Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration?
Abstract
:1. Introduction
2. Methods
Study Selection
3. Dietary Sources of the Essential Fatty Acids
4. Nutrient Bioavailability
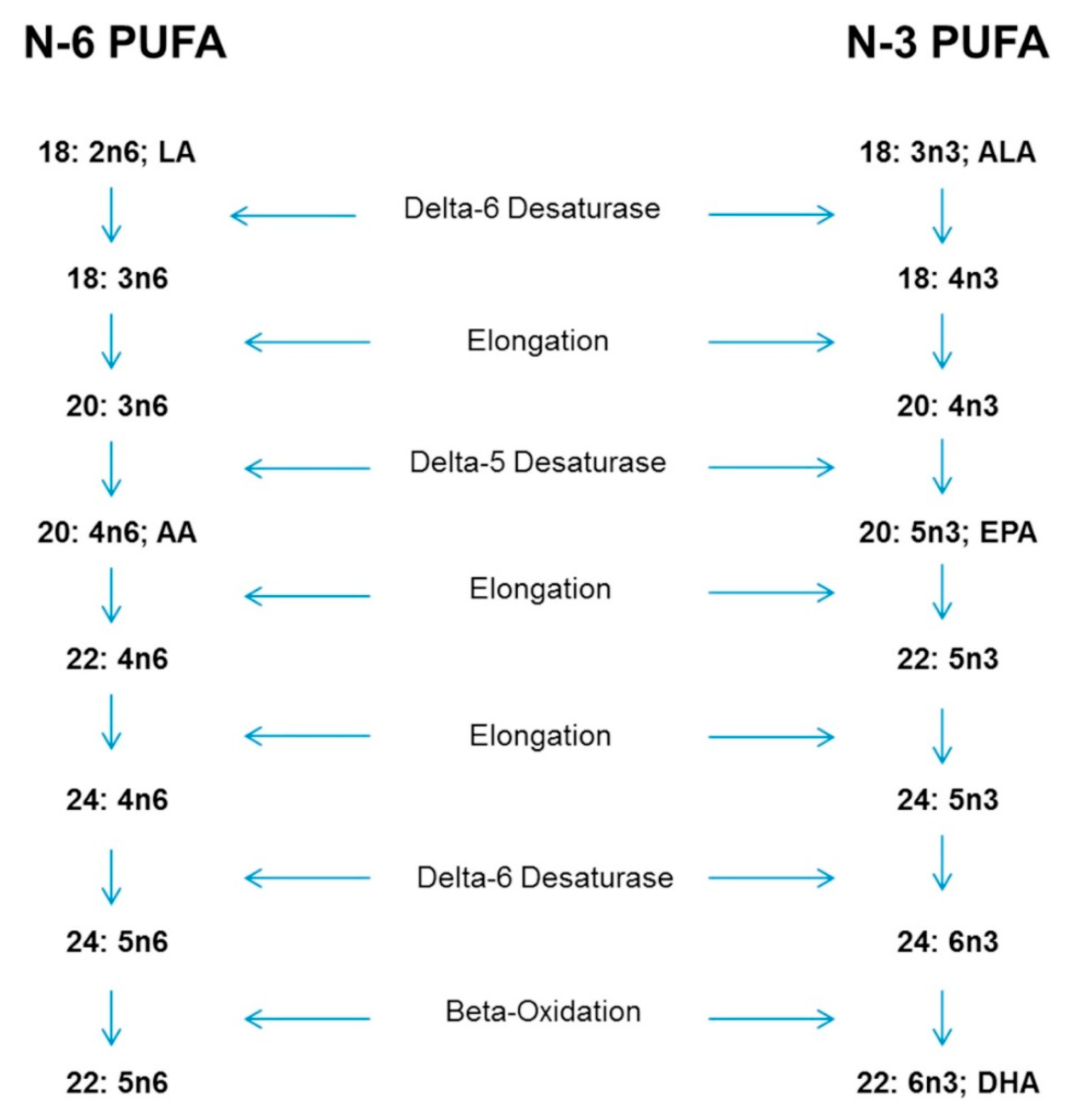
5. Physiological Reasons for Special Consideration-Conversion of ALA to EPA and DHA
5.1. Gender Differences
5.2. Influence of Trans-Fatty Acids on the Δ-6-Desaturase Enzyme
5.3. n-6 Fatty Acid Status
5.3.1. Dietary n-6 Fatty Acid Intakes
5.3.2. Plasma n-6 Concentrations
5.3.3. Serum n-6 Concentrations
5.3.4. Erythrocyte and Whole Blood n-6 Fatty Acid Status
5.3.5. Platelet n-6 Fatty Acid Status
5.3.6. Breastmilk n-6 Status
5.3.7. Adipose Tissue n-6 Status
5.4. N-3 Fatty Acid Status
5.4.1. Dietary Intakes of n-3 Fatty Acids in VGNs as Compared to Omnivores
5.4.2. Plasma n-3 Concentrations
5.4.3. Serum n-3 Concentrations
5.4.4. Erythrocyte and Whole Blood n-3 Status
5.4.5. Platelet n-3 Fatty Acid Status
5.4.6. Breastmilk n-3 Status
5.4.7. Adipose Tissue n-3 Status
5.5. Possible Health Risks of High Dietary LA Intake
5.6. Comparisons of High LA Biological Levels and n-3 Fatty Acid Status
6. Recommended Intakes of n-3 and the n-6:n-3 Ratio
Intake Suggestions to Meet the AI for n-3 and n-6 Fatty Acids
7. Additional Research
7.1. Conversion Rates
7.2. Diet and Biological Indicators
7.2.1. Biological Indicators and Tissue Status
7.2.2. Low n-3 Status/High n-6 Status and Risk
8. Conclusions
9. Main Findings from This Review
- Most studies indicate that VGNs consume higher amounts of LA compared to omnivores, with confirmation in tissues stores; however, there are inconsistent findings of AA tissue concentrations compared to omnivores.
- There are inconsistent results of ALA intake by VGNs compared to omnivores.
- Most studies show that VGNs consume low to zero amounts of EPA and DHA, unless they take supplements.
- Most studies indicate that plasma, serum, erythrocytes, adipose, and platelet levels of EPA and DHA are lower in VGNs than omnivores.
- VGNs may need an ALA increase of 2.2–4.4 g/day (or 1.1 g/day/1000 Kcals) depending on the amount of LA in the diet in order to achieve a 4:1 n-6:n-3 ratio, as well as a decrease of dietary LA if intake of LA is higher than recommended.
- Special consideration recommendations for both ALA and LA for adult VGNs should be considered by the AI/DRI.
Author Contributions
Funding
Conflicts of Interest
References
- Spector, A.; Kim, H.Y. Discovery of Essential Fatty Acids. J. Lipid Res. 2014, 56, 11–21. [Google Scholar] [CrossRef] [PubMed]
- The National Academies of Sciences Engineering Medicine. Dietary Reference Intakes tables and application. Available online: http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx (accessed on 18 August 2019).
- Institute of Medicine (IOM). Introduction to dietary reference intakes. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academics Press: Washington, DC, USA, 2005; pp. 29–37. [Google Scholar]
- Institute of Medicine (IOM). Dietary fats: Total fat and fatty acids. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academics Press: Washington, DC, USA, 2005; pp. 470–472, 030908525X (pbk.) 0309085373 (hardcover). [Google Scholar]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Alles, B.; Baudry, J.; Mejean, C.; Touvier, M.; Peneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Sante Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Sanders, T.A.; Kendall, A.C.; Nicolaou, A.; Gray, R.; Al-Khatib, H.; Hall, W.L. A comparison of heart rate variability, n-3 PUFA status and lipid mediator profile in age- and BMI-matched middle-aged vegans and omnivores. Br. J. Nutr. 2017, 117, 669–685. [Google Scholar] [CrossRef] [Green Version]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.; Allen, N.E.; Key, T.J. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef]
- Perrin, M.T.; Pawlak, R.; Dean, L.L.; Christis, A.; Friend, L. A cross-sectional study of fatty acids and brain-derived neurotropic factor (BDNF) in human milk from lactating women following vegan vegetarian and omnivore diets. Eur. J. Clin. Nutr. 2019, 58, 2401–2410. [Google Scholar] [CrossRef]
- Conquer, J.A.; Holub, B.J. Supplementation with an algae source of docosahexaenoic acid increases (n-3) fatty acid status and alters selected risk factors for heart disease in vegetarian subjects. J. Nutr. 1996, 126, 3032–3039. [Google Scholar] [CrossRef]
- Geppert, J.; Kraft, V.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid supplementation in vegetarians effectively increases omega-3 index: A randomized trial. Lipids 2005, 40, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 polyunsaturated fatty acids and vegetarian diets. Med. J. Aust. 2013, 199, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Sarter, B.; Kelsey, K.S.; Schwartz, T.A.; Harris, W.S. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: Associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clin. Nutr. 2015, 34, 212–218. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information, PubMed.gov. U.S. National Library of Medicine. National Institutes of Health. Database-PubMed. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 18 August 2019).
- U.S. Department of Agriculture (USDA). Nutrient Database. Available online: https://ndb.nal.usda.gov/ndb/nutrients/index (accessed on 11 June 2019).
- Ovega-3 i-Health Inc. Available online: https://www.ovega.com/ovega-3 (accessed on 25 October 2017).
- Deva Nutrition LLC. Available online: http://www.devanutrition.com/vegan-omega-3-dha-delayed-release.html (accessed on 25 October 2017).
- Nordic Naturals Inc. Available online: https://www.nordicnaturals.com/en/Products/Product_Details/514/?ProdID=1654 (accessed on 25 October 2017).
- Sanders, T.A.; Roshanai, F. Platelet phospholipid fatty acid composition and function in vegans compared with age- and sex-matched omnivore controls. Eur. J. Clin. Nutr. 1992, 46, 823–831. [Google Scholar] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Sauerwald, T.U.; Hachey, D.L.; Jensen, C.L.; Chen, H.; Anderson, R.E.; Heird, W.C. Effect of dietary alpha-linolenic acid intake on incorporation of docosahexaenoic and arachidonic acids into plasma phospholipids of term infants. Lipids 1996, 31, S131–S135. [Google Scholar] [CrossRef]
- Salem, N., Jr.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta 1994, 1213, 277–288. [Google Scholar] [CrossRef]
- Akerele, O.A.; Cheema, S.K. A balance of omega-3 and omega-6 polyunsaturated fatty acids is important in pregnancy. J. Nutr. Intermed. Metab. 2016, 5, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Domenichiello, A.; Kitson, A.; Bazinet, R. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L. The importance of the long-chain polyunsaturated fatty acid n-6 / n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Burdge, G.C.; Tan, S.Y.; Henry, C.J. Long-chain n-3 PUFA in vegetarian women: A metabolic perspective. J. Nutr. Sci. 2017, 6, e58. [Google Scholar] [CrossRef]
- Enig, M.G.; Atal, S.; Keeney, M.; Sampugna, J. Isomeric trans fatty acids in the U.S. diet. J. Am. Coll. Nutr. 1990, 9, 471–486. [Google Scholar] [CrossRef]
- Steinhart, H.; Rickert, R.; Winkler, K. Trans fatty acids (TFA): Analysis, occurrence, intake and clinical relevance. Eur. J. Med. Res. 2003, 8, 358–362. [Google Scholar]
- Valenzuela, A.; Morgado, N. Trans fatty acid isomers in human health and in the food industry. Biol. Res. 1999, 32, 273–287. [Google Scholar] [CrossRef]
- Hu, F.B.; van Dam, R.M.; Liu, S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef]
- Li, D.; Sinclair, A.; Wilson, A.; Nakkote, S.; Kelly, F.; Abedin, L.; Mann, N.; Turner, A. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am. J. Clin. Nutr. 1999, 69, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Kornsteiner, M.; Singer, I.; Elmadfa, I. Very low n-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Ann. Nutr. Metab. 2008, 52, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Lloren, J.I.C.; Haddad, E.; Jaceldo-Siegl, K.; Knutsen, S.; Sabate, J.; Fraser, G.E. Plasma, urine, and adipose tissue biomarkers of dietary intake differ between vegetarian and non-vegetarian diet groups in the Adventist Health Study-2. J. Nutr. 2019, 146, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Agren, J.J.; Tormala, M.L.; Nenonen, M.T.; Hanninen, O.O. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995, 30, 365–369. [Google Scholar] [CrossRef]
- Fokkema, M.R.; Brouwer, D.A.; Hasperhoven, M.B.; Martini, I.A.; Muskiet, F.A. Short-term supplementation of low-dose gamma-linolenic acid (GLA), alpha-linolenic acid (ALA), or GLA plus ALA does not augment LCP omega 3 status of Dutch vegans to an appreciable extent. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 287–292. [Google Scholar] [CrossRef]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes, M.A.; Wareham, N.J.; Khaw, K.T. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results from the EPIC-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1040–1051. [Google Scholar] [CrossRef]
- Mann, N.; Pirotta, Y.; O’Connell, S.; Li, D.; Kelly, F.; Sinclair, A. Fatty acid composition of habitual omnivore and vegetarian diets. Lipids 2006, 41, 637–646. [Google Scholar] [CrossRef]
- Sanders, T.A.; Ellis, F.R.; Dickerson, J.W. Studies of vegans: The fatty acid composition of plasma choline phosphoglycerides, erythrocytes, adipose tissue, and breast milk, and some indicators of susceptibility to ischemic heart disease in vegans and omnivore controls. Am. J. Clin. Nutr. 1978, 31, 805–813. [Google Scholar] [CrossRef]
- Fisher, M.; Levine, P.H.; Weiner, B.; Ockene, I.S.; Johnson, B.; Johnson, M.H.; Natale, A.M.; Vaudreuil, C.H.; Hoogasian, J. The effect of vegetarian diets on plasma lipid and platelet levels. Arch. Intern. Med. 1986, 146, 1193–1197. [Google Scholar] [CrossRef]
- Sanders, T.A.; Reddy, S. The influence of a vegetarian diet on the fatty acid composition of human milk and the essential fatty acid status of the infant. J. Pediatr. 1992, 120, S71–S77. [Google Scholar] [CrossRef]
- Stark, K.; VanElswyk, M.; Higgins, M.; Weatherford, C.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, T.; Weng, X.; Shou, T.; Wang, Q.; Zhou, X.; Hu, Q.; Li, D. Plasma n-3 and n-6 fatty acids and inflammatory markers in Chinese vegetarians. Lipids Health Dis. 2014, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S. Achieving optimal n-3 fatty acid status: The vegetarian’s challenge... or not. Am. J. Clin. Nutr. 2014, 100, 449S–452S. [Google Scholar] [CrossRef]
- Simopoulos, A. The importance of the omega6/omega 3 fatty acid ratio in cardiovascular disease and other diseases. Exp. Biol. Med. (Maywood) 2008, 223, 674–688. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar]
- Emken, E.A.; Adlof, R.O.; Duval, S.M.; Nelson, G.J. Effect of dietary arachidonic acid on metabolism of deuterated linoleic acid by adult male subjects. Lipids 1998, 33, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Mantzioris, E.; James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary substitution with an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am. J. Clin. Nutr. 1994, 59, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Indu, M. n-3 fatty acids in Indian diets—Comparison of the effects of precursor (alpha-linolenic acid) Vs product (long chain n-3 poly unsaturated fatty acids). Nutr. Res. 1992, 12, 569–582. [Google Scholar] [CrossRef]
- Mest, H.J.; Beitz, J.; Heinroth, I.; Block, H.U.; Forster, W. The influence of linseed oil diet on fatty acid pattern in phospholipids and thromboxane formation in platelets in man. Klin. Wochenschr. 1983, 61, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Younger, K.M. The effect of dietary supplements of omega 3 polyunsaturated fatty acids on the fatty acid composition of platelets and plasma choline phosphoglycerides. Br. J. Nutr. 1981, 45, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Bjerve, K.S.; Mostad, I.L.; Thoresen, L. Alpha-linolenic acid deficiency in patients on long-term gastric-tube feeding: Estimation of linolenic acid and long-chain unsaturated n-3 fatty acid requirement in man. Am. J. Clin. Nutr. 1987, 45, 66–77. [Google Scholar] [CrossRef]
- Regents of University of Minnesota. Nutrition Data Systems for Research. NDS-R Software. Available online: http://www.ncc.umn.edu/products/ (accessed on 2 October 2019).
| Nutrient 1 | Energy | Protein | Total Lipid | 18:2n-6 | 18:3n-3 | CHO | Total Fiber | |
|---|---|---|---|---|---|---|---|---|
| Unit | Kcal | g | g | g | g | g | g | |
| Oils | ||||||||
| Oil, Canola | 100 g | 884 | 0 | 100 | 18.64 | 9.137 | 0 | 0 |
| Oil, Flaxseed/Linseed (Panos) | 100 g | 884 | 0.110 | 99.98 | 14.25 | 53.37 | 0 | 0 |
| Oil, Soybean | 100 g | 763 | 0 | 100 | 50.42 | 6.789 | 0 | 0 |
| Oil, Walnut | 100 g | 884 | 0 | 100 | 52.90 | 10.40 | 0 | 0 |
| Oil, High Oleic Sunflower | 100 g | 884 | 0 | 100 | 3.61 * | 0.192 ** | 0 | 0 |
| Oil, High Oleic Safflower | 100 g | 884 | 0 | 100 | 12.72 | 0.096 | 0 | 0 |
| Oil, Culinary Algae | 100 g | 800 | 0 | 93.33 | n/a | n/a | 0 | 0 |
| Ovega-3 supplement 2 | 1 gel | n/a | 0 | n/a | n/a | 0.5 | 0 | 0 |
| Deva Vegan supplement 3 | 1 cap | 5 | 0 | 0.5 | n/a | 0.2 | 0 | 0 |
| Nordic Naturals supplement 4 | 2 gels | 10.0 | 0 | 1.00 | n/a | 0.715 | 0 | 0 |
| Food Sources | ||||||||
| Almonds Raw | 100 g | 579 | 21.15 | 49.93 | 12.30 | 0.003 | 21.55 | 12.50 |
| Amaranth | 100 g | 371 | 13.65 | 7.020 | 2.736 | 0.042 | 65.25 | 6.70 |
| Avocados, Raw, California | 100 g | 167 | 1.960 | 15.41 | 1.674 | 0.111 | 8.640 | 6.80 |
| Black Walnuts Dried | 100 g | 619 | 24.06 | 59.33 | 33.80 | 2.680 | 9.580 | 6.80 |
| Brazil Nuts, Dried | 100 g | 659 | 14.32 | 67.10 | 23.859 | 0.018 | 11.74 | 7.50 |
| Brown Rice Cooked | 100 g | 123 | 2.740 | 0.970 | 0.355 | 0.011 | 25.58 | 1.60 |
| Bulgur Cooked | 100 g | 83 | 3.08 | 0.240 | 0.094 | 0.004 | 18.58 | 4.50 |
| Cashews Raw | 100 g | 553 | 18.22 | 43.85 | 7.782 | 0.062 | 30.19 | 3.30 |
| Chia Seeds Dried | 100 g | 486 | 16.54 | 30.74 | 5.840 | 17.80 | 42.12 | 34.4 |
| English Walnuts Dried | 100 g | 654 | 15.23 | 65.21 | 38.09 | 9.08 | 13.71 | 6.70 |
| Flaxseed Raw | 100 g | 534 | 18.29 | 42.16 | 5.903 | 22.81 | 28.88 | 27.3 |
| Hempseed Hulled | 100 g | 553 | 31.56 | 48.75 | 1.340 | 8.864 | 8.670 | 4.00 |
| Millet Cooked | 100 g | 119 | 3.510 | 1.000 | 0.480 | 0.028 | 23.67 | 1.300 |
| Oat Bran Cooked | 100 g | 40 | 3.210 | 0.860 | 0.324 | 0.015 | 11.44 | 2.60 |
| Pistachio Raw | 100 g | 560 | 20.16 | 45.32 | 13.10 | 0.210 | 27.17 | 10.60 |
| Poppy Seeds | 100 g | 525 | 17.99 | 41.56 | 28.30 | 0.273 | 28.13 | 19.50 |
| Quinoa | 100 g | 368 | 14.12 | 6.070 | 2.977 | 0.260 | 64.16 | 7.00 |
| Rye | 100 g | 338 | 10.34 | 1.630 | 0.659 | 0.108 | 75.86 | 15.1 |
| Sesame Seeds dried | 100 g | 573 | 17.73 | 49.67 | 21.375 | 0.376 | 23.45 | 11.8 |
| Soybeans Raw | 100 g | 446 | 36.49 | 19.94 | 9.925 | 1.330 | 30.16 | 9.30 |
| Soybeans, Boiled | 100 g | 141 | 12.35 | 6.400 | 2.657 | 0.354 | 11.05 | 4.20 |
| Sunflower Seeds | 100 g | 584 | 20.78 | 51.50 | 23.05 * | 0.06 ** | 20.00 | 8.60 |
| Author, Year | Plasma | Serum | Erythrocyte and Whole Blood | Platelets | Breastmilk | Adipose |
|---|---|---|---|---|---|---|
| Rosell et al., 2005 [10] | Higher concentrations of LA in VGN plasma fatty acids than in meat-eaters, but found no significant differences in AA. | - | - | - | - | - |
| Li et al., 1999 [39] | Higher concentrations of LA in VGN plasma fatty acids than in meat-eaters, but found no significant differences in AA. | - | - | VGNs had higher levels of LA in platelets, but lower levels of AA than the high meat-eaters. | - | - |
| Mann et al., 2006 [45] | Higher concentrations of LA in VGN plasma fatty acids than in meat-eaters, but found no significant differences in AA. | - | - | - | - | - |
| Pinto et al., 2017 [9] | No significant differences between plasma LA or AA in VGNs or omnivores. | - | No difference for LA or AA in blood fatty acid profiles of VGNs and meat eaters. | - | - | - |
| Sanders et al., 1978 [46] | Higher LA and AA plasma choline phospholipids in VGNs compared to omnivores. | - | Higher levels of LA in VGNs than omnivores, but no significant differences for AA in erythrocytes. LA was higher in breastfed VGN infant erythrocytes compared to omnivore breast-fed infants, and AA was not significantly different. | - | - | Higher levels of LA in VGN adipose tissue compared to omnivore adipose tissue. |
| Elorinne et al., 2016 [11] | - | Higher LA, but no significant differences of AA in VGN serum compared to non-VGN serum. | - | - | - | - |
| Agren et al., 1995 [42] | - | Higher LA, but no significant differences of AA in VGN serum compared to non-VGN serum. | Higher levels of LA in VGNs than omnivores, and higher AA in omnivores. | Higher levels of LA in VGN platelets, but no differences of AA compared to omnivores. | - | - |
| Sarter et al., 2015 [16] | - | - | No difference for LA or AA in blood fatty acid profiles of VGNs and soldiers. | - | - | - |
| Kornsteiner et al., 2008 [40] | - | - | Higher levels of LA in VGNs than omnivores, and higher AA in omnivores. | - | - | - |
| Fisher et al., 1986 [47] | - | - | - | Higher platelet LA concentration, and lower AA in both the VGN and vegetarian subgroups as compared to omnivores. | - | - |
| Sanders et al., 1992 [48] | - | - | - | - | Higher levels of LA in VGN breastmilk compared to omnivores, but there were no significant differences for AA. | - |
| Perrin et al., 2018 [12] | - | - | - | - | No significant differences for LA or AA in VGNs as compared to omnivores. LA:ALA ratio was significantly lower in VGNs than omnivores. | - |
| Miles et al., 2019 [41] | - | - | - | - | - | LA was higher in VGNs than non-vegetarians. AA was significantly lower in VGNs than non-vegetarians. |
| Author, Year | Plasma | Serum | Erythrocyte and Whole Blood | Platelets | Breastmilk | Adipose |
|---|---|---|---|---|---|---|
| Rosell et al., 2005 [10] | Plasma ALA was not different between male VGNs and meat-eaters, whereas EPA and DHA were significantly lower in male VGNs than in meat-eaters. | - | - | - | - | - |
| Mann et al., 2006 [45] | ALA plasma was higher in male VGNs than the high meat group, whereas EPA and DHA were significantly lower in VGNs. | - | - | - | - | - |
| Agren et al., 1995 [42] | Lower levels of DHA and EPA in VGNs compared to omnivores. | Lower levels of DHA and EPA in VGN serum compared to omnivores. | DHA and EPA were lower in VGN erythrocytes compared to omnivores. | Low levels of DHA and EPA in VGN platelets compared to omnivores. | - | - |
| Sanders et al., 1978 [46] | EPA and DHA were significantly lower compared to omnivores. | - | Low EPA and DHA in VGNs and VGN breast-fed infant erythrocytes compared to omnivores. | - | Higher ALA levels in VGNs than omnivores, and lower DHA and EPA (not significant). | ALA was higher in adipose tissue of VGNs than omnivores (not significant). |
| Li et al., 1999 [39] | EPA and DHA were lower in male VGNs, and the n-3:n-6 ratio was lower, and AA:EPA was higher compared to male high meat eaters. | - | - | No differences in ALA composition between VGNs and omnivores; however, there was a significant decrease in EPA and DHA levels in VGNs. | - | - |
| Pinto et al., 2017 [9] | ALA was higher in VGNs than the high meat group; however, EPA and DHA were significantly lower in VGNs. | - | The omega-3 index was significantly lower in VGNs (2.71%) than in omnivores (5.42%). | - | - | - |
| Welch et al., 2010 [44] | ALA levels were not different for males or females as compared to the meat-eaters, but VGN DHA was higher in women than meat-eaters. In VGN males, plasma DHA was lower than omnivores. | - | - | - | - | - |
| Elorinne et al., 2016 [11] | - | No differences between VGNs and non-VGN serum ALA concentrations, however EPA and DHA serum concentrations were significantly lower in VGNs than omnivores. | - | - | - | - |
| Sanders et al., 1992 [48] | - | - | The proportion of DHA in erythrocytes of breast-fed VGN infants was 1.9% as compared to infants fed a milk formula (3.7%). | - | Levels of ALA were comparable to vegetarians, but concentrations of DHA were less than 50% for both vegetarians and omnivores. | - |
| Kornsteiner et al., 2008 [40] | - | - | DHA and EPA in adult erythrocytes were decreased in Austrian VGNs. | - | - | - |
| Sarter et al., 2015 [16] | - | - | Higher ALA and EPA levels in VGNs. No differences in DHA levels between VGNs and US soldiers in whole blood FA. | - | - | - |
| Sanders et al., 1992 [22] | - | - | - | No difference in ALA composition between VGNs and non-VGNs; however, there was a sig. decrease in EPA and DHA levels in VGNs. | - | - |
| Perrin et al., 2018 [12] | - | - | - | ALA was significantly higher in VGNs than in non-VGNs; DHA and EPA were not sig. different. The LA:ALA ratio was lower in VGNs than non-VGNs. | - | - |
| Miles et al., 2019 [41] | - | - | - | - | - | ALA was higher and EPA, DPA, and DHA were sig. lower in VGNs than non-VGNs. Total n-3 was highest in VGNs due to high ALA levels. |
| Author, Year | Recommendation: Males; Females Combined | 18:2n-6 LA | 18:3 n-3 ALA | 20:5 n-3 EPA | 22:6 n-3 DHA | n-6:n-3 or Omega-3 Index |
|---|---|---|---|---|---|---|
| Agnoli et al., 2017 [55] | Females | Limit intake of sources of n-6 FA, & TFA. Limit consumption of processed, deep-fried foods, and alcohol. | - | Pregnant/breastfeeding or women with increased requirement for long chain n-3 fatty acids, should be advised to consume an algae-based supplement of known nutrient content. | Pregnant/breastfeeding or women with increased requirement for long chain n-3 fatty acids, should be advised to consume an algae-based supplement of known nutrient content. | - |
| Combined-Males and Females | - | Improve n-3 nutritional status by regular consumption of good sources of alpha-linolenic acid. | - | - | - | |
| Burdge et al., 2017 [34] | Females | - | - | - | Need more cognitive studies for children of vegetarian females due to low DHA levels. | - |
| Pinto et al., 2017 [9] | Combined | - | Further research whether populations with low n-3 status are more at risk of having a pro-inflammatory profile. | - | - | - |
| Melina et al., 2016 [5] | Females | - | - | - | Low dose microalgae DHA supplements for pregnancy and lactation. | - |
| Combined | High intakes of LA may suppress ALA conversion. | May be prudent to ensure higher intakes of ALA. N-3 needs of healthy individuals can be met with ALA alone; endogenous synthesis of EPA and DHA from ALA is sufficient. | Clinical relevance of reduced EPA and DHA status in VGNs are unknown. Low-dose micro-algae based DHA supplements are available for those with increased needs. | Clinical relevance of reduced EPA and DHA status in VGNs are unknown. Low-dose micro-algae based DHA supplements are available for those with increased needs. | A ratio of LA/ALA not exceeding 4:1 has been suggested for optimal conversion. | |
| Harris, 2014 [56] | Combined | Consume more ALA and less LA to increase the Omega-3 Index. | Consume more ALA and less LA to increase the Omega-3 Index. | - | - | Omega-3 Index >8%. |
| Sarter et al., 2014 [16] | Combined | - | - | VGNs respond robustly to a relatively low dose of a vegetarian omega-3 supplement. | VGNs respond robustly to a relatively low dose of a vegetarian omega-3 supplement. | No direct evidence that omega-3 index confers additional health benefits over and above their already protective VGN diet. |
| Saunders et al., 2013 [15] | Combined | - | Double current AI of ALA if no direct sources of EPA and DHA are consumed. Increased needs or reduced conversion may benefit from DHA and EPA supplements derived from microalgae of 200–300 mg/day of DHA and EPA (pregnant and lactating women) and reduced conversion ability. | - | - | - |
| Craig, 2010; Review [51] | Combined | - | Regular consumption of plant foods naturally rich in n-3 fatty acid ALA, such as ground flaxseed, walnuts, soy products, and hemp seed beverages. | - | Pregnant and lactating women may benefit from DHA-fortified foods and microalgae-derived DHA supplements. | - |
| Welch et al., 2010 [44] | Combined | - | Further research for conversion of ALA to long chain n-3 PUFAs for maintenance of adequate status in non-fish and fish-oil consumers is required. | - | - | - |
| Simopoulos, 2008 [57] | Combined | - | - | - | - | Ratio of 4:1 is associated with 70% decrease in total mortality in secondary prevention of cardiovascular disease. |
| Kornsteiner et al., 2008 [40] | Combined | - | Erythrocyte phospholipid n-3 status of VGNs is critical. It is important to maintain n-3 fatty acid intake during adult life. | - | - | Ensure physical, mental and neurological health, reduce n-6/n-3 ratio with an additional intake of direct sources of EPA and DHA, regardless of age and gender. |
| Mann et al., 2006 and Li et al., 1999 (Mann used data collected from Li, 1999) [45] | Combined | - | Advised to increase intake of n-3 fatty acids to increase platelet PL n-3 PUFA and reduce platelet aggregability. | - | - | - |
| Rosell et al., 2005 [10] | Combined | - | Suggests (with caution) VGNs increase intake of ALA and limit intake of LA to optimize FA status. The importance of long chain fatty acids in the diet needs further investigation. | - | - | - |
| Davis et al., 2003 [53] | Combined | 5.5–8% Kcals from n-6 FA. Reduce high n-6 oils in the diet, decrease processed foods. Primary fat should be MUFA. | <1.5–2% of calories should be obtained from n-3. Decrease EtOH and trans fatty acids to increase conversion of ALA to EPA and DHA. Double intake of ALA; provide >1% of energy from n-3 or about 1.1 g/1000 calories. Increased needs require 2.2 g/1000 calories. Aim for 2–4 g of ALA/day. | Not essential, but it is important to ensure sufficient levels by relying on conversion from parent fatty acids. Two important steps to improve EPA status: 1) Maximize conversion of ALA to EPA and DHA and 2) Provide a direct source of EPA and DHA. | Not technically essential, but ensure sufficient levels by relying on conversion from parent fatty acids. Supplements of DHA should be 100–300 mg/day. DHA and ALA should also be adequate. | To achieve the 4:1 ratio, <1.5–2% of calories should be obtained from n-3 and 5.5–8% of calories from n-6 FA. |
| Simopoulos, 2000 [54] | Combined | - | - | - | - | Ratio was 1:1 to 2:1 in ancient diets. Present ratio is 10 to 1:20 to 25 to 1- indicates a deficiency in Western Diets of n-3. |
| Fokkema et al., 2000 [43] | Combined | - | - | - | - | Found a 3:1 ratio increased EPA from 0.3 to about 1.0% in VGN plasma. A 3.7 g/day ALA diet for 4 weeks (ratio of 3.8:1) augmented ALA about 1% more. Total n-3 levels increased from 2.3 to 3.4% in plasma. DHA status was not increased. |
| Agren et al., 1995 [42] | Combined | Depressed levels of n-3 FA are depressed due to very low diet levels and high LA and oleic acid intake. | - | - | - | - |
| Sanders et al., 1978 [46] | Females | - | More research is needed on low delta 4 desaturase in lactating women. | - | - | - |
| Combined | - | Further research needed to establish whether differences in the proportion of n-3 derivatives in tissues are of physiological importance. | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; San Pablo, G. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. https://doi.org/10.3390/nu11102365
Burns-Whitmore B, Froyen E, Heskey C, Parker T, San Pablo G. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients. 2019; 11(10):2365. https://doi.org/10.3390/nu11102365
Chicago/Turabian StyleBurns-Whitmore, Bonny, Erik Froyen, Celine Heskey, Temetra Parker, and Gregorio San Pablo. 2019. "Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration?" Nutrients 11, no. 10: 2365. https://doi.org/10.3390/nu11102365
APA StyleBurns-Whitmore, B., Froyen, E., Heskey, C., Parker, T., & San Pablo, G. (2019). Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients, 11(10), 2365. https://doi.org/10.3390/nu11102365





