Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review
Abstract
:1. Introduction
2. Materials and Methods
3. Distribution
4. Traditional Uses
5. Biological Activity
5.1. Anticancer Activity
5.2. Anti-Obesity Activity
5.3. Anti-HIV Activity
5.4. Antimicrobial Activity
5.5. Antioxidant Activity
5.6. Anti-Inflammatory Activity
5.7. Anticholinesterase Activity
5.8. Anti-Mutagenicity Activity
5.9. Effect on Cytochromes CYP 450
5.10. Vascular Activity
5.11. Adaptogenic Activity
5.12. Xanthine Oxidase Inhibitory Activity
5.13. Allergenic Activity
5.14. Neurological Activity
5.15. Nociceptive Activity.
5.16. Wound-Healing Activity
5.17. Effects on Sexual Performance
5.18. Miscellaneous
6. Chemical Metabolites of Kaempferia Species
6.1. Diterpenoids
6.1.1. Isopimarane-Type Diterpenoids
Biosynthesis of Isopimarane-Type Diterpenoids
6.1.2. Abietane-Type Diterpenoids
6.1.3. Labdane and Clerodane Diterpenoids
6.1.4. Flavonoids
6.1.5. Phenolic Compounds
6.1.6. Steroids and Triterpenes
6.1.7. Volatile Oils
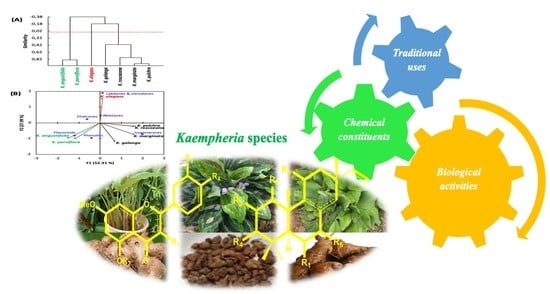
7. Principal Components Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC) for Kaempferia Species
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| K. | Kaempheria |
| Sp. | Species |
| PCA | Principal component analysis |
| AHC | Agglomerative hierarchical clustering |
| CCA | Significant Cholangiocarcinoma |
| HSC-2 | Mouth squamous cell carcinoma |
| EAC | Ehrlich ascites carcinoma cancer cells |
| HL-60 | Human leukemia cancer cells |
| CL-6 | Human cholangiocarcinoma cells |
| TKI | Tyrosine kinase inhibitors |
| BCRP | Breast cancer resistance protein |
| MTT | (3-[4,5,[4,5-Dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) assay |
| CH2Cl2 | Dichloromethane |
| BChE | Butyrylcholinesterase |
| NO | Nitric oxide |
| CUPRAC | Modified cupric reducing antioxidant capacity |
| PDE5 | Phosphodiesterase type 5 inhibitor |
| AP | Aerial parts |
| AChE | Acetylcholinesterase |
| RBL-2H3 | Rat Basophilic Leukemia cells |
| P38 | Type of mitogen-activated protein kinases |
| STAT1 and 3 | Signal transducers and activators of transcription 1 and 3 |
| MeOH | Methanol |
| EtOAc | Ethyl acetate |
| MCF-7 | Breast cancer cells |
| HT-29 | Colorectal adenocarcinoma cell |
| PMF | Polymethoxyflavonoid-rich fraction |
| TSOD | Tsumura Suzuki obese diabetes |
| GGPP | (E,E,E)-Geranylgeranyl diphosphate |
| CPP | Copalyl diphosphate |
| CPS | Copalyl diphosphate synthases |
| EtOH | Ethanol |
| AChE | Acetylcholinesterase |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) assay |
| Vpr | Viral protein R |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl assay |
| FRAP | Ferric reducing antioxidant power |
| CYP3A | Cytochrome P450, family 3, subfamily A |
| NF-κB | Nuclear factor pathway |
| Rh | Rhizomes |
| BChE | Butyrylcholinesterase |
| STZ | Streptozotocin |
| MPO | myeloperoxidase |
| DMF | Dimethylformamide |
| MAPKs | Type of mitogen-activated protein kinases |
| PGE2 | Prostaglandin E2 |
References
- Vinceti, B.; Loo, J.; Gaisberger, H.; van Zonneveld, M.J.; Schueler, S.; Konrad, H.; Kadu, C.A.; Geburek, T. Conservation priorities for Prunus africana defined with the aid of spatial analysis of genetic data and climatic variables. PLoS ONE 2013, 8, e59987. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Gálvez, C.; Ubera, J.L. Bioclimatic influence on essential oil composition in South Iberian Peninsular populations of Thymus zygis. J. Essent. Oil Res. 2012, 24, 71–81. [Google Scholar] [CrossRef]
- Elshamy, A.; Mohamed, T.A.; Al-Rowaily, S.L.; Abd El-Gawad, A.M.; Dar, B.A.; Shahat, A.A.; Hegazy, M.F. Euphosantianane E–G: Three new premyrsinane type 2 diterpenoids from Euphorbia sanctae-catharinae with 3 contribution to chemotaxonomy. Molecules 2019, 24, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Techaprasan, J.; Klinbunga, S.; Ngamriabsakul, C.; Jenjittikul, T. Genetic variation of Kaempferia (Zingiberaceae) in Thailand based on chloroplast DNA (psbA-trnH and petA-psbJ) sequences. Genet. Mol. Res. 2010, 9, 1957–1973. [Google Scholar] [CrossRef]
- Wutythamawech, W. Encyclopedia of Thai Herbs; OS Printing: Bangkok, Thailand, 1997; p. 365. [Google Scholar]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Aburada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011, 65, 73–80. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, K.; Deguchi, T.; Itoh, K.; Fujita, T.; Higashino, M.; Yoshioka, Y.; Matsumura, S.-I.; Tanaka, R.; Shinada, T. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011, 34, 1143–1146. [Google Scholar] [CrossRef]
- Sivarajan, V.; Balachandran, I. Ayurvedic Drugs and their Plant Sources Oxford and IBH Publishing Co; Oxford & IBH Pub. Co: New Delhi, India, 1994. [Google Scholar]
- Hidaka, M.; Horikawa, K.; Akase, T.; Makihara, H.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ibuki, A.; Matsumoto, Y. Efficacy of Kaempferia parviflora in a mouse model of obesity-induced dermatopathy. J. Nat. Med. 2017, 71, 59–67. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Matsui, T.; Aimaiti, S.; Kodama, T.; Ngwe, H.; Okamoto, Y.; Tanaka, M.; Asakawa, Y.; Abe, I. Isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorganic Med. Chem. Lett. 2016, 26, 1789–1793. [Google Scholar] [CrossRef]
- Yao, F.; Huang, Y.; Wang, Y.; He, X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.). Ind. Crop. Prod. 2018, 125, 454–461. [Google Scholar] [CrossRef]
- Vimala, S.; Norhanom, A.; Yadav, M. Anti-tumour promoter activity in Malaysian ginger rhizobia used in traditional medicine. Br. J. Cancer 1999, 80, 110. [Google Scholar] [CrossRef] [PubMed]
- Amuamuta, A.; Plengsuriyakarn, T.; Na-Bangchang, K.J. Anticholangiocarcinoma activity and toxicity of the Kaempferia galanga Linn. rhizome ethanolic extract. BMC Complementary Altern. Med. 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Swapana, N.; Tominaga, T.; Elshamy, A.I.; Ibrahim, M.A.; Hegazy, M.-E.F.; Singh, C.B.; Suenaga, M.; Imagawa, H.; Noji, M.; Umeyama, A. Kaemgalangol A: Unusual seco-isopimarane diterpenoid from aromatic ginger Kaempferia galanga. Fitoterapia 2018, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Win, N.N.; Ito, T.; Aimaiti, S.; Kodama, T.; Imagawa, H.; Ngwe, H.; Asakawa, Y.; Abe, I.; Morita, H. Kaempulchraols I–O: New isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their antiproliferative activity. Tetrahedron 2015, 71, 4707–4713. [Google Scholar] [CrossRef]
- Chawengrum, P.; Boonsombat, J.; Kittakoop, P.; Mahidol, C.; Ruchirawat, S.; Thongnest, S. Cytotoxic and antimicrobial labdane and clerodane diterpenoids from Kaempferia elegans and Kaempferia pulchra. Phytochem. Lett 2018, 24, 140–144. [Google Scholar] [CrossRef]
- Dash, P.R.; Nasrin, M.; Ali, M.S. In vivo cytotoxic and In vitro antibacterial activities of Kaempferia galanga. J. Pharmacogn. Phytochem. 2014, 3, 172–177. [Google Scholar]
- Ali, H.; Yesmin, R.; Satter, M.A.; Habib, R.; Yeasmin, T. Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells. J. King Saud Univ. Sci. 2018, 30, 386–392. [Google Scholar] [CrossRef]
- Bae, S.; D’cunha, R.; Shao, J.; An, G. Effect of 5, 7-dimethoxyflavone on Bcrp1-mediated transport of sorafenib in vitro and in vivo in mice. Eur. J. Pharm. Sci. 2018, 117, 27–34. [Google Scholar] [CrossRef]
- Ahmed, F.R.S.; Amin, R.; Hasan, I.; Asaduzzaman, A.; Kabir, S.R. Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol. 2017, 102, 952–959. [Google Scholar] [CrossRef]
- Omar, M.N.; Rahman, S.A.; Ichwan, S.; Hasali, N.; Rasid, F.A.; Halim, F.A. Cytotoxicity effects of extracts and essential oil of Kaempferia galanga on cervical cancer C33A cell line. Orient. J. Chem. 2017, 33, 1659–1664. [Google Scholar] [CrossRef]
- Tang, S.W.; Sukari, M.A.; Neoh, B.K.; Yeap, Y.S.Y.; Abdul, A.B.; Kifli, N.; Cheng Lian Ee, G. Phytochemicals from Kaempferia angustifolia Rosc. and their cytotoxic and antimicrobial activities. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Win, N.N.; Ngwe, H.; Abe, I.; Morita, H. Naturally occurring Vpr inhibitors from medicinal plants of Myanmar. J. Nat. Med. 2017, 71, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonsombat, J.; Mahidol, C.; Chawengrum, P.; Reuk-Ngam, N.; Chimnoi, N.; Techasakul, S.; Ruchirawat, S.; Thongnest, S. Roscotanes and roscoranes: Oxygenated abietane and pimarane diterpenoids from Kaempferia roscoeana. Phytochemistry 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Werngren, J.; Jose, L.; Suja, K.; Nair, M.S.; Varma, R.L.; Mundayoor, S.; Hoffner, S.; Kumar, R.A. Ethyl p-methoxycinnamate isolated from a traditional anti-tuberculosis medicinal herb inhibits drug resistant strains of Mycobacterium tuberculosis in vitro. Fitoterapia 2011, 82, 757–761. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, S.; Wang, F.; Li, Z.; Liu, L.; Yang, X.; Bao, Y.; Wu, Y.; Huang, Y.; Sun, L. Chemical composition and antibacterial activity of Kaempferia galanga essential oil. Int. J. Agric. Biol. 2018, 20, 457–462. [Google Scholar] [CrossRef]
- Panyakaew, J.; Sookkhee, S.; Rotarayanont, S.; Kittiwachana, S.; Wangkarn, S.; Mungkornasawakul, P. Chemical variation and potential of Kaempferia oils as larvicide against Aedes aegypti. J. Essent. Oil Bear. Plants 2017, 20, 1044–1056. [Google Scholar] [CrossRef]
- Malahayati, N.; Widowati, T.W.; Febrianti, A. Total phenolic, antioxidant and antibacterial activities of curcumin extract of kunci pepet (Kaempferia rotunda L). Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 129–135. [Google Scholar]
- Fauziyah, P.N.; Sukandar, E.Y.; Ayuningtyas, D.K. Combination effect of antituberculosis drugs and ethanolic extract of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Sci. Pharm. 2017, 85, 14. [Google Scholar] [CrossRef]
- Kochuthressia, K.; Britto, S.J.; Jaseentha, M.; Raphael, R. In vitro antimicrobial evaluation of Kaempferia galanga L. rhizome extract. Am. J. Biotechnol. Mol. Sci. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Yeap, Y.S.Y.; Kassim, N.K.; Ng, R.C.; Ee, G.C.L.; Saiful Yazan, L.; Musa, K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017, 20, 1158–1172. [Google Scholar] [CrossRef]
- Tang, S.W.; Sukari, M.A.; Rahmani, M.; Lajis, N.H.; Ali, A.M. A new abietene diterpene and other constituents from Kaempferia angustifolia Rosc. Molecules 2011, 16, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Parida, R.; Singh, S.; Padhy, R.N.; Nayak, S. Evaluation of yield, quality and antioxidant activity of essential oil of in vitro propagated Kaempferia galanga Linn. J. Acute Dis. 2014, 3, 124–130. [Google Scholar] [CrossRef]
- Kaewkroek, K.; Wattanapiromsakul, C.; Kongsaeree, P.; Tewtrakul, S. Nitric oxide and tumor necrosis factor-alpha inhibitory substances from the rhizomes of Kaempferia marginata. Nat. Prod. Commun. 2013, 8, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Kaewkroek, K.; Wattanapiromsakul, C.; Matsuda, H.; Nakamura, S.; Tewtrakul, S. Anti-inflammatory activity of compounds from Kaempferia marginata rhizomes. Songklanakarin J. Sci. Technol. 2017, 39, 91–99. [Google Scholar]
- Sematong, T.; Reutrakul, V.; Tuchinda, P.; Claeson, P.; Pongprayoon, U.; Nahar, N. Topical antiinflammatory activity of two pimarane diterpenes from Kaempferia pulchra. Phytother. Res. 1996, 10, 534–535. [Google Scholar] [CrossRef]
- Sae-wong, C.; Tansakul, P.; Tewtrakul, S. Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J. Ethnopharmacol. 2009, 124, 576–580. [Google Scholar] [CrossRef]
- Lee, M.-h.; Han, A.-R.; Jang, M.; Choi, H.-K.; Lee, S.-Y.; Kim, K.-T.; Lim, T.-G. Antiskin inflammatory activity of black ginger (Kaempferia parviflora) through antioxidative activity. Oxidative Med. Cell. Longev. 2018, 2018, 5967150. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Sato, K.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ichinose, K.; Aburada, M.; Ochiai, W.; Sugiyama, K. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018, 72, 136–144. [Google Scholar] [CrossRef]
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Majid, A.M.S.A.; Al-Suede, F.S.R.; Hassan, L.E.A.; Altaf, R.; Ahamed, M.B.K. Ethyl-p-methoxycinnamate isolated from Kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α, and angiogenesis by blocking endothelial functions. Clinics 2014, 69, 134–144. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S. Effects of compounds from Kaempferia parviflora on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha productions in RAW264. 7 macrophage cells. J. Ethnopharmacol. 2008, 120, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, P.C.; Latha, K.P.; Mudgal, J.; Nampurath, G.K. Extraction, characterization and evaluation of Kaempferia galanga L.(Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016, 194, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Azuma, T.; Kayano, S.-i.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Ochiai, W.; Kobayashi, H.; Kitaoka, S.; Kashiwada, M.; Koyama, Y.; Nakaishi, S.; Nagai, T.; Aburada, M.; Sugiyama, K. Effect of the active ingredient of Kaempferia parviflora, 5, 7-dimethoxyflavone, on the pharmacokinetics of midazolam. J. Nat. Med. 2018, 72, 607–614. [Google Scholar] [CrossRef]
- Yorsin, S.; Kanokwiroon, K.; Radenahmad, N.; Jansakul, C. Effects of Kaempferia parviflora rhizomes dichloromethane extract on vascular functions in middle-aged male rat. J. Ethnopharmacol. 2014, 156, 162–174. [Google Scholar] [CrossRef]
- Othman, R.; Ibrahim, H.; Mohd, M.A.; Mustafa, M.R.; Awang, K. Bioassay-guided isolation of a vasorelaxant active compound from Kaempferia galanga L. Phytomedicine 2006, 13, 61–66. [Google Scholar] [CrossRef]
- Wattanapitayakul, S.K.; Chularojmontri, L.; Herunsalee, A.; Charuchongkolwongse, S.; Chansuvanich, N. Vasorelaxation and antispasmodic effects of Kaempferia parviflora ethanolic extract in isolated rat organ studies. Fitoterapia 2008, 79, 214–216. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kamiya, T.; Kusaba, N.; Yamaguchi, K.; Takagaki, K.; Kameya, T.; Sugie, H.; Saito, M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. 2015, 61, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Muchimapura, S.; Tong-Un, T.; Saenghong, N.; Thukhum-Mee, W.; Sripanidkulchai, B. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid. Based Complementary Altern. Med. 2012, 2012, 732816. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Plaingam, W.; Sangsuthum, S.; Angkhasirisap, W.; Tencomnao, T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complementary Med. 2017, 7, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Dash, P.R.; Nasrin, M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complementary Altern. Med. 2015, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Ridtitid, W.; Sae-Wong, C.; Reanmongkol, W.; Wongnawa, M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J. Ethnopharmacol. 2008, 118, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, T.V.; Sharma, C.; Adiga, S.; Bairy, K.; Shenoy, S.; Shenoy, G. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J. Physiol. Pharmacol. 2006, 50, 384–390. [Google Scholar]
- Temkitthawon, P.; Hinds, T.R.; Beavo, J.A.; Viyoch, J.; Suwanborirux, K.; Pongamornkul, W.; Sawasdee, P.; Ingkaninan, K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J. Ethnopharmacol. 2011, 137, 1437–1441. [Google Scholar] [CrossRef]
- Stein, R.A.; Schmid, K.; Bolivar, J.; Swick, A.G.; Joyal, S.V.; Hirsh, S.P. Kaempferia parviflora ethanol extract improves self-assessed sexual health in men: A pilot study. J. Integr. Med. 2018, 16, 249–254. [Google Scholar] [CrossRef]
- Horigome, S.; Maeda, M.; Ho, H.-J.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Foods 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Lert-Amornpat, T.; Maketon, C.; Fungfuang, W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia 2017, 49, e12770. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.-B.; Hwang, J.-K. Standardized Kaempferia parviflora Wall. ex Baker (Zingiberaceae) extract inhibits fat accumulation and muscle atrophy in ob/ob mice. Evid. Based Complementary Altern. Med. 2018, 2018, 8161042. [Google Scholar] [CrossRef]
- Ono, S.; Yoshida, N.; Maekawa, D.; Kitakaze, T.; Kobayashi, Y.; Kitano, T.; Fujita, T.; Okuwa-Hayashi, H.; Harada, N.; Nakano, Y. 5-Hydroxy-7-methoxyflavone derivatives from Kaempferia parviflora induce skeletal muscle hypertrophy. Food Sci. Nutr. 2019, 7, 312–321. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. Kaempferia parviflora extract as a potential anti-acne agent with anti-inflammatory, sebostatic and anti-propionibacterium acnes activity. Int. J. Mol. Sci. 2018, 19, 3457. [Google Scholar] [CrossRef]
- Kongdang, P.; Jaitham, R.; Thonghoi, S.; Kuensaen, C.; Pradit, W.; Ongchai, S. Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine 2019, 59, 152755. [Google Scholar] [CrossRef]
- Dash, P.R.; Mou, K.M.; Erina, I.N.; Ripa, F.A.; Al Masud, K.N.; Ali, M.S. Study of anthelmintic and insecticidal activities of different extracts of Kaempferia galanga. Int. J. Pharm. Sci. Res. 2017, 8, 729–733. [Google Scholar]
- Jaipetch, T.; Reutrakul, V.; Tuntiwachwuttikul, P.; Santisuk, T. Flavonoids in the black rhizomes of Boesenbergia panduta. Phytochemistry 1983, 22, 625–626. [Google Scholar] [CrossRef]
- Herunsalee, A.; Pancharoen, O.; Tuntiwachwuttikul, P. Further studies of flavonoids of the black rhizome Boesenbergia pandurata. J. Sci. Soc. Thail. 1987, 13, 119–122. [Google Scholar]
- Trakoontivakorn, G.; Nakahara, K.; Shinmoto, H.; Takenaka, M.; Onishi-Kameyama, M.; Ono, H.; Yoshida, M.; Nagata, T.; Tsushida, T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J. Agric. Food Chem. 2001, 49, 3046–3050. [Google Scholar] [CrossRef]
- Raina, A.P.; Abraham, Z. Chemical profiling of essential oil of Kaempferia galanga L. germplasm from India. J. Essent. Oil Res. 2016, 28, 29–34. [Google Scholar] [CrossRef]
- Wong, K.; Ong, K.; Lim, C. Compositon of the essential oil of rhizomes of kaempferia galanga L. Flavour Fragr. J. 1992, 7, 263–266. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Aimaiti, S.; Imagawa, H.; Ngwe, H.; Abe, I.; Morita, H. Kaempulchraols A–H, diterpenoids from the rhizomes of Kaempferia pulchra collected in Myanmar. J. Nat. Prod. 2015, 78, 1113–1118. [Google Scholar] [CrossRef]
- Thongnest, S.; Mahidol, C.; Sutthivaiyakit, S.; Ruchirawat, S. Oxygenated pimarane diterpenes from Kaempferia marginata. J. Nat. Prod. 2005, 68, 1632–1636. [Google Scholar] [CrossRef]
- Prawat, U.; Tuntiwachwuttikul, P.; Taylor, W.C.; Engelhardt, L.M.; Skelton, B.; White, A. Diterpenes from a Kaempferia species. Phytochemistry 1993, 32, 991–997. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef]
- Umar, M.I.; Asmawi, M.Z.B.; Sadikun, A.; Altaf, R.; Iqbal, M.A. Phytochemistry and medicinal properties of Kaempferia galanga L. (Zingiberaceae) extracts. Afr. J. Pharm. Pharmacol. 2011, 5, 1638–1647. [Google Scholar] [CrossRef]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef]
- Davis, E.M.; Croteau, R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top. Curr. Chem. 2000, 209, 53–95. [Google Scholar]
- Xu, M.; Hillwig, M.L.; Tiernan, M.S.; Peters, R.J. Probing labdane-related diterpenoid biosynthesis in the fungal genus Aspergillus. J. Nat. Prod. 2017, 80, 328–333. [Google Scholar] [CrossRef]
- Li, Y.C.; Ji, H.; Li, X.H.; Zhang, H.X.; Li, H.T. Isolation of nematicidal constituents from essential oil of Kaempferia galanga L rhizome and their activity against Heterodera avenae Wollenweber. Trop. J. Pharm. Res. 2017, 16, 59–65. [Google Scholar] [CrossRef]
- Munda, S.; Saikia, P.; Lal, M. Chemical composition and biological activity of essential oil of Kaempferia galanga: A review. J. Essent. Oil Res. 2018, 30, 303–308. [Google Scholar] [CrossRef]
- Labrooy, C.D.; Abdullah, T.L.; Stanslas, J. Identification of ethnomedicinally important Kaempferia L. (Zingiberaceae) species based on morphological traits and suitable DNA region. Curr. Plant Biol. 2018, 14, 50–55. [Google Scholar] [CrossRef]




 | |||||||||||
| No | Name | R | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Plant | Ref |
| 1 | Sandaracopimaradiene | H | H | H | H | H | H | H | H | K. galanga K. roscoeana K. marginata | [4,16,17,25,26,36,74,75,76] |
| 2 | Sandaracopimaradiene-9α-ol | α-OH | H | H | H | H | H | H | H | ||
| 3 | 8(14),15-Sandaracopimaradiene-1α,9α-diol | α-OH | H | α-OH | H | H | H | H | H | K. galanga K. pulchra K. sp. | |
| 4 | 1,11-Dihydroxypimara-8(14),15-diene | H | H | α-OH | H | H | α-OH | H | H | ||
| 5 | 6β-Hydroxypimara-8(14),15-diene-1-one | H | β-OH | =O | H | H | H | H | H | K. galanga K. marginata | |
| 6 | Sandaracopimaradien-6β,9α-diol-l-one | α-OH | β-OH | =O | H | H | H | H | H | K. galanga | |
| 7 | Boesenberol I | α-OH | H | =O | H | H | H | α-OH | H | ||
| 8 | Boesenberol J | α-OH | β-OH | =O | H | H | H | H | H | K. galanga | |
| 9 | Sandaracopimaradien-1α,2α-diol | H | H | α-OH | α-OH | H | H | H | H | K. roscoeana K. pulchra K. marginata | [26,38,75] |
| 10 | 2α-Acetoxy-sandaracopimaradien-1α-ol | H | H | α-OH | α-OAc | H | H | H | H | K. pulchra K. marginata | |
| 11 | Kaempulchraol E | α-H | β-OH | α-OH | H | H | H | H | H | K. galanga K. pulchra | |
| 12 | Kaempulchraol F | H | H | α-OH | H | α-OH | H | H | H | K. pulchra | [4,16,17,25,26,74] |
| 13 | Kaempulchraol H | H | β-OH | α-OH | H | α-OH | H | H | H | ||
| 14 | Kaempulchraol I | H | H | α-OH | H | H | H | H | H | K. galanga K. pulchra K. roscoeana | |
| 15 | Kaempulchraol J | H | H | α-OH | H | H | H | =O | K. pulchra | ||
| 16 | Kaempulchraol K | α-OH | β-OAc | H | H | H | H | H | H | ||
| 17 | Kaempulchraol L | α-OMe | β-OH | H | H | H | H | H | H | K. galanga K. pulchra | |
| 18 | Kaempulchraol M | α-OH | H | α-OH | α-OH | H | H | H | H | K. pulchra | |
| 19 | Kaempulchraol P | H | β-OH | H | H | H | H | H | H | ||
| 20 | Kaempulchraol Q | α-OAc | β-OH | H | H | H | H | H | H | ||
| 21 | Kaempulchraol R | α-OH | H | H | H | H | H | α-OAc | H | ||
| 22 | Kaempulchraol T | H | β-OH | H | H | H | H | α-OAc | H | ||
| 23 | Kaempulchraol V | α-OH | β-OH | H | H | H | H | β-OAc | H | ||
| 24 | Kaempulchraol W | α-OH | β-OH | H | H | H | H | β-OH | H | ||
| 25 | 9 α-Hydroxyisopimara-8(14),15-dien-7-one | α-OH | H | H | H | H | H | =O | H | ||
| 26 | 7β,9 α-Dihydroxypimara-8(14),15-diene | α-OH | H | H | H | H | H | β-OH | H | ||
| 27 | Isopimara-8(14),15-dien-7-one | H | H | H | H | H | H | =O | H | K. roscoeana | [26] |
| 28 | (1S,5S,9S,10S,11R,13R)-1,11-Dihydroxypimara-8(14),15-diene | H | H | α-OH | H | H | α-OH | H | H | K. roscoeana K. marginata K. pulchra | [4,17,25,26,74,75] |
| 29 | (1R,2S,5S,9S,10S,11R,13R)-1,2,11-Trihydroxypimara-8(14),15-diene | H | H | α-OH | α-OH | H | α-OH | H | H | ||
| 30 | 7α-Hydroxyisopimara-8(14),15-diene | H | H | H | H | H | H | α-OH | H | K. roscoeana K. pulchra | |
| 31 | Sandaracopimaradien- 9α-ol-l-one | α-OH | H | =O | H | H | H | H | H | K. sp | [76] |
| 32 | 6β-Acetoxysandaracopimaradien-9α-ol-l-one | α-OH | β-OAc | =O | H | H | H | H | H | ||
| 33 | Sandaracopimaradien-6β,9α-diol-l-one | α-OH | β-OH | =O | H | H | H | H | H | ||
| 34 | 6β-Acetoxysandaracopimaradien-lα,9α-diol | α-OH | β-OAc | α-OH | H | H | H | H | H | ||
| 35 | Sandaracopimaradien- lα,6β,9α-triol | α-OH | β-OH | α-OH | H | H | H | H | H | ||
| 36 | Roscorane B | H | H | H | H | H | α-OH | H | OH | K. roscoeana | [26] |
| 37 | Roscorane C | H | β-OH | H | OH | H | H | OH | H | ||
| 38 | Roscorane D | H | H | H | OH | H | H | OH | OH | ||
| 39 | (1R,2S,5S,7S,9R,10S,13R)-1,2,7-Trihydroxypimara-8(14),15-diene | H | H | H | α-OH | H | H | β-OH | H | K. marginata | [75] |
| 40 | (1S,5S,7R,9R,10S,11R,13R)-1,7,11-Trihydroxypimara-8(14),15-diene | H | β-OH | H | H | H | H | α-OH | H | ||
| 41 | (1R,2S,5S,7S,9R,10S,13R)-1,2-Dihydroxypimara-8(14),15diene-7-one | H | H | H | α-OH | H | H | H | H | ||
 52–54 |  42–51 | ||||||||||
| No | Name | R1 | R2 | R3 | R4 | Plant | Ref | ||||
| 42 | Kaempulchraol A | H | β-OH | H | α-OMe | K. pulchra | [4,17,25,74] | ||||
| 43 | Kaempulchraol B | H | β-OH | H | β-OMe | ||||||
| 44 | Kaempulchraol C | H | β-OH | H | α-OH | ||||||
| 45 | Kaempulchraol D | H | β-OH | H | β-OH | ||||||
| 46 | Kaempulchraol G | H | β-OH | H | =O | ||||||
| 47 | Kaempulchraol N | α-OH | β-OH | H | α-OH | ||||||
| 48 | Kaempulchraol O | α-OH | β-OH | H | β-OMe | ||||||
| 49 | Kaempulchraol S | H | H | =O | α-OH | ||||||
| 50 | Kaempulchraol U | H | H | H | α-OH | ||||||
| 51 | Isopimara-8(9),15-dien-7-one | H | H | =O | H | K. roscoeana | [26] | ||||
| 52 | 8(14),15-Isopimaradiene-6α-ol | H | α-OH | H | --- | K. marginata | [36] | ||||
| 53 | 1α-Acetoxy-sandaracopimaradiene | α-OAc | H | H | - | ||||||
| 54 | 1α-Acetoxy-sandaraco pimaradien-2-one | α-OAc | =O | H | - | ||||||
| No | Name | Structure | Plant | Ref | |||||||
| 55 | (2R)-ent-2-Hydroxyisopimara-8(14),15-diene |  | K. pulchra | [4,17,25,74] | |||||||
| 56 | Kaemgalangol A |  | K. galanga | [16] | |||||||
| 57 | Roscorane A |  | K. roscoeana | [26] | |||||||
| 58 | R=OMe; Roscotane A |  | K. roscoeana | [26] | |||||||
| 59 | R=H; Roscotane B | ||||||||||
| 60 | Roscotane C |  | |||||||||
| 61 | Roscotane D |  | |||||||||
| 62 | R=H; Ar-abietatriene |  | |||||||||
| 63 | R=[=O]; 7-Dehydroabietanone | ||||||||||
| 64 | R=α-OH; Abieta-8,11,13-trien-7α-ol | ||||||||||
| 65 | Kaempfolienol |  | K. angustifolia | [33,34] | |||||||
| 66 | (12Z,14R)-Labda-8(17),12-dien-14,15,16-triol |  | K. roscoeana | [26] | |||||||
| 67 | Propadane A |  | K. elegans | [18] | |||||||
| 68 | R=H --- 8(17)-Labden-15-ol |  | |||||||||
| 69 | R=OH; Propadane B | ||||||||||
| 70 | Propadane C |  | K. pulchra | ||||||||
| 71 | Cleroda-2,4(18),14-trien-13-ol |  | K. pulchra | ||||||||
| 72 | R=H; Anticopalic acid |  | K. elegans | ||||||||
| 73 | R=Me; Methyl anticopalate | ||||||||||
| 74 | (+)-15,16-Eoxy-8(17),13(16),14-labdatriene |  | [18] | ||||||||
| 75 | (+)-Pumiloxide |  | |||||||||
| 76 | 13-Oxo-14,15-bis-nor-labd-8(17)-ene |  | |||||||||
| 77 | Anticopalol |  | K. elegans | ||||||||
| 78 | Labda-8(17),13(14)-diene-15,16-olide |  | |||||||||
| 79 | (+)-Labda-8(17),13(Z)-diene-15,16-diol |  | |||||||||
| 80 | Calcaratarin A |  | K. pulchra | ||||||||
| 81 | R=H; (-)-Kolavelool |  | [18] | ||||||||
| 82 | R= β-OH; (-)-2β-Hydroxykolavelool | ||||||||||
| 83 | R=β-OMe; Dysoxydensin E | ||||||||||
| 84 | 13-Epi-roseostachenone |  | |||||||||
| 85 | (+)-13-Epi-2α-hydroxykolavelool (13-epi-roseostachenol) |  | K. pulchra | ||||||||
 86–97 |  98–101 |  102–103 | |||||
| No | Name | R1 | R2 | R3 | R4 | Plant | Ref |
| 86 | 5,7-Dimethoxyflavone | H | Me | H | H | K. parviflora | [9,55,71,77] |
| 87 | 4‘,5,7-Trimethoxyflavone | H | Me | H | OMe | ||
| 88 | 3‘,4‘,5,7-Tetramethoxyflavone | H | Me | OMe | OMe | ||
| 89 | 3,5,7-Trimethoxyflavone | OMe | Me | H | H | ||
| 90 | 3,5,7,4‘-Tetramethoxyflavone | OMe | Me | H | OMe | ||
| 91 | 3,5,7,3‘,4‘-Pentamethoxyflavone | OMe | Me | OMe | OMe | ||
| 92 | 5-Hydroxy-7-methoxyflavone | H | H | H | H | ||
| 93 | 5-hydroxy-7,4‘-dimethoxyflavone | H | H | H | OMe | ||
| 94 | 5-Hydroxy-3,7-dimethoxyflavone | OMe | H | H | H | ||
| 95 | 5-Hydroxy-3,7,4‘-trimethoxyflavone | OMe | H | H | OMe | ||
| 96 | 5-Hydroxy-3,7,3‘,4‘-tetramethoxy flavone | OMe | H | OMe | OMe | ||
| 97 | 5,3‘-Dihydroxy-3,7,4‘-trimethoxyflavone | OMe | H | OH | OMe | ||
| 98 | Kaempferol | H | OH | - | - | K. galanga | [42] |
| 99 | Kaempferide | H | OMe | - | |||
| 100 | Tectochrysin | Me | H | - | - | K. parviflora | |
| 101 | Genkwanin | Me | OH | - | - | [46] | |
| 102 | Pinocembin | H | - | - | - | K. parviflora K. angustifolia | [71] |
| 103 | Pinostrobin | Me | - | - | - | ||
| 104 | Sakuranetin |  | |||||
| 105 | 2″,2″-Dimethylpyrano-[5″,6″:8,7]-flavone |  | K. pulchra | [18] | |||
 106–109 |  110–113 |  114–115 | |||||
 116–117 |  118–119 | ||||||
| No | Name | R1 | R2 | R3 | Plant | Ref | |
| 106 | Ethyl trans-p-methoxycinnamate | H | OMe | CH2Me | K. galanga | [13] | |
| 107 | Ferulic acid | OMe | OH | H | |||
| 108 | trans-p-Hydroxy-cinnamic acid | H | OH | H | |||
| 109 | trans-p-Methoxy cinnamic acid | H | H | CH2Me | |||
| 110 | p-Hydroxybenzoic acid | H | OH | COOH | [13,50] | ||
| 111 | p-Methoxybenzoic acid | H | OMe | COOH | |||
| 112 | Vanillic acid | OMe | OH | COOH | |||
| 113 | Methyl 3,4-dihydroxybenzoate | OH | OH | COOMe | |||
| 114 | Methyl (2R,3S)-2,3-dihydroxy-3-(4-methoxyphenyl) propanoate | Me | - | - | |||
| 115 | Ethyl-(2R,3S)-2,3-dihydroxy-3-(4-methoxyphenyl) propanoate | CH2Me | - | - | |||
| 116 | (1R,3R,5R)-1,5-Epoxy-3-hydroxy-1-(3,4-dihydroxyphenyl)-7-(3,4-dihydroxy phenyl) heptane | H | - | - | [13] | ||
| 117 | (1R,3R,5R)-1,5-Epoxy-3-hydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl) heptane 3-O-β-D-glucopyranoside | D-glc | - | - | |||
| 118 | 2‘-hydroxy-4‘,6‘-dimethoxychalcone | H | - | - | K. parviflora K. angustifolia | [24,71] | |
| 119 | 2‘-hydroxy-4,4‘,6‘-trimethoxychalcone | Me | - | - | |||
| No | Name | Structure | Plant | Ref | |||
| 120 | Desmethoxyyangonin |  | K. marginata | [36] | |||
| 121 | Bisdemethoxycurcumin |  | |||||
| 122 | 1-(4-Hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)heptane-1,2,3,5,6-pentaol |  | K. galanga | [13] | |||
| 123 | (3R,5S)-3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxy phenyl) heptane |  | K. galanga | ||||
| 124 | Phaeoheptanoxide |  | |||||
| 125 | Hedycoropyran B |  | |||||
| 126 | 1-O-4-Carbonxylphenyl-(6-O-4-hydroxybenzoyl)-β-D-glucopyranoside |  | |||||
| 127 | Dihydro-5,6-dehydrokawain |  | K. parviflora | [13,50] | |||
| 128 | R=OH; (-)-Hydroxypanduratin A |  | |||||
| 129, | R=OMe; (-)-Panduratin A | ||||||
| 130 | Cinnamaldehyde |  | K. galanga | [78] | |||
| 131 | R=Me; Crotepoxide |  | K. angustifolia | [24,33] | |||
| 132 | R=Benzen; Boesenboxide | ||||||
| 133 134 | R=H; Zeylenol R=Me; 6-methylzeylenol |  | |||||
| 135 | rel-(5aS,10bS)-5a,10b-Dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1–d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside] |  R=α-L-rha-(1→6)-β-D-glc | K. parviflora | [79] | |||
| 136 | rel-(5aS,10bR)-5a,10b-Dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1–d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside] |  R=α-L-rha-(1→6)-β-D-glc | |||||
| 137 | (2R,3S,4S)-3-O-[α-l-Rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-3′-O-methyl-ent-epicatechin-(2α→O→3,4α→ 4)-(5aS,10bS)-5a,10b-dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1,2-d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→ 6)-β-d-glucopyranoside] |  R=α-L-rha-(1→6)-β-D-glc | |||||
 138–139 | ||||
| No | Name | Structure | Plant | Ref |
| 138 | β-Sitosterol | R=H | K.marginata K. angustifolia | [24,33,36] |
| 139 | β-Sitosterol-β-D-glucoside | R=β-D-glc | ||
| 140 | Stigmasterol |  | ||
| 141 | (24S)-24-methyl-lanosta-9(11), 25-dien-3β-ol |  | K. angustifolia | [24] |
| No | Name | Plant | Ref |
|---|---|---|---|
| 142 | δ-3-Carene | K. galanga | [29,35,82,83] |
| 143 | E-Ethyl cinnamate | ||
| 144 | Ethyl-p-methoxycinnamate | ||
| 145 | γ-Cadinene | ||
| 146 | 1,8-Cineole | ||
| 147 | Trans-cinnamaldehyde | ||
| 148 | Borneol | ||
| 149 | Pentadecane | ||
| 150 | γ-car-3-ene | ||
| 151 | Linoleoyl chloride | ||
| 152 | Caryophyllene oxide | ||
| 153 | Cubenol | ||
| 154 | Caryophyllene | ||
| 155 | Limonene | ||
| 156 | Camphene | ||
| 157 | α-Pinene |
| K. angustifolia | K. elegans | K. galanga | K. marginata | K. parviflora | K. pulchra | |
|---|---|---|---|---|---|---|
| K. elegans | −0.539 | |||||
| K. galanga | −0.042 | −0.339 | ||||
| K. marginata | −0.500 | −0.241 | 0.615 | |||
| K. parviflora | 0.833 | −0.312 | 0.075 | −0.280 | ||
| K. pulchra | −0.675 | 0.053 | 0.378 | 0.938 | −0.372 | |
| K. roscoeana | −0.643 | −0.225 | 0.206 | 0.771 | −0.513 | 0.766 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-El Gawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients 2019, 11, 2396. https://doi.org/10.3390/nu11102396
Elshamy AI, Mohamed TA, Essa AF, Abd-El Gawad AM, Alqahtani AS, Shahat AA, Yoneyama T, Farrag ARH, Noji M, El-Seedi HR, et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients. 2019; 11(10):2396. https://doi.org/10.3390/nu11102396
Chicago/Turabian StyleElshamy, Abdelsamed I., Tarik A. Mohamed, Ahmed F. Essa, Ahmed M. Abd-El Gawad, Ali S. Alqahtani, Abdelaaty A. Shahat, Tatsuro Yoneyama, Abdel Razik H. Farrag, Masaaki Noji, Hesham R. El-Seedi, and et al. 2019. "Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review" Nutrients 11, no. 10: 2396. https://doi.org/10.3390/nu11102396
APA StyleElshamy, A. I., Mohamed, T. A., Essa, A. F., Abd-El Gawad, A. M., Alqahtani, A. S., Shahat, A. A., Yoneyama, T., Farrag, A. R. H., Noji, M., El-Seedi, H. R., Umeyama, A., Paré, P. W., & Hegazy, M.-E. F. (2019). Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients, 11(10), 2396. https://doi.org/10.3390/nu11102396