The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
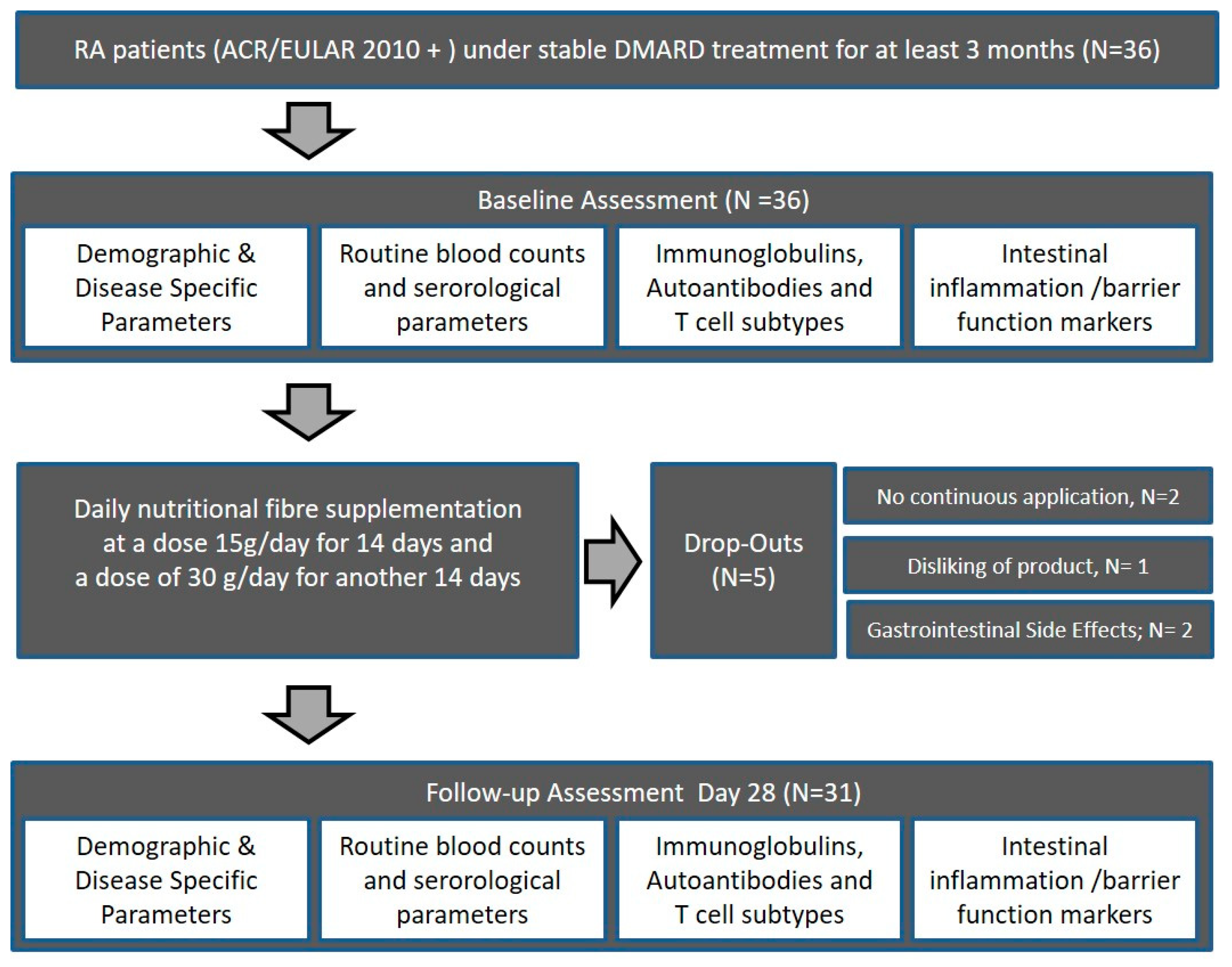
2.1. Study Subjects
2.2. Intervention
2.3. Assessment of Safety (Adverse Events, Concomitant Medication, and Tolerability)
2.4. Data analysis and Statistics
2.5. Sample Collection and Processing
2.5.1. Cell Handling and Cryoconservation
2.5.2. Flow Cytometry
2.5.3. Enzyme-linked Immunosorbent Assay (ELISA)
2.5.4. Stool Sample Processing
2.5.5. Modified Vimentin 58-GRVYATRSSAVR-69 (p18)
3. Results
3.1. Patient Charactristics
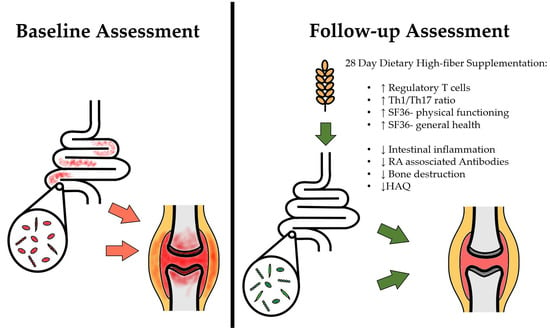
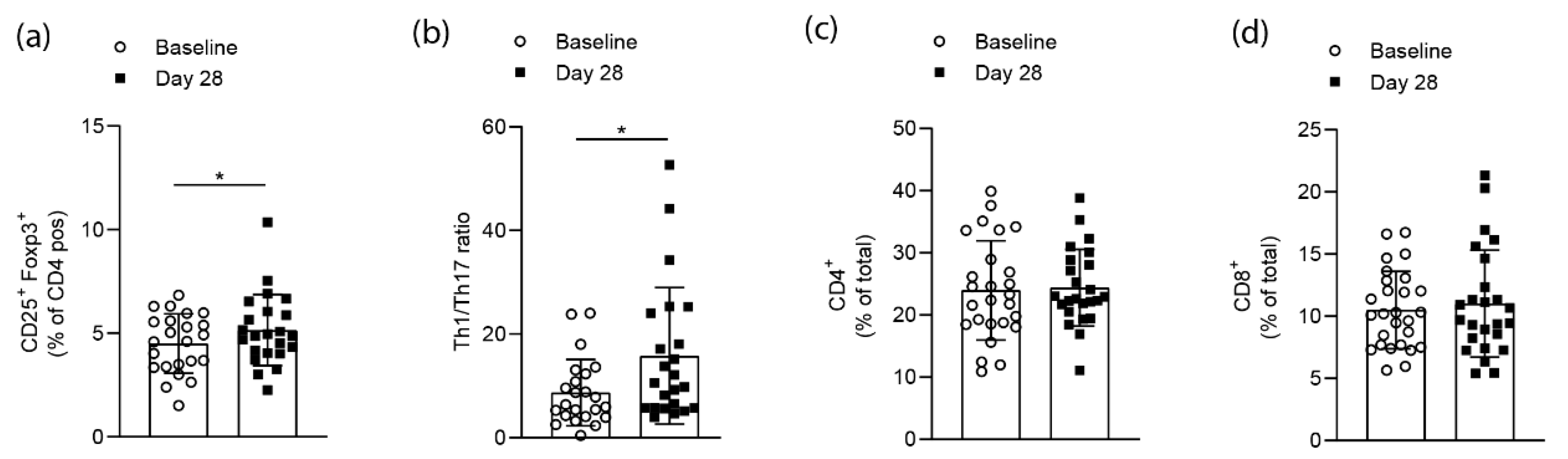
3.2. Effects on T cell Homeostasis
3.3. Effects on Signs and Symptoms of Arthritis
3.4. Effects on Routine Laboratory Parameters and Markers of Bone Resorption
3.5. Effects on Immunological Parameters
3.6. Effects on Intestinal Markers of Inflammation and Barrier Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Incidence and Prevalence of Rheumatoid Arthritis, Based on the 1987 American College of Rheumatology Criteria: A Systematic Review. Semin. Arthritis Rheum. 2006, 36, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Abdel-Nasser, A. Epidemiology of rheumatic musculoskeletal disorders in the developing world. Best Pr. Res. Clin. Rheumatol. 2008, 22, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Stastny, P. Association of the B-Cell Alloantigen DRw4 with Rheumatoid Arthritis. N. Engl. J. Med. 1978, 298, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.-F. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; Macauley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002, 41, 222–227. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef]
- Binder, H.J.; Mehta, P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 1989, 96, 989–996. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Genet. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Brüssow, H.; Parkinson, S.J. You are what you eat. Nat. Biotech. 2014, 32, 243. [Google Scholar]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Darlington, L.; Ramsey, N.; Mansfield, J. Placebo-Controlled, blind study of dietary manipulation therapy in rheumatoid arthritis. Lancet 1986, 327, 236–238. [Google Scholar] [CrossRef]
- Cleland, L.G.; James, M.J. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin. Arthritis Rheum. 1997, 27, 85–97. [Google Scholar]
- Kremer, J.; Michalek, A.; Lininger, L.; Huyck, C.; Bigauoette, J.; Timchalk, M.; Rynes, R.; Zieminski, J.; Bartholomew, L. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet 1985, 1, 184–187. [Google Scholar] [CrossRef]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; Digiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheumnol. 1990, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Panush, R.S.; Carter, R.L.; Katz, P.; Kowsari, B.; Longley, S.; Finnie, S. Diet therapy for rheumatoid arthritis. Arthritis Rheumnol. 1983, 26, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Borchgrevink, C.; Laerum, E.; Haugen, M.; Eek, M.; Førre, O.; Mowinkel, P.; Hovi, K.; Frre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Udén, A.M.; Trang, L.; Venizelos, N.; Palmblad, J. Neutrophil functions and clinical performance after total fasting in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1983, 42, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Førre, Ø. Vegetarian diet for patients with rheumatoid arthritis — Status: Two years after introduction of the diet. Clin. Rheumatol. 1994, 13, 475–482. [Google Scholar] [CrossRef]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Powrie, F. Feed your Tregs more fiber. Science 2013, 341, 463–464. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Rapin, A.; Lebon, L.; Dubey, L.K.; Mosconi, I.; Sarter, K.; Piersigilli, A.; Menin, L.; Walker, A.W.; Rougemont, J.; et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity 2015, 43, 998–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, W.; Yu, D.; Cao, Z.; Zhao, X. Butyrate inhibit collagen-induced arthritis via Treg/IL-10/Th17 axis. Int. Immunopharmacol. 2019, 68, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Bottcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Kronke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.-Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.M.; Axmann, R.; Zwerina, J.; Polzer, K.; Gückel, E.; Skapenko, A.; Horwood, N.; Cope, A.; Schett, G.; Schulze-Koops, H.; et al. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheumnol. 2007, 56, 4104–4112. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Frey, B.; Hess, A.; Zwerina, J.; Luther, J.; Nimmerjahn, F.; Engelke, K.; Kollias, G.; Hünig, T.; Schett, G.; et al. Regulatory T Cells Protect from Local and Systemic Bone Destruction in Arthritis. J. Immunol. 2010, 184, 7238–7246. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Sarter, K.; Hess, A.; Engelke, K.; Böhm, C.; Nimmerjahn, F.; Voll, R.; Schett, G.; David, J.-P.; David, J. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheumnol. 2010, 62, 2328–2338. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Jury, E.C.; Mauri, C.; Ehrenstein, M.R. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2008, 105, 19396–19401. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumnol. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.P. Antimodified protein antibody response pattern influences the risk for disease relapse in patients with rheumatoid arthritis tapering disease modifying antirheumatic drugs. Ann. Rheum. Dis. 2017, 76, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.; Bang, H.; Hammar, F.; Reimer, U.; Dyke, B.; Sahbudin, I.; Buckley, C.D.; Fisher, B.; Filer, A.; Raza, K. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann. Rheum. Dis. 2016, 75, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Van Tuyl, L.H.; Voskuyl, A.E.; Boers, M.; Geusens, P.; Landewé, R.B.; Dijkmans, B.A.; Lems, W.F. Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1623–1628. [Google Scholar] [CrossRef]
- Van der Helm-van, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.; Huizinga, T.W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Deehan, E.C.; Walter, J. The Fiber Gap and the Disappearing Gut Microbiome: Implications for Human Nutrition. Trends Endocrinol. Metab. 2016, 27, 239–242. [Google Scholar] [CrossRef]
- Cuvelier, C.; Barbatis, C.; Mielants, H.; De Vos, M.; Roels, H.; Veys, E. Histopathology of intestinal inflammation related to reactive arthritis. Gut 1987, 28, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, O.; Kuwata, J.; Umehara, K.; Uemura, F.; Takahashi, T.; Ozawa, A. Susceptibility to adjuvant-induced arthritis among germfree, specific-pathogen-free, and conventional rats. Infect. Immun. 1979, 26, 791–794. [Google Scholar] [PubMed]
- Kohashi, O.; Kohashi, Y.; Takahashi, T.; Ozawa, A.; Shigematsu, N. Suppressive effect ofEscherichia coli on adjuvant-induced arthritis in germ-free rats. Arthritis Rheumnol. 1986, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.C.; Herfarth, H.H.; Ikeda, J.S.; Grenther, W.B.; Hamm, T.E.; Balish, E.; Taurog, J.D.; Hammer, R.E.; Wilson, K.H.; Sartor, R.B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Investig. 1996, 98, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Sinkorova, Z.; Capkova, J.; Niederlová, J.; Štěpánková, R.; Sinkora, J. Commensal intestinal bacterial strains trigger ankylosing enthesopathy of the ankle in inbred B10.BR (H-2k) male mice. Hum. Immunol. 2008, 69, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Evans-Marin, H.; Rogier, R.; Koralov, S.B.; Manasson, J.; Roeleveld, D.; Van Der Kraan, P.M.; Scher, J.U.; Koenders, M.I.; Abdollahi-Roodsaz, S. Microbiota-Dependent Involvement of Th17 Cells in Murine Models of Inflammatory Arthritis. Arthritis Rheumatol. 2018, 70, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Jubair, W.K.; Hendrickson, J.D.; Severs, E.L.; Schulz, H.M.; Adhikari, S.; Ir, D.; Pagan, J.D.; Anthony, R.M.; Robertson, C.E.; Frank, D.N.; et al. Modulation of Inflammatory Arthritis in Mice by Gut Microbiota Through Mucosal Inflammation and Autoantibody Generation. Arthritis Rheumatol. 2018, 70, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Edwards, C.J. Commensal gut bacteria and the etiopathogenesis of rheumatoid arthritis. J. Rheumatol. 2008, 35, 1477–14797. [Google Scholar]
- Vaahtovuo, J.; Munukka, E.; Korkeamäki, M.; Luukkainen, R.; Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 1500–1505. [Google Scholar]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Arvikar, S.L.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Investig. 2017, 127, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Bazzazi, H. Th1-Th17 Ratio as a New Insight in Rheumatoid Arthritis Disease. Iran. J. Allergy Asthma Immunol. 2018, 17, 68–77. [Google Scholar]
- Kotake, S.; Nanke, Y.; Yago, T.; Kawamoto, M.; Kobashigawa, T.; Yamanaka, H. Elevated Ratio of Th17 Cell-Derived Th1 Cells (CD161(+)Th1 Cells) to CD161(+)Th17 Cells in Peripheral Blood of Early-Onset Rheumatoid Arthritis Patients. Biomed. Res. Int. 2016, 2016, 4186027. [Google Scholar] [CrossRef]
- Aleyd, E.; Al, M.; Tuk, C.W.; van der Laken, C.J.; van Egmond, M. IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcalphaRI. J. Immunol. 2016, 197, 4552–4559. [Google Scholar] [CrossRef]
- Revell, P.A.; Mayston, V.J. Immunoglobulin classes in plasma cells of the synovial membrane in chronic inflammatory joint disease. Ann. Rheum. Dis. 1986, 45, 405–408. [Google Scholar] [CrossRef]
- Kinslow, J.D.; Blum, L.K.; Deane, K.D.; Demoruelle, M.K.; Okamoto, Y.; Parish, M.C.; Kongpachith, S.; Lahey, L.J.; Norris, J.M.; Holers, V.M. Elevated IgA Plasmablast Levels in Subjects at Risk of Developing Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 2372–2383. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Després, N.; Lapointe, E.; Van Der Heijden, A.; Lora, M.; Senshu, T.; Van Venrooij, W.J.; Ménard, H.A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 2004, 6, R142–R150. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Gómara, M.J.; Gómez-Puerta, J.A.; Sanmartí, R.; Haro, I. The Use of Chimeric Vimentin Citrullinated Peptides for the Diagnosis of Rheumatoid Arthritis. J. Med. Chem. 2011, 54, 7486–7492. [Google Scholar] [CrossRef] [PubMed]
- Falkenburg, W.J.; Van Schaardenburg, D. Evolution of autoantibody responses in individuals at risk of rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2017, 31, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Bromberg, R.; Deane, K.D.; Lahey, L.J.; Derber, L.A.; Chandra, P.E.; Edison, J.D.; Gilliland, W.R.; Tibshirani, R.J.; Norris, J.M.; et al. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLoS ONE 2012, 7, e35296. [Google Scholar] [CrossRef]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Sapone, A.; De Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.; Berti, I.; Sapone, A.; Gerarduzzi, T.; Not, T.; Zielke, R.; Fasano, A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc. Natl. Acad. Sci. USA 2005, 102, 2916–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, B.M.; Lammers, K.M.; Arrieta, M.C.; Fasano, A.; Meddings, J.B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: A proof of concept study. Aliment. Pharmacol. Ther. 2007, 26, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Andreev, D.; Oeser, K.; Krljanac, B.; Hueber, A.; Kleyer, A.; Voehringer, D.; Schett, G.; Bozec, A. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 2016, 7, 11596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographic characteristics (N = 36) | |
| Age, mean ± SD, years | 56.19 ± 7.7 |
| Females, N (%) | 20 (64.51%) |
| BMI, mean ± SD, units | 26.63 ± 6.4 |
| Ever smoker, N (%) | 6 (19.35%) |
| Disease-specific characteristics (N = 36) | |
| Disease duration, mean ± SD, years | 11.64 ± 9.39 |
| Disease activity score (DAS) 28, mean ± SD, units | 2.54 ± 0.28 |
| Anti-CCP-IgG antibody positive, N (%) | 15 (48.38%) |
| Rheumatoid Factor IgM positive, N (%) | 12 (38.7%) |
| Concomitant anti-rheumatic treatment (N = 36) | |
| Methotrexate, N (%) | 16 (51.61%) |
| Glucocorticoids, N (%) | 5 (16.12%) |
| Other csDMARDS, N (%) | 1 (3.22%) |
| Biological DMARDs, N (%) | 23 (74.10%) |
| Tumor Necrosis Factor Inhibitors, N (%) | 5 (16.12%) |
| Tocilizumab, N (%) | 8 (25.8%) |
| Abatacept, N (%) | 3 (9.67%) |
| Rituximab, N (%) | 7 (22.58%) |
| JAK-inhibitors, N (%) | 4 (12.9%) |
| Baseline | Day 28 | |
|---|---|---|
| Disease Activity | ||
| Disease activity score (DAS) 28, mean ± SD, units | 2.56 ± 0.28 | 2.53 ± 0.28 |
| Swollen joint count, mean ± SD, N | 0.61 ± 0.23 | 0.50 ± 0.22 |
| Tender joints, mean ± SD, N | 2.29 ± 0.60 | 2.25 ± 0.62 |
| Visual analogue scale (VAS) for pain, mean ± SD, cm | 25.54 ± 4.78 | 26.96 ± 4.74 |
| VAS for patients’ global disease activity, mean ± SD, cm | 24.54 ± 4.22 | 26.18 ± 4.25 |
| VAS for physicians´global disease activity, mean ± SD, cm | 20.31 ± 4.26 | 17.85 ± 4.08 |
| Physical Function | ||
| Health Assessment Questionaire (HAQ), mean ± SD, units | 0.54 ± 0.08 | 0.43 ± 0.09 ** |
| Quality of Life | ||
| SF 36- physical functioning | 66.37 ± 5.30 | 73.74 ± 4.79 *** |
| SF 36- role limitation/physical, mean ± SD, units | 57.69 ± 8.52 | 58.65 ± 8.54 |
| SF 36- role limitation/emotional, mean ± SD, units | 64.04 ± 7.84 | 60.23 ± 8.68 |
| SF 36- energy/fatigue, mean ± SD, units | 55.77 ± 4.60 | 58.27 ± 4.33 |
| SF 36- emotional wellbeing, mean ± SD, units | 70.96 ± 3.96 | 71.20 ± 3.94 |
| SF 36- social functioning, mean ± SD, units | 76.10 ± 4.38 | 75.16 ± 4.08 |
| SF 36- pain, mean ± SD, units | 63.48 ± 3.69 | 61.87 ± 3.91 |
| SF 36- general health, mean ± SD, units | 40.37 ± 3.58 | 50.53 ± 4.47 * |
| SF 36- health change, mean ± SD, units | 61.00 ± 4.58 | 55.00 ± 4.08 |
| Laboratory Tests | Baseline | Day 28 |
|---|---|---|
| C-reactive protein, mean ± SD, mg/L | 6.74 ± 0.61 | 6.01 ± 0.36 |
| Erythrocytes, mean ± SD, N × 106/µL | 4.60 ± 0.07 | 4.60 ± 0.07 |
| Hematocrit, mean ± SD, % | 41.25 ± 0.52 | 40.96 ± 0.49 |
| Leukocytes, mean ± SD, N × 103/µL | 6.41 ± 0.46 | 6.50 ± 0.50 |
| Thrombocytes, mean ± SD, N × 103/µL | 250.2 ± 10.65 | 262.2 ± 11.83 |
| Neutrophils, mean ± SD, N × 103/µL | 4.07 ± 0.43 | 3.83 ± 0.36 |
| Monocytes, mean ± SD, N × 103/µL | 0.54 ± 0.03 | 0.52 ± 0.08 |
| Lymphocytes, mean ± SD, N × 103/µL | 1.54 ± 0.10 | 1.63 ± 0.11 |
| Eosinophils, mean ± SD, N × 103/µL | 0.13 ± 0.01 | 0.16 ± 0.02 |
| Basophils, mean ± SD, N × 103/µL | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Creatinin, mean ± SD, mg/dL | 0.76 ± 0.03 | 0.75 ± 0.03 |
| Uric acid, mean ± SD, mg/dL | 5.28 ± 0.22 | 5.19 ± 0.25 |
| Sodium, mean ± SD, mmol/ L | 139.8 ± 0.28 | 140.0 ± 0.33 |
| Potassium, mean ± SD, mmol/L | 4.41 ± 0.08 | 4.42 ± 0.07 |
| LDH, mean ± SD, U/L | 274.3 ± 12.10 | 264.2 ± 8.81 |
| Creatine kinase, mean ± SD, U/L | 130.2 ± 16.28 | 158.3 ± 29.53 |
| ALT, mean ± SD, U/L | 26.42 ± 2.94 | 29.04 ± 2.77 |
| Alkaline phosphatase, mean ± SD, U/L | 78.39 ± 3.96 | 81.52 ± 4.69 |
| LDL- cholesterol, mean ± SD, mg/dL | 160.3 ± 7.17 | 153.5 ± 7.53 |
| HDL- Cholesterol, mean ± SD, mg/dL | 70.31 ± 2.39 | 68.94 ± 3.48 |
| Triglycerides, mean ± SD, mg/dL | 105.9 ± 10.51 | 94.38 ± 8.86 |
| Hba1c, mean ± SD, % | 5.50 ± 0.11 | 5.51 ± 0.11 |
| Immunological Parameters | Baseline | Day 28 |
|---|---|---|
| RF- IgM, mean ± SD, Units | 211.9 ± 98.26 | 185.7 ± 79.35 |
| RF IgA, mean ± SD, Units | 142.4 ± 62.80 | 127.9 ± 50.85 |
| ACPA-IgG (CCP2 test), mean ± SD, Units | 231.2 ± 76.94 | 170.7 ± 53.21 |
| IgA1, mean ± SD, OD | 3911 ± 537.5 | 3599 ± 515.1 * |
| IgA2, mean ± SD, OD | 215.2 ± 32.88 | 211.9 ± 39.06 |
| Total IgA, mean ± SD, OD | 4206 ± 558.9 | 3873 ± 538.7 * |
| ACPA (CCP2 test) IgA1, mean ± SD, µg/mL | 3.12 ± 0.36 | 2.83 ± 0.35 |
| ACPA (CCP2 test) IgA2, mean ± SD, µg/mL | 0.68 ± 0.10 | 0.64 ± 0.10 |
| Total ACPA IgA, mean ± SD, µg/mL | 4.08 ± 0.31 | 3.78 ± 0.37 |
| Anti-citrullinated VIM p18, mean ± SD, OD | 654.5 ± 116.4 | 460.3 ± 89.25 ** |
| Anti- Acetylated ornithine VIM p18, mean ± SD, OD | 311.0 ± 100.7 | 303.7 ± 107.3 |
| Carbamylated vimentin VIM p18, mean ± SD, OD | 284.6 ± 126.4 | 229.3 ± 97.45 |
| Acetylated lysine VIM p18, mean ± SD, OD | 299.0 ± 141.8 | 276.4 ± 132.3 |
| Calprotectin, mean ± SD, ng/mL | 6.06 ± 0.71 | 4.52 ± 0.31 ** |
| Zonulin, mean ± SD, ng/mL | 4.01 ± 0.51 | 2.91 ± 0.32 * |
| Crosslaps, mean ± SD, ng/mL | 0.42 ± 0.05 | 0.36 ± 0.05 * |
| Osteocalcin, mean ± SD, ng/mL | 16.31 ± 1.16 | 16.73 ± 1.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. https://doi.org/10.3390/nu11102392
Häger J, Bang H, Hagen M, Frech M, Träger P, Sokolova MV, Steffen U, Tascilar K, Sarter K, Schett G, et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients. 2019; 11(10):2392. https://doi.org/10.3390/nu11102392
Chicago/Turabian StyleHäger, Julian, Holger Bang, Melanie Hagen, Michael Frech, Pascal Träger, Maria V. Sokolova, Ulrike Steffen, Koray Tascilar, Kerstin Sarter, Georg Schett, and et al. 2019. "The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study" Nutrients 11, no. 10: 2392. https://doi.org/10.3390/nu11102392
APA StyleHäger, J., Bang, H., Hagen, M., Frech, M., Träger, P., Sokolova, M. V., Steffen, U., Tascilar, K., Sarter, K., Schett, G., Rech, J., & Zaiss, M. M. (2019). The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients, 11(10), 2392. https://doi.org/10.3390/nu11102392