The Metabolic Concept of Meal Sequence vs. Satiety: Glycemic and Oxidative Responses with Reference to Inflammation Risk, Protective Principles and Mediterranean Diet
Abstract
:1. Introduction
2. Materials and Methods
3. Results
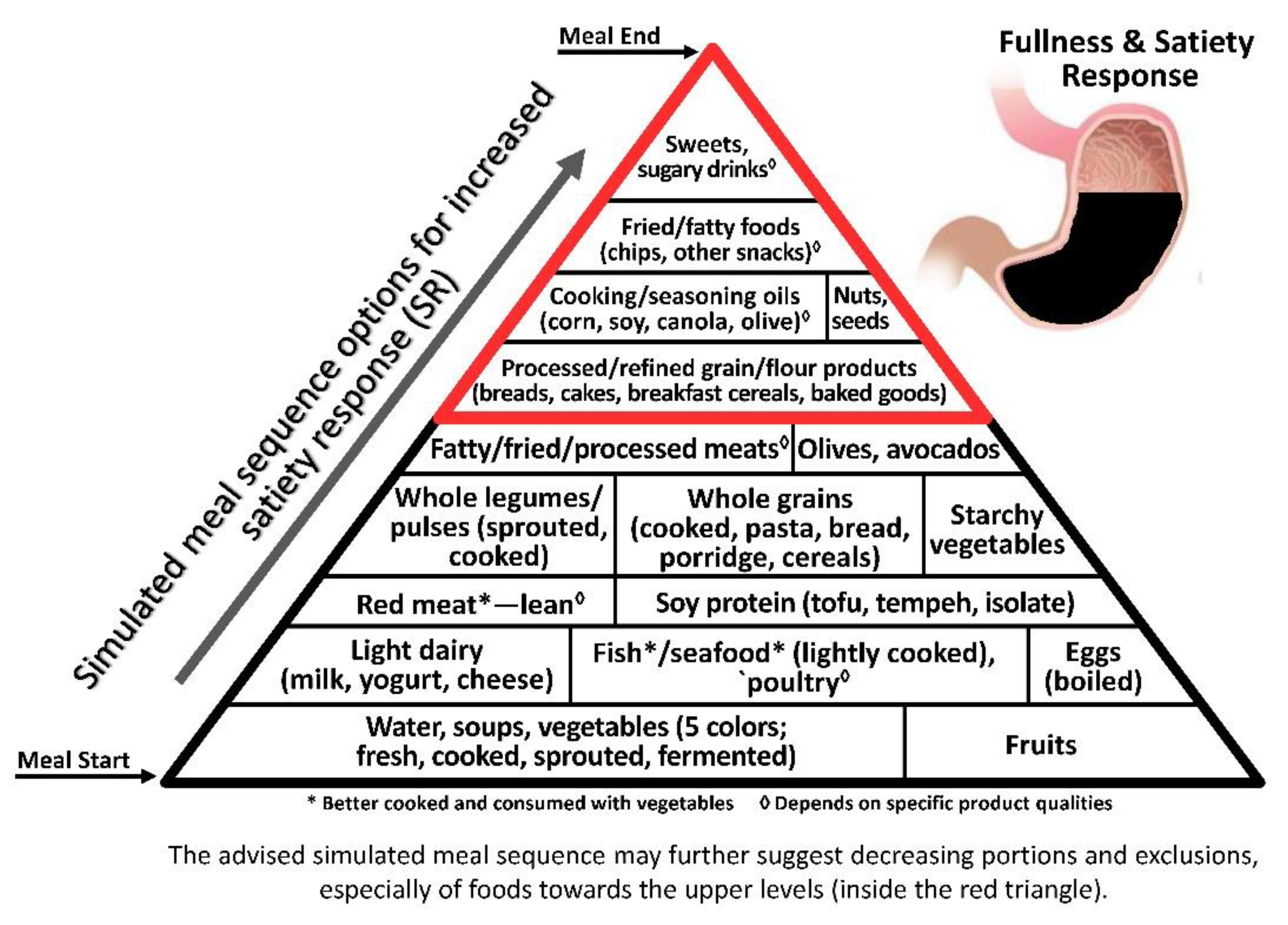
3.1. Satiety–Obesity Axis
3.1.1. Non-Caloric Component
Added and Incorporated Water
3.1.2. Caloric Components
Fibre
Protein
Carbohydrate
Fat
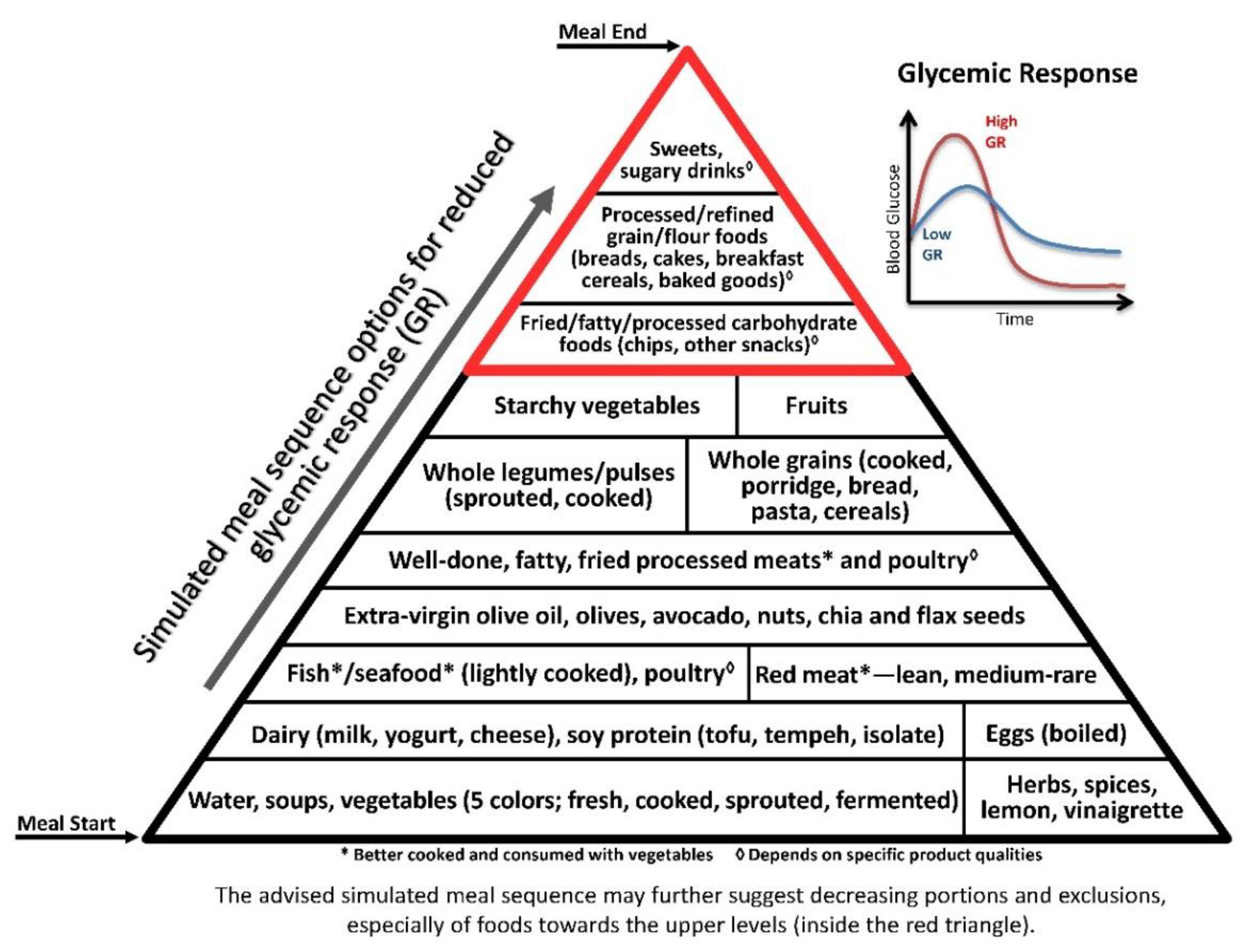
3.2. Glycaemic Axis
3.2.1. Macronutrients
Carbohydrate
Fibre
Protein
Fat
3.2.2. Vegetables, Spices and Phytonutrients
3.2.3. Fermentation and Organic Acids
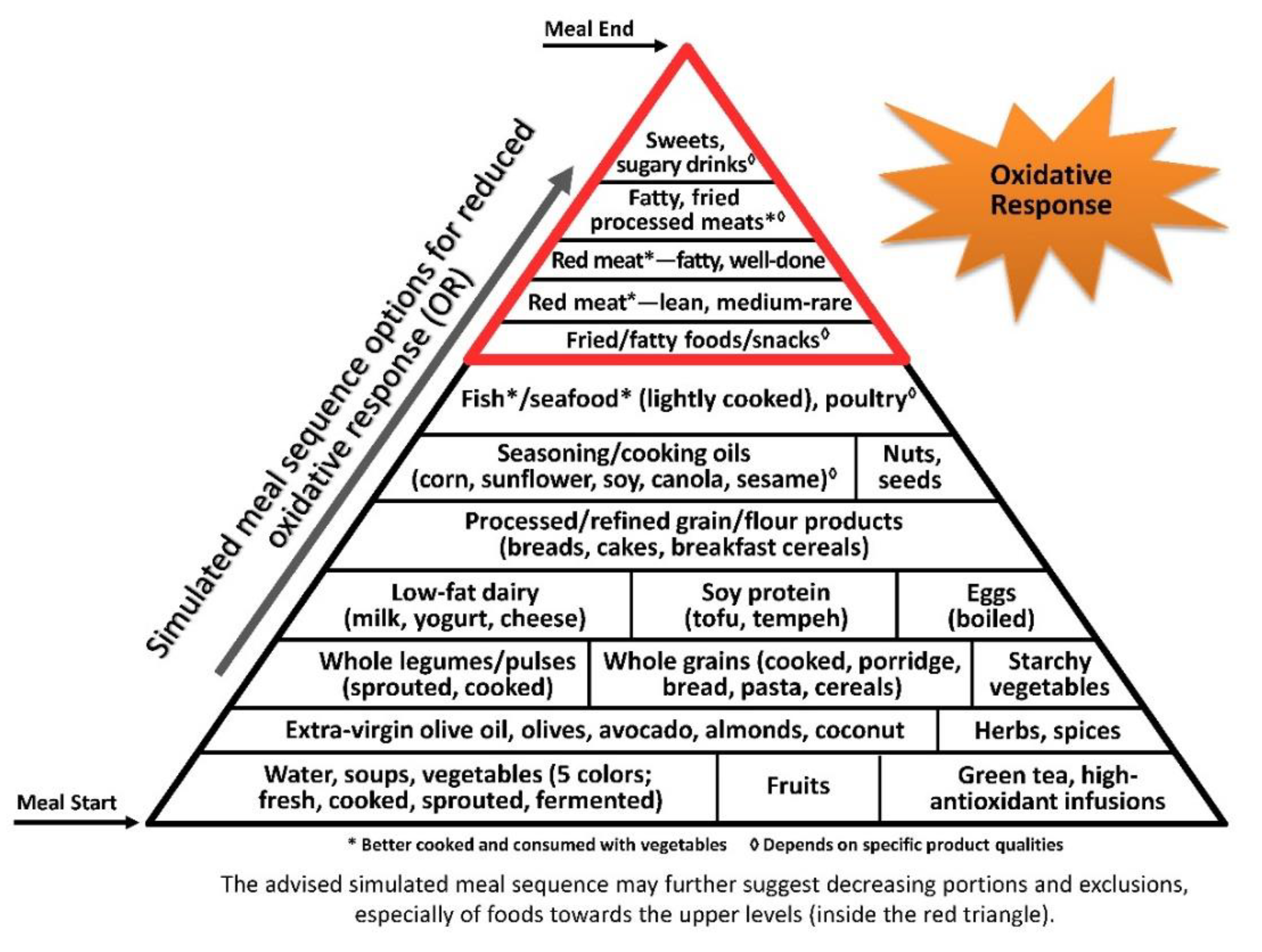
3.3. Oxidative Stress Axis
3.3.1. Macronutrients
Protein
Starchy Carbohydrate
Fats and Fatty Acids
3.3.2. Food Antioxidants
Spices and Seasonings
3.3.3. Food Preparation and Processing
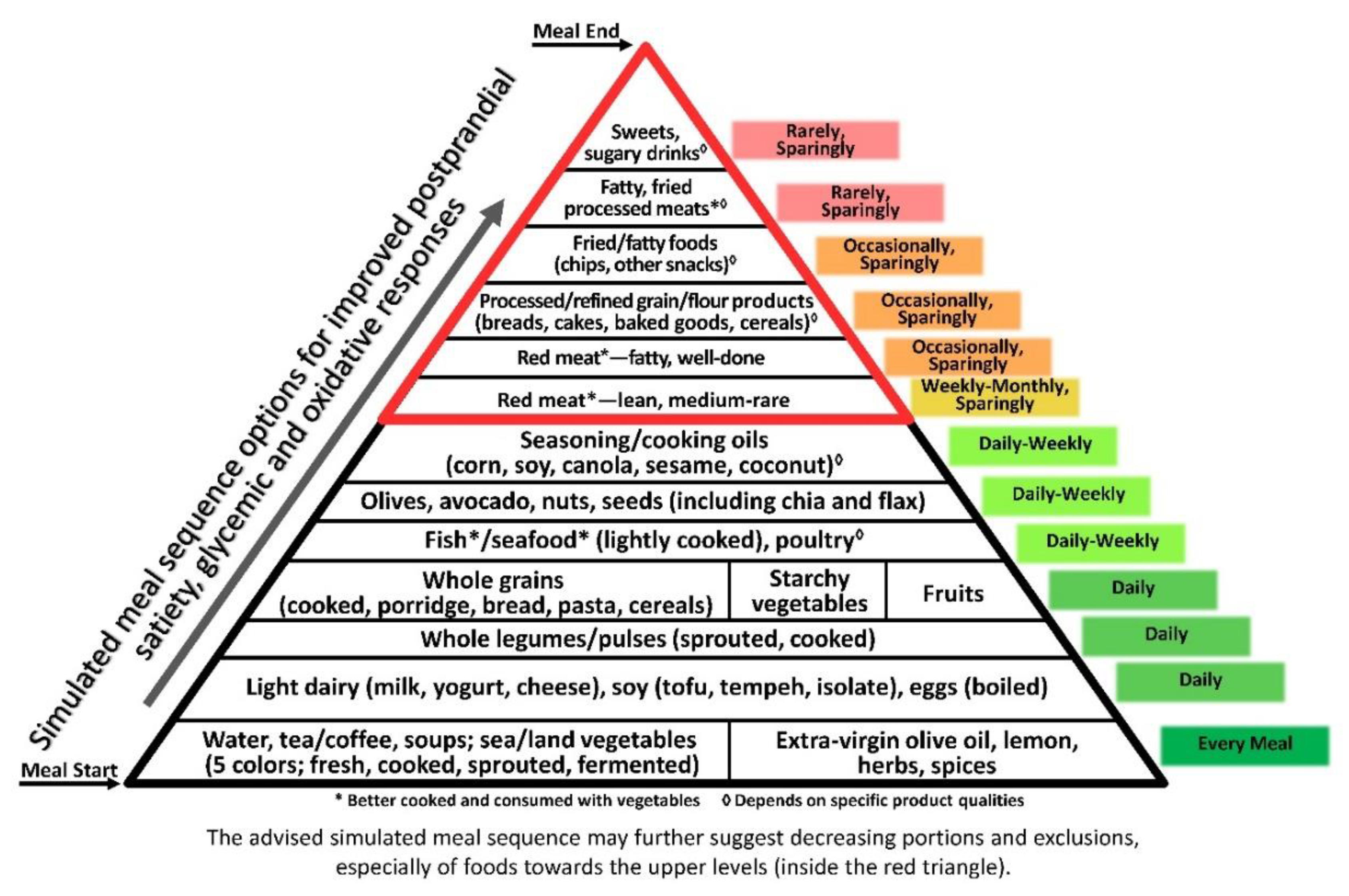
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef]
- Lipovetzky, N.; Hod, H.; Roth, A.; Kishon, Y.; Sclarovsky, S.; Green, M.S. Heavy meals as a trigger for a first event of the acute coronary syndrome: A case-crossover study. Isr. Med Assoc. J. 2004, 6, 728–731. [Google Scholar]
- De Vries, M.A.; Klop, B.; Janssen, H.W.; Njo, T.L.; Westerman, E.M.; Castro Cabezas, M. Postprandial inflammation: Targeting glucose and lipids. Adv. Exp. Med. Biol. 2014, 824, 161–170. [Google Scholar] [CrossRef]
- Klop, B.; Proctor, S.D.; Mamo, J.C.; Botham, K.M.; Castro Cabezas, M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int. J. Vasc. Med. 2012, 2012, 947417. [Google Scholar] [CrossRef]
- Schmid, A.; Petry, N.; Walther, B.; Butikofer, U.; Luginbuhl, W.; Gille, D.; Chollet, M.; McTernan, P.G.; Gijs, M.A.; Vionnet, N.; et al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br. J. Nutr. 2015, 113, 1853–1861. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med. 2010, 42, 245–253. [Google Scholar] [CrossRef]
- Margioris, A.N. Fatty acids and postprandial inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J.; Wirth, M.D.; Shivappa, N.; Hebert, J.R. Lower Dietary Inflammatory Index Scores Are Associated with Lower Glycemic Index Scores among College Students. Nutrients 2018, 10, 182. [Google Scholar] [CrossRef]
- Ehses, J.A.; Lacraz, G.; Giroix, M.H.; Schmidlin, F.; Coulaud, J.; Kassis, N.; Irminger, J.C.; Kergoat, M.; Portha, B.; Homo-Delarche, F.; et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA 2009, 106, 13998–14003. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Volund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef]
- Stienstra, R.; Joosten, L.A.; Koenen, T.; van Tits, B.; van Diepen, J.A.; van den Berg, S.A.; Rensen, P.C.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef] [Green Version]
- Van Asseldonk, E.J.; Stienstra, R.; Koenen, T.B.; Joosten, L.A.; Netea, M.G.; Tack, C.J. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2011, 96, 2119–2126. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thevenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jonsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef]
- Brunstrom, J.M. Mind over platter: Pre-meal planning and the control of meal size in humans. Int. J. Obes. 2014, 38, S9. [Google Scholar] [CrossRef]
- Fay, S.H.; Ferriday, D.; Hinton, E.C.; Shakeshaft, N.G.; Rogers, P.J.; Brunstrom, J.M. What determines real-world meal size? Evidence for pre-meal planning. Appetite 2011, 56, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Ducrot, P.; Mejean, C.; Aroumougame, V.; Ibanez, G.; Alles, B.; Kesse-Guyot, E.; Hercberg, S.; Peneau, S. Meal planning is associated with food variety, diet quality and body weight status in a large sample of French adults. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 12. [Google Scholar] [CrossRef]
- Cunningham, E. Where Can I Find Resources to Assist Clients with At-Home Meal Planning for Therapeutic Diets? J. Acad. Nutr. Diet. 2015, 115, 2056. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Stubbs, R.J.; Harbron, C.G. Effect of overfeeding macronutrients on day-to-day food intake in man. Eur. J. Clin. Nutr. 1996, 50, 418–430. [Google Scholar]
- Flood, J.E.; Rolls, B.J. Soup preloads in a variety of forms reduce meal energy intake. Appetite 2007, 49, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Tey, S.L.; Salleh, N.; Henry, C.J.; Forde, C.G. Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose. Nutrients 2018, 10, 161. [Google Scholar] [CrossRef]
- Van Walleghen, E.L.; Orr, J.S.; Gentile, C.L.; Davy, B.M. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity (Silver Spring) 2007, 15, 93–99. [Google Scholar] [CrossRef]
- Rolls, B.J.; Hetherington, M.M.; Stoner, S.A.; Andersen, A.E. Effects of preloads of differing energy and macronutrient content on eating behavior in bulimia nervosa. Appetite 1997, 29, 353–367. [Google Scholar] [CrossRef]
- Rolls, B.J. Dietary energy density: Applying behavioural science to weight management. Nutr. Bull. 2017, 42, 246–253. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015. [Google Scholar] [CrossRef]
- Bonora, E.; Muggeo, M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: The epidemiological evidence. Diabetologia 2001, 44, 2107–2114. [Google Scholar] [CrossRef]
- Parks, E.J.; Skokan, L.E.; Timlin, M.T.; Dingfelder, C.S. Dietary sugars stimulate fatty acid synthesis in adults. J. Nutr. 2008, 138, 1039–1046. [Google Scholar] [CrossRef]
- Swarbrick, M.M.; Stanhope, K.L.; Elliott, S.S.; Graham, J.L.; Krauss, R.M.; Christiansen, M.P.; Griffen, S.C.; Keim, N.L.; Havel, P.J. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br. J. Nutr. 2008, 100, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [PubMed]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2018, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Peiro, C.; Romacho, T.; Azcutia, V.; Villalobos, L.; Fernandez, E.; Bolanos, J.P.; Moncada, S.; Sanchez-Ferrer, C.F. Inflammation, glucose, and vascular cell damage: The role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Moncayo, S.; Insenser, M.; Montes-Nieto, R.; Fernandez-Duran, E.; Alvarez-Blasco, F.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Postprandial inflammatory responses after oral glucose, lipid and protein challenges: Influence of obesity, sex and polycystic ovary syndrome. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Cook, N.R.; Stampfer, M.J.; Ridker, P.M.; Rexrode, K.M.; Buring, J.E.; Manson, J.E.; Liu, S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008, 57, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Buring, J.E.; Stampfer, M.J.; Willett, W.C.; Ridker, P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002, 75, 492–498. [Google Scholar] [CrossRef]
- Sun, L.; Ranawana, D.V.; Leow, M.K.; Henry, C.J. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur. J. Nutr. 2014, 53, 1719–1726. [Google Scholar] [CrossRef]
- Trico, D.; Filice, E.; Trifiro, S.; Natali, A. Manipulating the sequence of food ingestion improves glycemic control in type 2 diabetic patients under free-living conditions. Nutr. Diabetes 2016, 6, e226. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Milan, A.M.; Pundir, S.; Pileggi, C.A.; Markworth, J.F.; Lewandowski, P.A.; Cameron-Smith, D. Comparisons of the Postprandial Inflammatory and Endotoxaemic Responses to Mixed Meals in Young and Older Individuals: A Randomised Trial. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Lopez-Moreno, J.; Garcia-Carpintero, S.; Jimenez-Lucena, R.; Haro, C.; Rangel-Zuniga, O.A.; Blanco-Rojo, R.; Yubero-Serrano, E.M.; Tinahones, F.J.; Delgado-Lista, J.; Perez-Martinez, P.; et al. Effect of Dietary Lipids on Endotoxemia Influences Postprandial Inflammatory Response. J. Agric. Food Chem. 2017, 65, 7756–7763. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Pinto Ferreira, L.R.; Ferreira, F.M.; Neto, E.C.; Sampaio, G.R.; Rogero, M.M. Circulating plasma microRNAs dysregulation and metabolic endotoxemia induced by a high-fat high-saturated diet. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Monfort-Pires, M.; Crisma, A.R.; Bordin, S.; Ferreira, S.R.G. Greater expression of postprandial inflammatory genes in humans after intervention with saturated when compared to unsaturated fatty acids. Eur. J. Nutr. 2018, 57, 2887–2895. [Google Scholar] [CrossRef]
- Alayon, A.N.; Rivadeneira, A.P.; Herrera, C.; Guzman, H.; Arellano, D.; Echeverri, I. Metabolic and inflammatory postprandial effect of a highly saturated fat meal and its relationship to abdominal obesity. Biomedica 2018, 38, 93–100. [Google Scholar] [CrossRef]
- Maranhao, P.A.; de Souza, M.; Panazzolo, D.G.; Nogueira Neto, J.F.; Bouskela, E.; Kraemer-Aguiar, L.G. Metabolic Changes Induced by High-Fat Meal Evoke Different Microvascular Responses in Accordance with Adiposity Status. Biomed. Res. Int. 2018, 2018, 5046508. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef]
- Mora, S.; Rifai, N.; Buring, J.E.; Ridker, P.M. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008, 118, 993–1001. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, T.; Yunoki, K.; Ito, H. Postprandial hyperlipidemia as a potential residual risk factor. J. Cardiol. 2016, 67, 335–339. [Google Scholar] [CrossRef]
- Gogebakan, O.; Kohl, A.; Osterhoff, M.A.; van Baak, M.A.; Jebb, S.A.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Weickert, M.O.; et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (DiOGenes) study: A randomized, controlled trial. Circulation 2011, 124, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Gibbs, A.L.; Mehling, C.; Chiasson, J.L.; Connelly, P.W.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W.; et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein. Am. J. Clin. Nutr. 2008, 87, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Schwarz, Y.; Wang, C.; Breymeyer, K.; Coronado, G.; Wang, C.Y.; Noar, K.; Song, X.; Lampe, J.W. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J. Nutr. 2012, 142, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Beulen, Y.; Martinez-Gonzalez, M.A.; van de Rest, O.; Salas-Salvado, J.; Sorli, J.V.; Gomez-Gracia, E.; Fiol, M.; Estruch, R.; Santos-Lozano, J.M.; Schroder, H.; et al. Quality of Dietary Fat Intake and Body Weight and Obesity in a Mediterranean Population: Secondary Analyses within the PREDIMED Trial. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martinez-Gonzalez, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Aros, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19, Erratum. Diabetes Care 2018, 41, 2259–2260. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martinez-Gonzalez, M.A.; Estruch, R.; Salas-Salvado, J.; Fito, M.; Martinez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, E.; Rico-Cabanas, L.; Rosgaard, N.; Estruch, R.; Bach-Faig, A. Mediterranean diet and cardiodiabesity: A review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Safouris, A.; Tsivgoulis, G.; Sergentanis, T.N.; Psaltopoulou, T. Mediterranean Diet and Risk of Dementia. Curr. Alzheimer Res. 2015, 12, 736–744. [Google Scholar] [CrossRef]
- Tamburrelli, C.; Gianfagna, F.; D’Imperio, M.; De Curtis, A.; Rotilio, D.; Iacoviello, L.; de Gaetano, G.; Donati, M.B.; Cerletti, C. Postprandial cell inflammatory response to a standardised fatty meal in subjects at different degree of cardiovascular risk. Thromb. Haemost. 2012, 107, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Stofkova, A. Leptin and adiponectin: From energy and metabolic dysbalance to inflammation and autoimmunity. Endocr. Regul. 2009, 43, 157–168. [Google Scholar] [PubMed]
- Rolls, B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009, 97, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, S.H.; Miller, J.C.; Petocz, P.; Farmakalidis, E. A satiety index of common foods. Eur. J. Clin. Nutr. 1995, 49, 675–690. [Google Scholar]
- Rolls, B.J.; Drewnowski, A.; Ledikwe, J.H. Changing the energy density of the diet as a strategy for weight management. J. Am. Diet. Assoc. 2005, 105, S98–S103. [Google Scholar] [CrossRef]
- Rouhani, M.H.; Surkan, P.J.; Azadbakht, L. The effect of preload/meal energy density on energy intake in a subsequent meal: A systematic review and meta-analysis. Eat. Behav. 2017, 26, 6–15. [Google Scholar] [CrossRef]
- Rolls, B.J.; Bell, E.A.; Waugh, B.A. Increasing the volume of a food by incorporating air affects satiety in men. Am. J. Clin. Nutr. 2000, 72, 361–368. [Google Scholar] [CrossRef]
- Blundell, J.E.; Burley, V.J.; Cotton, J.R.; Lawton, C.L. Dietary fat and the control of energy intake: Evaluating the effects of fat on meal size and postmeal satiety. Am. J. Clin. Nutr. 1993, 57, 772S–777S. [Google Scholar] [CrossRef]
- Blundell, J.E.; MacDiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar] [CrossRef]
- Rolls, B.J.; Roe, L.S.; Meengs, J.S. Salad and satiety: Energy density and portion size of a first-course salad affect energy intake at lunch. J. Am. Diet. Assoc. 2004, 104, 1570–1576. [Google Scholar] [CrossRef]
- Flood-Obbagy, J.E.; Rolls, B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009, 52, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dove, E.R.; Hodgson, J.M.; Puddey, I.B.; Beilin, L.J.; Lee, Y.P.; Mori, T.A. Skim milk compared with a fruit drink acutely reduces appetite and energy intake in overweight men and women. Am. J. Clin. Nutr. 2009, 90, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corney, R.A.; Sunderland, C.; James, L.J. Immediate pre-meal water ingestion decreases voluntary food intake in lean young males. Eur. J. Nutr. 2016, 55, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Bell, E.A.; Thorwart, M.L. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am. J. Clin. Nutr. 1999, 70, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Davis, P.A.; Schneeman, B.O. Plasma cholecystokinin is associated with subjective measures of satiety in women. Am. J. Clin. Nutr. 2002, 76, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, K.R.; Purhonen, A.K.; Salmenkallio-Marttila, M.; Lahteenmaki, L.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Rogovik, A.L.; Jovanovski, E.; Jenkins, A.L. Fiber facts: Benefits and recommendations for individuals with type 2 diabetes. Curr. Diabetes Rep. 2009, 9, 405–411. [Google Scholar] [CrossRef]
- Rebello, C.J.; Chu, Y.F.; Johnson, W.D.; Martin, C.K.; Han, H.; Bordenave, N.; Shi, Y.; O’Shea, M.; Greenway, F.L. The role of meal viscosity and oat beta-glucan characteristics in human appetite control: A randomized crossover trial. Nutr. J. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M. Glycomacropeptide (GMP) is not critical to whey-induced satiety, but may have a unique role in energy intake regulation through cholecystokinin (CCK). Physiol. Behav. 2008, 93, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeley, N.; Blouet, C. Central Amino Acid Sensing in the Control of Feeding Behavior. Front. Endocrinol. (Lausanne) 2016, 7, 148. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, S.C.; Johnstone, A.M.; Lobley, G.E.; Adamson, J.; Hickson, K.; Holtrop, G.; Fyfe, C.; Clark, L.F.; Pearson, D.W.; Abraham, P.; et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in Type 2 diabetes. Diabet. Med. 2013, 30, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Bertenshaw, E.J.; Lluch, A.; Yeomans, M.R. Perceived thickness and creaminess modulates the short-term satiating effects of high-protein drinks. Br. J. Nutr. 2013, 110, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiol. Behav. 2013, 116–117, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tome, D.; Schwarz, J.; Darcel, N.; Fromentin, G. Protein, amino acids, vagus nerve signaling, and the brain. Am. J. Clin. Nutr. 2009, 90, 838S–843S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, S.C. Metabolic signals and food intake. Forty years of progress. Appetite 2013, 71, 440–444. [Google Scholar] [CrossRef]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Moran, T.H.; Dailey, M.J. Intestinal feedback signaling and satiety. Physiol. Behav. 2011, 105, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Bellisle, F. Nutrients, satiety, and control of energy intake. Appl. Physiol. Nutr. Metab. 2015, 40, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lluch, A.; Hanet-Geisen, N.; Salah, S.; Slas-Salvado, J.; L’Heureux-Bouron, D.; Halford, J.C.G. Short-term appetite-reducing effects of a low-fat dairy product enriched with protein and fibre. Food Qual. Prefer. 2010, 21, 402–409. [Google Scholar] [CrossRef]
- Kristensen, M.D.; Bendsen, N.T.; Christensen, S.M.; Astrup, A.; Raben, A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork)—A randomized cross-over meal test study. Food Nutr. Res. 2016, 60, 32634. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, A.L.; Altschwager, D.; Thomas, W.; Slavin, J.L. The Effects of a Beef-Based Meal Compared to a Calorie Matched Bean-Based Meal on Appetite and Food Intake. J. Food Sci. 2015, 80, H2088–H2093. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Almiron-Roig, E.; Lluch, A.; Guyonnet, D.; Drewnowski, A. Higher satiety ratings following yogurt consumption relative to fruit drink or dairy fruit drink. J. Am. Diet. Assoc. 2006, 106, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Anderson, H.; Luhovyy, B.; Akhavan, T.; Panahi, S. Milk Proteins in the Regulation of Body Weight, Satiety, Food Intake and Glycemia; Karger Publishers: Basel, Switzerland, 2011; Volume 67, pp. 147–159. [Google Scholar] [CrossRef]
- Gustafson, D.R.; McMahon, D.J.; Morrey, J.; Nan, R. Appetite is not influenced by a unique milk peptide: Caseinomacropeptide (CMP). Appetite 2001, 36, 157–163. [Google Scholar] [CrossRef]
- Panahi, S.; El Khoury, D.; Kubant, R.; Akhavan, T.; Luhovyy, B.L.; Goff, H.D.; Anderson, G.H. Mechanism of action of whole milk and its components on glycemic control in healthy young men. J. Nutr. Biochem. 2014, 25, 1124–1131. [Google Scholar] [CrossRef]
- Rubio-Martin, E.; Garcia-Escobar, E.; Ruiz de Adana, M.S.; Lima-Rubio, F.; Pelaez, L.; Caracuel, A.M.; Bermudez-Silva, F.J.; Soriguer, F.; Rojo-Martinez, G.; Olveira, G. Comparison of the Effects of Goat Dairy and Cow Dairy Based Breakfasts on Satiety, Appetite Hormones, and Metabolic Profile. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Jacobsen, R.; Lorenzen, J.K.; Toubro, S.; Krog-Mikkelsen, I.; Astrup, A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int. J. Obes. 2005, 29, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, J.; Leite, J.O.; de Ogburn, R.; Puglisi, M.J.; VanHeest, J.; Fernandez, M.L. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 h in adult men. Nutr. Res. 2010, 30, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Missimer, A.; DiMarco, D.M.; Andersen, C.J.; Murillo, A.G.; Vergara-Jimenez, M.; Fernandez, M.L. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Pombo-Rodrigues, S.; Calame, W.; Re, R. The effects of consuming eggs for lunch on satiety and subsequent food intake. Int. J. Food Sci. Nutr. 2011, 62, 593–599. [Google Scholar] [CrossRef]
- Rebello, C.J.; Liu, A.G.; Greenway, F.L.; Dhurandhar, N.V. Dietary strategies to increase satiety. Adv. Food Nutr. Res. 2013, 69, 105–182. [Google Scholar] [CrossRef]
- Borzoei, S.; Neovius, M.; Barkeling, B.; Teixeira-Pinto, A.; Rossner, S. A comparison of effects of fish and beef protein on satiety in normal weight men. Eur. J. Clin. Nutr. 2006, 60, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Torris, C.; Molin, M.; Smastuen, M.C. Lean Fish Consumption Is Associated with Beneficial Changes in the Metabolic Syndrome Components: A 13-Year Follow-Up Study from the Norwegian Tromso Study. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Uhe, A.M.; Collier, G.R.; O’Dea, K. A comparison of the effects of beef, chicken and fish protein on satiety and amino acid profiles in lean male subjects. J. Nutr. 1992, 122, 467–472. [Google Scholar] [CrossRef]
- Parra, D.; Ramel, A.; Bandarra, N.; Kiely, M.; Martinez, J.A.; Thorsdottir, I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008, 51, 676–680. [Google Scholar] [CrossRef]
- Nielsen, L.V.; Kristensen, M.D.; Klingenberg, L.; Ritz, C.; Belza, A.; Astrup, A.; Raben, A. Protein from Meat or Vegetable Sources in Meals Matched for Fiber Content has Similar Effects on Subjective Appetite Sensations and Energy Intake-A Randomized Acute Cross-Over Meal Test Study. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Grunwald, G.K.; Melanson, E.L.; Saris, W.H.; Hill, J.O. The role of low-fat diets in body weight control: A meta-analysis of ad libitum dietary intervention studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A. Carbohydrate quantity and quality in relation to body mass index. J. Am. Diet. Assoc. 2007, 107, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Holt, S.H.; Pawlak, D.B.; McMillan, J. Glycemic index and obesity. Am. J. Clin. Nutr. 2002, 76, 281S–285S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Axelsen, M.; Kendall, C.W.; Augustin, L.S.; Vuksan, V.; Smith, U. Dietary fibre, lente carbohydrates and the insulin-resistant diseases. Br. J. Nutr. 2000, 83 (Suppl. 1), S157–S163. [Google Scholar] [CrossRef]
- Bellisle, F.; Drewnowski, A.; Anderson, G.H.; Westerterp-Plantenga, M.; Martin, C.K. Sweetness, satiation, and satiety. J. Nutr. 2012, 142, 1149S–1154S. [Google Scholar] [CrossRef]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef]
- Blundell, J.E.; Green, S.; Burley, V. Carbohydrates and human appetite. Am. J. Clin. Nutr. 1994, 59, 728S–734S. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.E.; Tremblay, A. Appetite control and energy (fuel) balance. Nutr. Res. Rev. 1995, 8, 225–242. [Google Scholar] [CrossRef]
- Lowe, M.R.; Levine, A.S. Eating motives and the controversy over dieting: Eating less than needed versus less than wanted. Obes Res. 2005, 13, 797–806. [Google Scholar] [CrossRef]
- Blundell, J.E.; Stubbs, R.J.; Golding, C.; Croden, F.; Alam, R.; Whybrow, S.; Le Noury, J.; Lawton, C.L. Resistance and susceptibility to weight gain: Individual variability in response to a high-fat diet. Physiol. Behav. 2005, 86, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J. Carbohydrates, fats, and satiety. Am. J. Clin. Nutr. 1995, 61, 960S–967S. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Noda, Y.; Ohno, Y.; Sugiyama, H.; Oe, H.; Nakamura, K.; Kohno, K.; Ito, H. Omega-3 fatty acids improve postprandial lipemia and associated endothelial dysfunction in healthy individuals—A randomized cross-over trial. Biomed. Pharmacother. 2014, 68, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Shaefer, C.F.; Anderson, J. The importance of postprandial glycemic control: Optimizing add-on therapy to basal insulin. Postgrad. Med. 2016, 128, 137–144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation 2011; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Lind, M.; Tuomilehto, J.; Uusitupa, M.; Nerman, O.; Eriksson, J.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Peltonen, M.; Pivodic, A.; Lindstrom, J. The association between HbA1c, fasting glucose, 1-h glucose and 2-h glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS ONE 2014, 9, e109506. [Google Scholar] [CrossRef]
- Garber, A.J. Postprandial dysmetabolism and the heart. Heart Fail. Clin. 2012, 8, 563–573. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Masala, G.; Ceroti, M.; Pala, V.; Krogh, V.; Vineis, P.; Sacerdote, C.; Saieva, C.; Salvini, S.; Sieri, S.; Berrino, F.; et al. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br. J. Nutr. 2007, 98, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Hlebowicz, J.; Darwiche, G.; Bjorgell, O.; Almer, L.O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Quek, R.; Henry, C.J. Influence of polyphenols from lingonberry, cranberry, and red grape on in vitro digestibility of rice. Int. J. Food Sci. Nutr. 2015, 66, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.D.; Parry Strong, A.; Cresswell, P.; Reynolds, A.N.; Hanna, A.; Haeusler, S. A randomised trial of the feasibility of a low carbohydrate diet vs. standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac. J. Clin. Nutr. 2016, 25, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S., Jr.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S., Jr.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Guisado, J.; Munoz-Serrano, A.; Alonso-Moraga, A. Spanish Ketogenic Mediterranean Diet: A healthy cardiovascular diet for weight loss. Nutr. J. 2008, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A.; Lodi, A.; Bosco, G. Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Wolever, T.M. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: Comparison with white bread. Eur. J. Clin. Nutr. 1998, 52, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Keogh, J.B.; Pedersen, E.; Clifton, P.M. Fructose acute effects on glucose, insulin, and triglyceride after a solid meal compared with sucralose and sucrose in a randomized crossover study. Am. J. Clin. Nutr. 2016, 103, 1453–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, A.P.; Iliescu, R.G.; Thomas, C.E.; Aronne, L.J. Food Order Has a Significant Impact on Postprandial Glucose and Insulin Levels. Diabetes Care 2015, 38, e98–e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 13, 175–187. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Dehghan, P.; Gargari, B.P.; Asghari-Jafarabadi, M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: A randomised controlled clinical trial. Br. J. Nutr. 2015, 113, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Gudmundsdottir, F.D.; Thorsdottir, I. Effects of two different types of fast food on postprandial metabolism in normal and overweight subjects. Eur. J. Clin. Nutr. 2012, 66, 1193–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrugger, S.; Maerkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frokiaer, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.; Bell, K.J.; O’Connell, S.M.; Smart, C.E.; Shafat, A.; King, B. The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Curr. Diabetes Rep. 2015, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Hatonen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winham, D.M.; Hutchins, A.M.; Thompson, S.V. Glycemic response to black beans and chickpeas as part of a rice meal: A randomized cross-over trial. Nutrients. 2017, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Filice, E.; Baldi, S.; Frascerra, S.; Mari, A.; Natali, A. Sustained effects of a protein and lipid preload on glucose tolerance in type 2 diabetes patients. Diabetes Metab. 2016, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response of people with type 2 diabetes to a high protein diet. Nutr. Metab. 2004, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef]
- Panahi, S.; Tremblay, A. The Potential Role of Yogurt in Weight Management and Prevention of Type 2 Diabetes. J. Am. Coll. Nutr. 2016, 35, 717–731. [Google Scholar] [CrossRef]
- Turner, K.M.; Keogh, J.B.; Clifton, P.M. Acute effect of red meat and dairy on glucose and insulin: A randomized crossover study. Am. J. Clin. Nutr. 2016, 103, 71–76. [Google Scholar] [CrossRef]
- Gunnerud, U.; Holst, J.J.; Ostman, E.; Bjorck, I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr. J. 2012, 11, 83. [Google Scholar] [CrossRef]
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Afaghi, A.; Sarreshtehdari, M. Effect of low glycemic load diet on glycated hemoglobin (HbA1c) in poorly-controlled diabetes patients. Glob. J. Health Sci. 2011, 4, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kamada, C.; Yoshimura, H.; Okumura, R.; Iimuro, S.; Ohashi, Y.; Araki, A.; Umegaki, H.; Sakurai, T.; Yoshimura, Y.; et al. Effects of total and green vegetable intakes on glycated hemoglobin A1c and triglycerides in elderly patients with type 2 diabetes mellitus: The Japanese Elderly Intervention Trial. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 50–58. [Google Scholar] [CrossRef]
- Imai, S.; Fukui, M.; Kajiyama, S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 54, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coe, S.; Ryan, L. Impact of polyphenol-rich sources on acute postprandial glycaemia: A systematic review. J. Nutr. Sci. 2016, 5, e24. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Cloarec, M. Regular consumption of Fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the Mediterranean diet, improves metabolic ageing of obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2015, 66, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Quagliano, S.; White, S. Vinegar ingestion at mealtime reduced fasting blood glucose concentrations in healthy adults at risk for type 2 diabetes. J. Funct. Foods 2013, 5, 2007–2011. [Google Scholar] [CrossRef]
- Ostman, E.; Granfeldt, Y.; Persson, L.; Bjorck, I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur. J. Clin. Nutr. 2005, 59, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Östman, E.; Nilsson, M.; Elmståhl, H.L.; Molin, G.; Björck, I. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Östman, E.M.; Liljeberg Elmståhl, H.G.; Björck, I.M. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am. J. Clin. Nutr. 2001, 74, 96–100. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, H.G.; Seo, K.H.; Yokoyama, W.; Kim, H. Antiobesity Effect of Prebiotic Polyphenol-Rich Grape Seed Flour Supplemented with Probiotic Kefir-Derived Lactic Acid Bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Castellani, P.; Balza, E.; Rubartelli, A. Inflammation, DAMPs, tumor development, and progression: A vicious circle orchestrated by redox signaling. Antioxid. Redox Signal. 2014, 20, 1086–1097. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef]
- Fu, Z.; Deming, S.L.; Fair, A.M.; Shrubsole, M.J.; Wujcik, D.M.; Shu, X.O.; Kelley, M.; Zheng, W. Well-done meat intake and meat-derived mutagen exposures in relation to breast cancer risk: The Nashville Breast Health Study. Breast Cancer Res. Treat. 2011, 129, 919–928. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Prasad, K.; Dhar, I. Oxidative stress as a mechanism of added sugar-induced cardiovascular disease. Int. J. Angiol. 2014, 23, 217–226. [Google Scholar] [CrossRef]
- Mohanty, P.; Hamouda, W.; Garg, R.; Aljada, A.; Ghanim, H.; Dandona, P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J. Clin. Endocrinol. Metab. 2000, 85, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Panahi, G.; Pasalar, P.; Zare, M.; Rizzuto, R.; Meshkani, R. High glucose induces inflammatory responses in HepG2 cells via the oxidative stress-mediated activation of NF-kappaB, and MAPK pathways in HepG2 cells. Arch. Physiol. Biochem. 2018, 124, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Milne, G.L.; Park, Y.M.; Sandler, D.P.; Nichols, H.B. Dietary Glycemic Index and Glycemic Load Are Positively Associated with Oxidative Stress among Premenopausal Women. J. Nutr. 2018, 148, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Della Corte, E.; Bonetti, E.; Abbondanza, A.; Abbati, A.; De Stefano, F. Fructose-induced hyperuricaemia. Lancet 1970, 2, 1310–1311. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Santos, A.M.; Junqueira, V.B.; Augusto, O. Peroxynitrite-mediated formation of free radicals in human plasma: EPR detection of ascorbyl, albumin-thiyl and uric acid-derived free radicals. Biochem J. 1996, 314 Pt 3, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Bagnati, M.; Perugini, C.; Cau, C.; Bordone, R.; Albano, E.; Bellomo, G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: A study using uric acid. Biochem. J. 1999, 340 Pt 1, 143–152. [Google Scholar] [CrossRef]
- Krieger-Brauer, H.I.; Kather, H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J. Clin. Invest. 1992, 89, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. 8th Scandinavian Congress of Clinical Physiology. 14–15 June 1989, Helsinki, Sweden. Proceedings. Clin. Physiol. 1990, 10, 257–303. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller do Nascimento, C.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediat. Inflamm. 2013, 2013, 137579. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hu, F.B.; Martinez-Gonzalez, M.A.; Fito, M.; Bullo, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gomez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Lopez, S.; Bermudez, B.; Abia, R.; Villar, J.; Muriana, F.J. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid Peroxidation in a Stomach Medium Is Affected by Dietary Oils (Olive/Fish) and Antioxidants: The Mediterranean versus Western Diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Mitjavila, M.T.; Estruch, R.; Marti, A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur. J. Clin. Nutr. 2009, 63, 1387–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Steck, S.E.; Guinter, M.; Zheng, J.; Thomson, C.A. Index-based dietary patterns and colorectal cancer risk: A systematic review. Adv. Nutr. 2015, 6, 763–773. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. Food Processing and the Mediterranean Diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef]
- Anderson, R.A.; Evans, L.M.; Ellis, G.R.; Khan, N.; Morris, K.; Jackson, S.K.; Rees, A.; Lewis, M.J.; Frenneaux, M.P. Prolonged deterioration of endothelial dysfunction in response to postprandial lipaemia is attenuated by vitamin C in Type 2 diabetes. Diabet Med. 2006, 23, 258–264. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Zhang, Y.; Rahnama, N.; Zerlin, A.; Thames, G.; Tseng, C.H.; Heber, D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type 2 diabetes mellitus. Diabet Med. 2013, 30, 590–595. [Google Scholar] [CrossRef]
- Zhang, Y.; Henning, S.M.; Lee, R.P.; Huang, J.; Zerlin, A.; Li, Z.; Heber, D. Turmeric and black pepper spices decrease lipid peroxidation in meat patties during cooking. Int. J. Food Sci. Nutr. 2015, 66, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef]
- Kim, I.S.; Jin, S.K.; Yang, M.R.; Chu, G.M.; Park, J.H.; Rashid, R.H.; Kim, J.Y.; Kang, S.N. Efficacy of tomato powder as antioxidant in cooked pork patties. Asian Australas. J. Anim. Sci. 2013, 26, 1339–1346. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Bonanni, A.; Costanzo, S.; De Lucia, F.; Pounis, G.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Adherence to a Mediterranean diet is associated with a better health-related quality of life: A possible role of high dietary antioxidant content. BMJ Open 2013, 3. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Gatenby, P.; Gerbi, V.; Janssens, J.; Johnston, B.; Kas, K.; La Vecchia, C.; Mainguet, P. Cancer prevention in Europe: The Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 2013, 22, 90–95. [Google Scholar] [CrossRef]
- Carollo, C.; Caimi, G. Wine consumption in the mediterranean diet: Old concepts in a new sight. Food Nutr. Sci. 2012, 3, 1726–1733. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Lagiou, A. Mediterranean diet and coronary heart disease: Are antioxidants critical? Nutr. Rev. 1999, 57, 253–255. [Google Scholar]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, Y.J.; Choi, Y.I.; Lee, J.J. Effects of Lemon Balm on the Oxidative Stability and the Quality Properties of Hamburger Patties during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2014, 34, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health--a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 2008, 87, 258S–268S. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Popkin, B.M. Energy density, portion size, and eating occasions: Contributions to increased energy intake in the United States, 1977-2006. PLoS Med. 2011, 8, e1001050. [Google Scholar] [CrossRef] [PubMed]
- De Kok, T.M.; de Waard, P.; Wilms, L.C.; van Breda, S.G. Antioxidative and antigenotoxic properties of vegetables and dietary phytochemicals: The value of genomics biomarkers in molecular epidemiology. Mol. Nutr. Food Res. 2010, 54, 208–217. [Google Scholar] [CrossRef]
- Maruyama, C.; Kikuchi, N.; Masuya, Y.; Hirota, S.; Araki, R.; Maruyama, T. Effects of green-leafy vegetable intake on postprandial glycemic and lipidemic responses and alpha-tocopherol concentration in normal weight and obese men. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 264–271. [Google Scholar] [CrossRef]
- Wang, G.J.; Tomasi, D.; Backus, W.; Wang, R.; Telang, F.; Geliebter, A.; Korner, J.; Bauman, A.; Fowler, J.S.; Thanos, P.K.; et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008, 39, 1824–1831. [Google Scholar] [CrossRef]
- Li, Y.; Zong, Y.; Qi, J.; Liu, K. Prebiotics and oxidative stress in constipated rats. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 447–452. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Abou-Samra, R.; Keersmaekers, L.; Brienza, D.; Mukherjee, R.; Mace, K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr. J. 2011, 10, 139. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef]
- Van Hecke, T.; Jakobsen, L.M.; Vossen, E.; Guéraud, F.; De Vos, F.; Pierre, F.; Bertram, H.C.; De Smet, S. Short-term beef consumption promotes systemic oxidative stress, TMAO formation and inflammation in rats, and dietary fat content modulates these effects. Food Funct. 2016, 7, 3760–3771. [Google Scholar] [CrossRef]
- Liu, G.; Zong, G.; Wu, K.; Hu, Y.; Li, Y.; Willett, W.C.; Eisenberg, D.M.; Hu, F.B.; Sun, Q. Meat Cooking Methods and Risk of Type 2 Diabetes: Results From Three Prospective Cohort Studies. Diabetes Care 2018, 41, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.; Ross, R.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- The Oldways Mediterranean Diet Pyramid. Available online: http://www.oldwayspt.org (accessed on 28 May 2018).
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Mediterranean Diet. Available online: https://ich.unesco.org/en/Rl/mediterranean-diet-00884 (accessed on 17 December 2018).
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapira, N. The Metabolic Concept of Meal Sequence vs. Satiety: Glycemic and Oxidative Responses with Reference to Inflammation Risk, Protective Principles and Mediterranean Diet. Nutrients 2019, 11, 2373. https://doi.org/10.3390/nu11102373
Shapira N. The Metabolic Concept of Meal Sequence vs. Satiety: Glycemic and Oxidative Responses with Reference to Inflammation Risk, Protective Principles and Mediterranean Diet. Nutrients. 2019; 11(10):2373. https://doi.org/10.3390/nu11102373
Chicago/Turabian StyleShapira, Niva. 2019. "The Metabolic Concept of Meal Sequence vs. Satiety: Glycemic and Oxidative Responses with Reference to Inflammation Risk, Protective Principles and Mediterranean Diet" Nutrients 11, no. 10: 2373. https://doi.org/10.3390/nu11102373
APA StyleShapira, N. (2019). The Metabolic Concept of Meal Sequence vs. Satiety: Glycemic and Oxidative Responses with Reference to Inflammation Risk, Protective Principles and Mediterranean Diet. Nutrients, 11(10), 2373. https://doi.org/10.3390/nu11102373



