, and BMI |
| Cheney [33], KORA | 2018, Germany | 53.5 (Both) | 2003 (69) | 7 y | enzyme immunoassay | identified using a standardized interview (FF4) by trained medical personnel. | age, sex, BMI, season of blood draw, physical activity, smoking status, smoking status alcohol consumption and vitamin D supplementation |
| Boer [45], CHS | 2012, USA | 74.0 (Both) | 1621 (335) | 15 y | high-performance liquid chromatography tandem mass spectrometry | obtained from available hospital discharge summaries, diagnostic test records, and consultation reports | age, sex, clinical site, smoking, body mass index, and physical activity |
| Michaelsson [44], ULSAM | 2010 Sweden | 71 (M) | 1194 (373) | 12.7 y | high-performance liquid chromatography–tandem mass spectrometry | obtained from Swedish National Cancer Registry and Cause of Death Registry | age, weight, height, calcium intake, season of blood draw, social class, smoking status, leisure physical activity, self-perceived health, diabetes mellitus, other endocrine disease, hematologic diseases, dermatoses, infectious disease, musculoskeletal disease, psychiatric disease, respiratory disease, kidney or urinary disease, gastrointestinal disease, supplemental vitamin D use, total vitamin D intake, fish intake, plasma parathyroid hormone, plasma cystatin C, plasma C-reactive protein, serum calcium, serum phosphate, plasma troponin I, plasma N-terminal pro brain natriuretic peptide, plasma cholesterol, plasma triglycerides, plasma HDL cholesterol, plasma retinol, plasma insulin, total energy intake, and alcohol intake and systolic blood pressure, diastolic blood pressure, lipid-lowering treatment, and antihypertensive treatment. |
| Ordonez -Mena [27] | 2013 Germany | 63 (Both) | 9007 (873) | 8 y | liquid chromatography/tandem mass spectrometry | obtained from Saarland Cancer Registry | Age, sex, multivitamin use, fish consumption, red meat consumption, daily fruit intake, daily vegetables intake, BMI, scholarly education, physical activity, smoking, and family history of cancer |
| First Author | Publication Year and Region | Mean Age (Gender) | Subjects (Cases) | Follow-Up Period | Exposure Measure | Outcome Ascertainment | Covariates Adjusted |
|---|---|---|---|---|---|---|---|
| Lee [46], EMAS | 2013, Europe | 60 (M) | 2816 (28) | 4.3 y | radioimmunoassay | Obtained from death certificates, death registers and medical/hospital records. | age, center, smoking status, alcohol consumption, self-reported morbidities, Physical Activity Scale for the Elderly score, Reuben’s Physical Performance Test rating and serum creatinine |
| Lin [47], GPTL | 2012, China | 56.5 (Both) | 110 (217) | 24 y | enzyme immunoassay | Obtained from records of the village doctors, evaluated and verified by a panel of Chinese experts | age and sex, with additional adjustment by separate continuous age variables for each stratum as well as sex, hypertension, tobacco smoking, BMI, and alcohol consumption. |
| Wong [48], CAIFOS | 2015, US | 75.1 (F) | 1188 (84) | 10 y | liquid chromatography tandem mass spectrometry | Obtained from the hospital death certificates and previous medical history, and the coded discharge diagnosis data | age, diastolic blood pressure, systolic blood pressures, previous and current smoker, BMI, daily alcohol use, co-morbidities, season at recruitment, treatment allocation, laboratory measurements |
| Weinstein [34], ATBC | 2018, Finland | 59.5 (M) | 4616 (2884) | 28 y | competitive chemiluminescence immunoassay/radioimmunoassay/liquid chromatography tandem mass spectrometry | obtained from Finnish Cancer Registry | BMI, number of cigarettes smoked per day, years of smoking, physical activity, serum cholesterol, history of diabetes, family history of cancer, systolic blood pressure, trial intervention group, and calendar year of diagnosis |
| Cawthon [49], MrOS | 2010 USA | 73.7 (M) | 1490 (97) | 7.3 y | chemiluminescence immunoassay | obtained from six U.S. clinical centers through death certificates and discharge summaries | age, clinic, season of blood collection, serum calcium and phosphate, GFR, percentage body fat, weight, race, health status, presence of at least one medical condition, alcohol use, education, activity level, marital status, and presence of a functional or mobility limitation |
| Hutchinson [42], TS | 2010 Norway | Nonsmoker 61 Smoker 57.2 (Both) | 7161 (498) | 13 y | mass spectrometry | obtained from Norway Cancer Registry | age, gender, season, BMI, physical activity score, diabetes, hypertension, serum creatinine, prior cardiovascular disease and prior cancer |
| Freedman [51], NHANES III | 2010 USA | 44 (Both) | 16,819 (884) | 12.5 y | electrochemiluminescence immunoassay | obtained from National Center for Health Statistics of the Centers for Disease Control and Prevention | age, race/ ethnicity, smoking history, and BMI. |
| Pilz [52], LURIC | 2008 Germany | 62.7 (Both) | 3257 (95) | 7.75 y | radioimmunoassay | obtained from local person registries | age, gender, season, BMI, active smokers, retinol, exercise tertiles, beer and wine consumption, and diabetes mellitus. |
| Eaton [53], WHI | 2011 USA | 65.8 (F) | 2429 (62) | 8 y | high-performance liquid chromatography | obtained from the Women’s Health Initiative | age, season, ethnicity, CaD trial indicator, education, smoking status, current aspirin use, history of fracture, waist circumference, BMI, physical activity, and use of vitamin D supplements. |
| Skaaby [54], Monica10 and Inter99. | 2012 Denmark | 49.8 (Both) | 9146 (301) | 10 y | chemiluminescence immunoassay | obtained from Danish Registry of Causes of Death | study group (no intervention (participants from Monica10), lifestyle counseling (group B from Inter99), lifestyle and group counseling (group A from Inter99)), gender, education, season of blood sample, intake of fish, physical activity, smoking, BMI and alcohol consumption. |
| Schöttker [55], ESTHER | 2013 Germany | 62 (Both) | 9578 (433) | 9.5 y | competitive protein-binding assay | identified by inquiry at the residents’ registration offices | age, sex, season of blood draw, regularly intake of multi-vitamin supplements, fish consumption, BMI, scholarly education, physical activity, smoking, systolic blood pressure, chronic kidney disease, serum CRP concentrations and total cholesterol |
| Signorello [56], SCCS | 2012 USA | 59.5 (Both) | 3704 (954) | 7 y | chemiluminescence immunoassay | identified by Social Security Administration’s Death Master File and the National Death Index | gender, race, age, community health center enrollment site, date of blood collection, BMI, smoking, physical activity, and household income. |
| Khaw [57], - | 2014 UK | 62 (Both) | 14,641 (1086) | 13 y | mass spectrometry | obtained fromNational Cancer Registry for incident Cancer | age, sex, month, BMI, physical activity, smoking, alcohol, vitamin C, diabetes, history of cardiovascular disease, history of cancer, social class, and education |
| Kritchevsky [58], Health ABC | 2012 US | 74.7 (Both) | 2638 (218) | 8.5 y | immunoassay | identified by medical records, death certificates, proxy information, and autopsy reports | age, gender, race, education, season, field center, smoking status, pack years, alcohol consumption, body mass index, time walking, usual 20m walking speed, estimated glomerular filtration rate, cognition, depressive symptoms, IL-6, cholesterol, and prevalent diabetes, hypertension, cardiovascular disease, cancer, or lung disease. |
| Rohrmann [59], Swiss MONICA | 2012 | 47.1 (Both) | 3198 (188) | 18 y | protein-bound assay | obtained from Swiss National Cohort | age, sex, sunlight exposure, systolic blood pressure, smoking status, nationality |
| Michaelsso [44], ULSAM | 2010 Sweden | 71 (M) | 1194 (164) | 12.7 y | high-performance liquid chromatography–tandem mass spectrometry | obtained from Swedish National Cancer Registry and Cause of Death Registry | age, weight, height, calcium intake, season of blood draw, social class, smoking status, leisure physical activity, self-perceived health, diabetes mellitus, other endocrine disease, hematologic diseases, dermatoses, infectious disease, musculoskeletal disease, psychiatric disease, respiratory disease, kidney or urinary disease, gastrointestinal disease, supplemental vitamin D use, total vitamin D intake, fish intake, plasma parathyroid hormone, plasma cystatin C, plasma C-reactive protein, serum calcium, serum phosphate, plasma troponin I, plasma N-terminal pro brain natriuretic peptide, plasma cholesterol, plasma triglycerides, plasma HDL cholesterol, plasma retinol, plasma insulin, total energy intake, and alcohol intake and systolic blood pressure, diastolic blood pressure, lipid-lowering treatment, and antihypertensive treatment. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Guo, X.; Yu, X.; Liu, S.; Cui, X.; Zhang, B.; Liang, H. 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2019, 11, 2295. https://doi.org/10.3390/nu11102295
Han J, Guo X, Yu X, Liu S, Cui X, Zhang B, Liang H. 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients. 2019; 11(10):2295. https://doi.org/10.3390/nu11102295
Chicago/Turabian StyleHan, Jianmin, Xiaofei Guo, Xiao Yu, Shuang Liu, Xinyue Cui, Bo Zhang, and Hui Liang. 2019. "25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies" Nutrients 11, no. 10: 2295. https://doi.org/10.3390/nu11102295
APA StyleHan, J., Guo, X., Yu, X., Liu, S., Cui, X., Zhang, B., & Liang, H. (2019). 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients, 11(10), 2295. https://doi.org/10.3390/nu11102295




