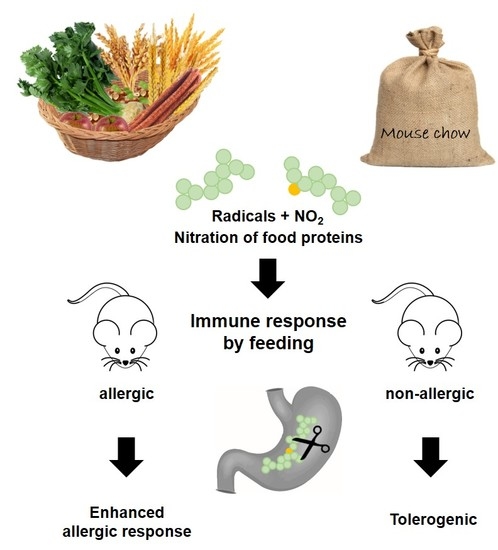
Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Extraction from Food and Stomach Content
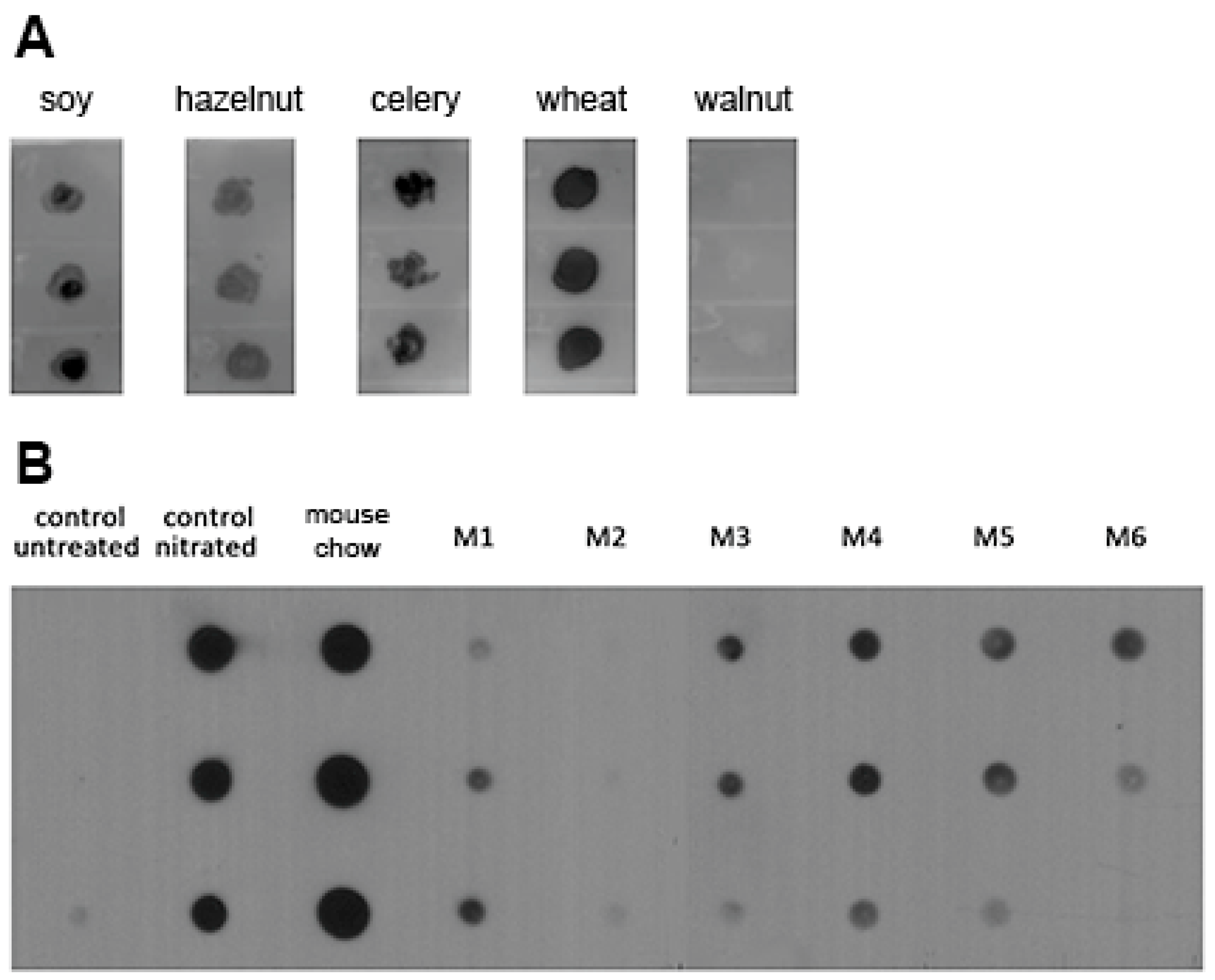
2.2. Dot Blot Experiments
2.3. Nitration of BLG
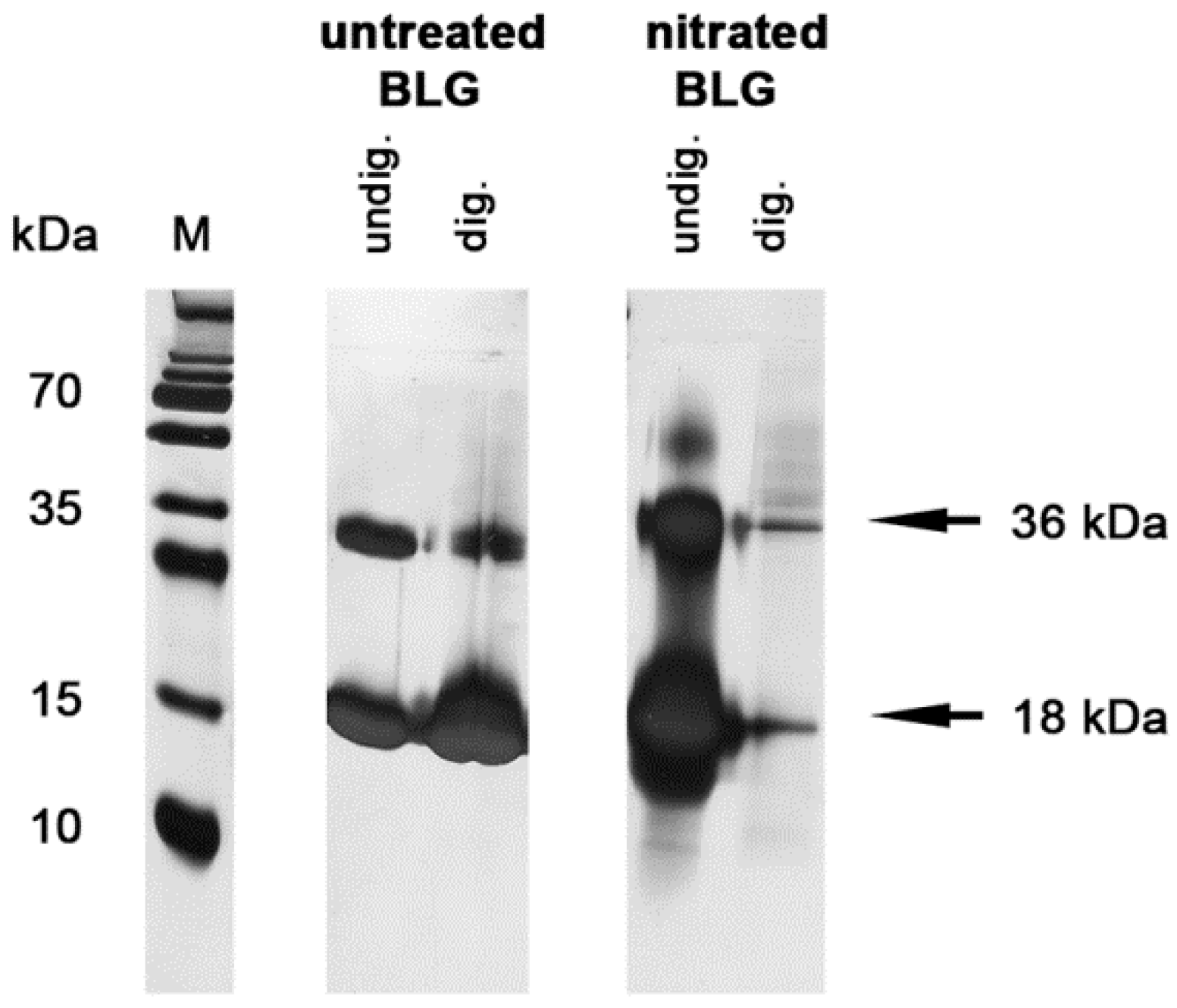
2.4. In Vitro Digestion Experiments
2.5. Animals
2.6. Oral Gavages to Non-Allergic Mice
2.7. Sensitization Protocol and Treatment of Allergic Mice
2.8. Measurement of Cytokine Release from Spleen Cells
2.9. Flow Cytometry Analysis of Regulatory T Cells
2.10. Detection of Allergen-Specific IgE, IgA, IgG1, and IgG2a in Serum
2.11. β-hexosaminidase Release Assay from Rat Basophil Leukemia (RBL-2H3) Cells
2.12. Detection of Allergen-Specific IgE and IgA in Intestinal Lavages
2.13. Detection of mMCP-1
2.14. Statistical Analysis
3. Results
3.1. Nitrated Proteins Are Present in Food and Stomach Content Extracts of Naïve Mice
3.2. Nitration Decreased Stability of BLG in Simulated Gastric Fluid
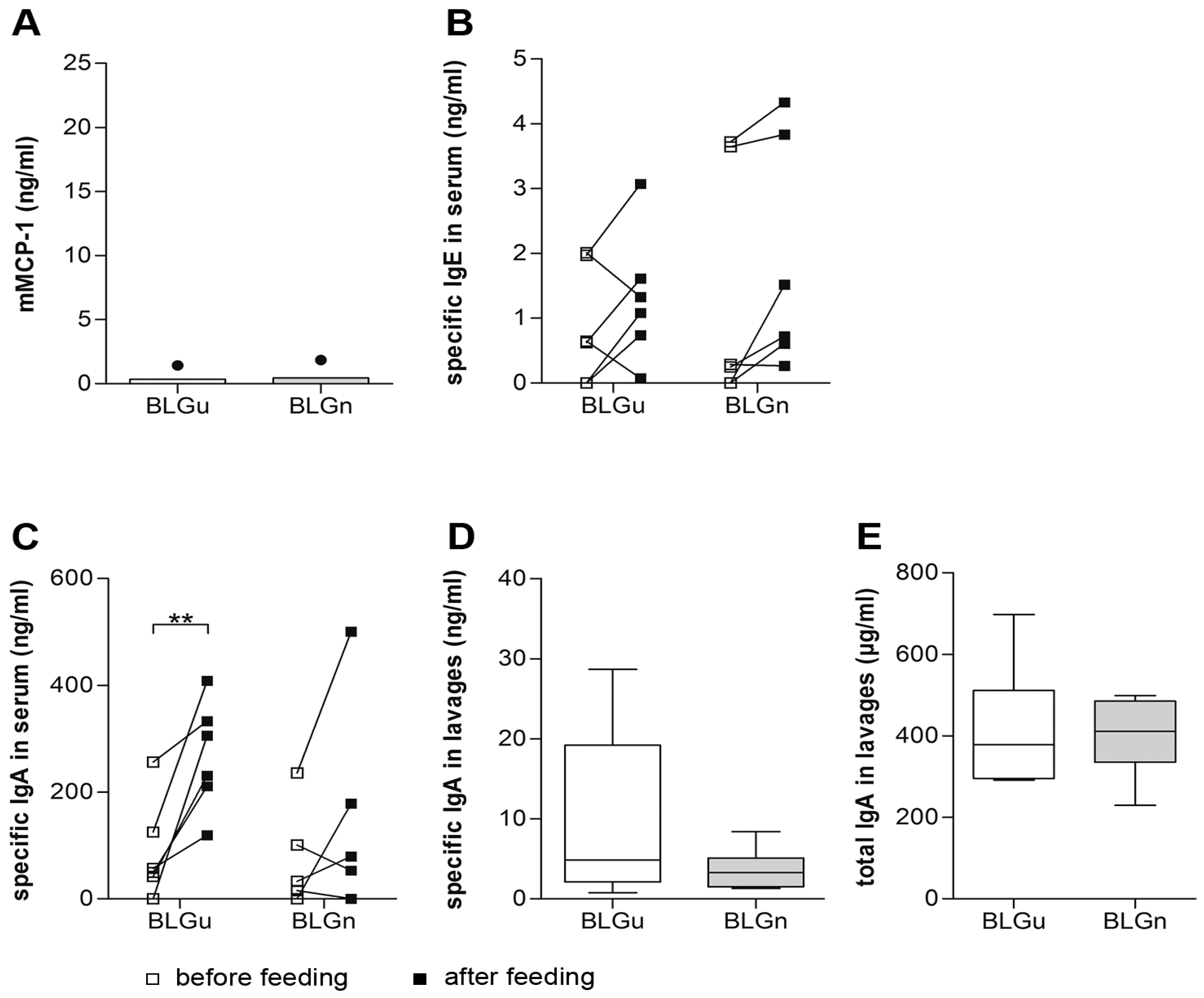
3.3. In Non-allergic Animals, BLGn Induced IgA, IL-10, and IFN-γ
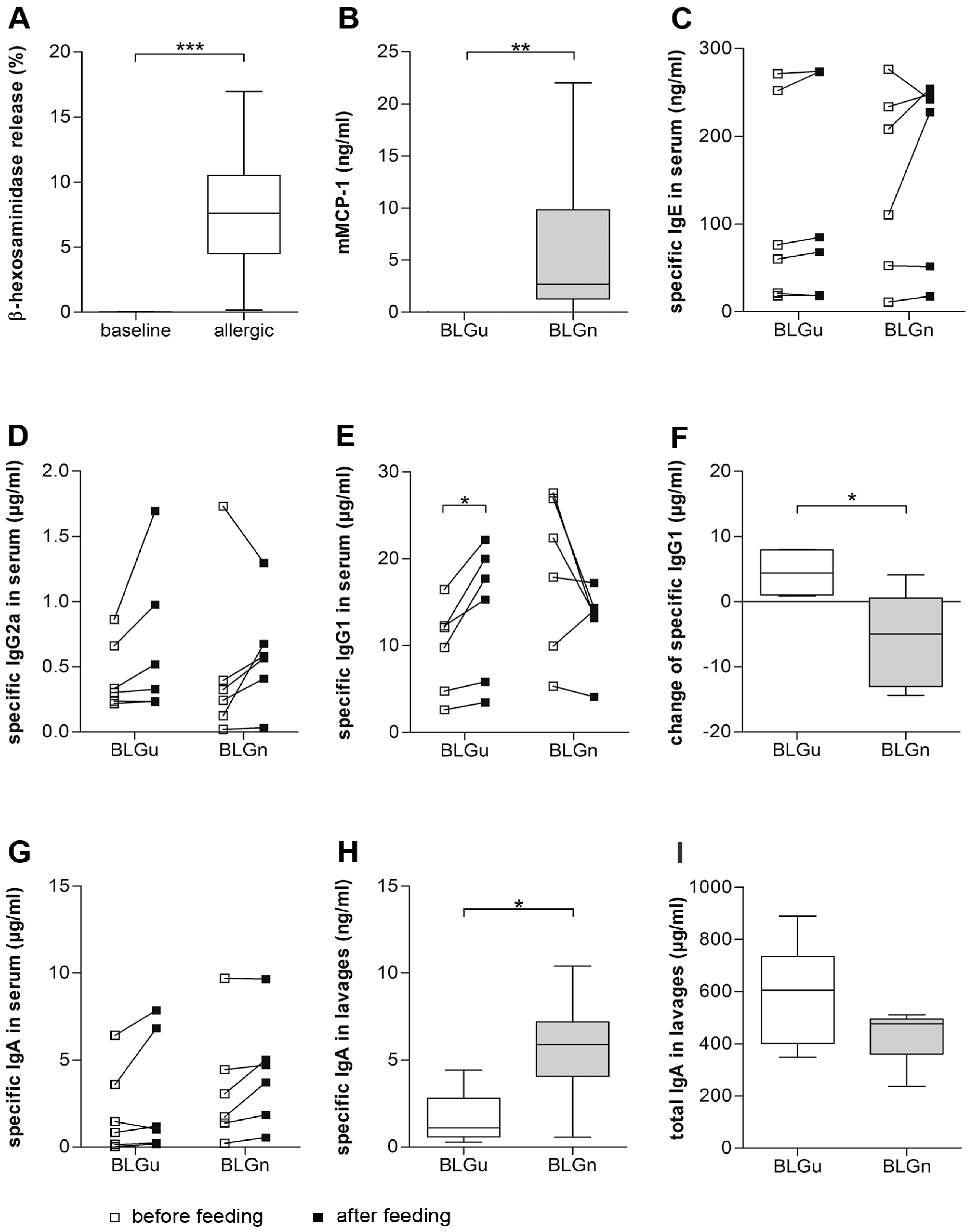
3.4. In BLGu-Allergic Mice, Ingestion of BLGn Led to Significantly Altered Antibody Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McClain, S.; Bowman, C.; Fernandez-Rivas, M.; Ladics, G.S.; Ree, R. Allergic sensitization: Food- and protein-related factors. Clin. Transl. Allergy 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Heilmann, M.; Ilchmann, A.; Vieths, S. The Maillard reaction and food allergies: Is there a link? Clin. Chem. Lab. Med. 2014, 52, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hilmenyuk, T.; Bellinghausen, I.; Heydenreich, B.; Ilchmann, A.; Toda, M.; Grabbe, S.; Saloga, J. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology 2010, 129, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Diesner, S.C.; Oostingh, G.J.; Selzle, K.; Pfaller, T.; Schultz, C.; Zhang, Y.; Krishnamurthy, D.; Starkl, P.; Knittelfelder, R.; et al. Nitration of the Egg-Allergen Ovalbumin Enhances Protein Allergenicity but Reduces the Risk for Oral Sensitization in a Murine Model of Food Allergy. PLoS ONE 2010, 5, e14210. [Google Scholar] [CrossRef]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Lundberg, J.O.; Radi, R.; Laranjinha, J. Intragastric nitration by dietary nitrite: Implications for modulation of protein and lipid signaling. Free Radic. Biol. Med. 2012, 52, 693–698. [Google Scholar] [CrossRef]
- Mills, E.N.C.; Sancho, A.I.; Rigby, N.M.; Jenkins, J.A.; Mackie, A.R. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009, 53, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Bottari, S.P. Protein tyrosine nitration: A signaling mechanism conserved from yeast to man. Proteomics 2015, 15, 185–187. [Google Scholar] [CrossRef]
- Ischiropoulos, H. Protein tyrosine nitration—An update. Arch. Biochem. Biophys. 2009, 484, 117–121. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Sosedova, Y.; Rouviere, A.; Yang, H.; Zhang, Y.; Abbatt, J.P.; Ammann, M.; Poschl, U. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat. Chem. 2011, 3, 291–295. [Google Scholar] [CrossRef]
- Lang-Yona, N.; Shuster-Meiseles, T.; Mazar, Y.; Yarden, O.; Rudich, Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: Outdoor exposure study supported by laboratory experiments. Sci. Total Environ. 2016, 541, 365–371. [Google Scholar] [CrossRef]
- Villaverde, A.; Morcuende, D.; Estevez, M. Effect of curing agents on the oxidative and nitrosative damage to meat proteins during processing of fermented sausages. J. Food Sci. 2014, 79, C1331–C1342. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.; Parra, V.; Estevez, M. Oxidative and nitrosative stress induced in myofibrillar proteins by a hydroxyl-radical-generating system: Impact of nitrite and ascorbate. J. Agric. Food Chem. 2014, 62, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Vossen, E.; De Smet, S. Protein oxidation and protein nitration influenced by sodium nitrite in two different meat model systems. J. Agric. Food Chem. 2015, 63, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Stagsted, J.; Bendixen, E.; Andersen, H.J. Identification of specific oxidatively modified proteins in chicken muscles using a combined immunologic and proteomic approach. J. Agric. Food Chem. 2004, 52, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, V.H.; Otles, S. Determination of 3-nitrotyrosine in food protein suspensions. Talanta 2017, 171, 81–89. [Google Scholar] [CrossRef]
- Ara, J.; Przedborski, S.; Naini, A.B.; Jackson-Lewis, V.; Trifiletti, R.R.; Horwitz, J.; Ischiropoulos, H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc. Natl. Acad. Sci. USA 1998, 95, 7659–7663. [Google Scholar] [CrossRef] [Green Version]
- Yeo, W.-S.; Kim, Y.J.; Kabir, M.H.; Kang, J.W.; Kim, K.P. Mass spectrometric analysis of protein tyrosine nitration in aging and neurodegenerative diseases. Mass Spectrom. Rev. 2014, 34, 166–183. [Google Scholar] [CrossRef]
- Oldreive, C.; Rice-Evans, C. The mechanisms for nitration and nitrotyrosine formation in vitro and in vivo: Impact of diet. Free Radic. Res. 2001, 35, 215–231. [Google Scholar] [CrossRef]
- Gruijthuijsen, Y.K.; Grieshuber, I.; Stöcklinger, A.; Tischler, U.; Fehrenbach, T.; Weller, M.G.; Vogel, L.; Vieths, S.; Pöschl, U.; Duschl, A. Nitration Enhances the Allergenic Potential of Proteins. Int. Arch. Allergy Immunol. 2006, 141, 265–275. [Google Scholar] [CrossRef]
- Birnboim, H.C.; Lemay, A.M.; Lam, D.K.; Goldstein, R.; Webb, J.R. Cutting edge: MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J. Immunol. 2003, 171, 528–532. [Google Scholar] [CrossRef]
- Diesner, S.C.; Schultz, C.; Ackaert, C.; Oostingh, G.J.; Ondracek, A.; Stremnitzer, C.; Singer, J.; Heiden, D.; Roth-Walter, F.; Fazekas, J.; et al. Nitration of beta-Lactoglobulin but Not of Ovomucoid Enhances Anaphylactic Responses in Food Allergic Mice. PLoS ONE 2015, 10, e0126279. [Google Scholar] [CrossRef] [PubMed]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of food allergens to digestion in vitro. Nat. Biotechnol. 1996, 14, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Pali-Scholl, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Diesner, S.C.; Knittelfelder, R.; Krishnamurthy, D.; Pali-Scholl, I.; Gajdzik, L.; Jensen-Jarolim, E.; Untersmayr, E. Dose-dependent food allergy induction against ovalbumin under acid-suppression: A murine food allergy model. Immunol. Lett. 2008, 121, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2012, 51, 311–321. [Google Scholar]
- Li, X.-M.; Schofield, B.H.; Huang, C.-K.; Kleiner, G.I.; Sampson, H.A. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999, 103, 206–214. [Google Scholar] [CrossRef]
- Dibbern, D.A., Jr.; Palmer, G.W.; Williams, P.B.; Bock, S.A.; Dreskin, S.C. RBL cells expressing human Fc epsilon RI are a sensitive tool for exploring functional IgE-allergen interactions: Studies with sera from peanut-sensitive patients. J. Immunol. Methods 2003, 274, 37–45. [Google Scholar] [CrossRef]
- Ischiropoulos, H. Biological Tyrosine Nitration: A Pathophysiological Function of Nitric Oxide and Reactive Oxygen Species. Arch. Biochem. Biophys. 1998, 356, 1–11. [Google Scholar] [CrossRef]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef]
- Singer, I.I.; Kawka, D.W.; Scott, S.; Weidner, J.R.; Mumford, R.A.; Riehl, T.E.; Stenson, W.F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996, 111, 871–885. [Google Scholar] [CrossRef]
- Dremina, E.S.; Sharov, V.S.; Schöneich, C. Protein tyrosine nitration in rat brain is associated with raft proteins, flotillin-1 and α-tubulin: Effect of biological aging. J. Neurochem. 2005, 93, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Andersen, J.K. Nitrosylation and nitration of mitochondrial complex I in Parkinson’s disease. Free Radic. Res. 2011, 45, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Correia, M.G.; Fernandes, R.C.; Goncalves, J.S.; Laranjinha, J. Dietary nitrite induces occludin nitration in the stomach. Free Radic. Res. 2016, 50, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Lundberg, J.O.; Mann, G.E.; Radi, R.; Laranjinha, J. Pepsin is nitrated in the rat stomach, acquiring antiulcerogenic activity: A novel interaction between dietary nitrate and gut proteins. Free Radic. Biol. Med. 2013, 58, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Franze, T.; Weller, M.G.; Niessner, R.; Pöschl, U. Protein Nitration by Polluted Air. Environ. Sci. Technol. 2005, 39, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Franze, T.; Weller, M.G.; Niessner, R.; Poschl, U. Enzyme immunoassays for the investigation of protein nitration by air pollutants. Analyst 2003, 128, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Bruschi, M.; Citterio, S.; Bolzacchini, E.; Ferrero, L.; Sangiorgi, G.; Asero, R.; Perrone, M.G. Nitration of pollen aeroallergens by nitrate ion in conditions simulating the liquid water phase of atmospheric particles. Sci. Total Environ. 2016, 573, 1589–1597. [Google Scholar] [CrossRef]
- Hong, Q.; Zhou, S.; Zhao, H.; Peng, J.; Li, Y.; Shang, Y.; Wu, M.; Zhang, W.; Lu, S.; Li, S.; et al. Allergenicity of recombinant Humulus japonicus pollen allergen 1 after combined exposure to ozone and nitrogen dioxide. Environ. Pollut. 2018, 234, 707–715. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Wheat Data-All Years. Available online: https://www.ers.usda.gov/data-products/wheat-data/ (accessed on 21 August 2019).
- Weidmann, E.; Samadi, N.; Klems, M.; Heiden, D.; Seppova, K.; Ret, D.; Untersmayr, E. Mouse Chow Composition Influences Immune Responses and Food Allergy Development in a Mouse Model. Nutrients 2018, 10, 1775. [Google Scholar] [CrossRef]
- Fu, T.-J. Digestion Stability as a Criterion for Protein Allergenicity Assessment. Ann. N. Y. Acad. Sci. 2002, 964, 99–110. [Google Scholar] [CrossRef]
- Fu, T.J.; Abbott, U.R.; Hatzos, C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J. Agric. Food Chem. 2002, 50, 7154–7160. [Google Scholar] [CrossRef] [PubMed]
- Trout, G.E.; Fruton, J.S. The side-chain specificity of pepsin. Biochemistry 1969, 8, 4183–4190. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Jensen-Jarolim, E. Mechanisms of type I food allergy. Pharmacol. Ther. 2006, 112, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroek, L.A.; van Esch, B.C.; Hofman, G.A.; den Hartog Jager, C.F.; Nauta, A.J.; Willemsen, L.E.; Bruijnzeel-Koomen, C.A.; Garssen, J.; van Hoffen, E.; Knippels, L.M. Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow’s milk allergy. Pediatr. Allergy Immunol. 2013, 24, 656–664. [Google Scholar] [CrossRef]
- Pecquet, S.; Bovetto, L.; Maynard, F.; Fritsché, R. Peptides obtained by tryptic hydrolysis of bovine β-lactoglobulin induce specific oral tolerance in mice. J. Allergy Clin. Immunol. 2000, 105, 514–521. [Google Scholar] [CrossRef]
- Akdis, C.A.; Blaser, K. Role of IL-10 in allergen-specific immunotherapy and normal response to allergens. Microbes Infect. 2001, 3, 891–898. [Google Scholar] [CrossRef]
- Vila, L.; Beyer, K.; Järvinen, K.M.; Chatchatee, P.; Bardina, L.; Sampson, H.A. Role of conformational and linear epitopes in the achievement of tolerance in cow’s milk allergy. Clin. Exp. Allergy 2001, 31, 1599–1606. [Google Scholar] [CrossRef]
- Haddad, Z.H.; Kalra, V.; Verma, S. IgE antibodies to peptic and peptic-tryptic digests of betalactoglobulin: Significance in food hypersensitivity. Ann. Allergy 1979, 42, 368–371. [Google Scholar]
- Strait, R.T.; Mahler, A.; Hogan, S.; Khodoun, M.; Shibuya, A.; Finkelman, F.D. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 982–989. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
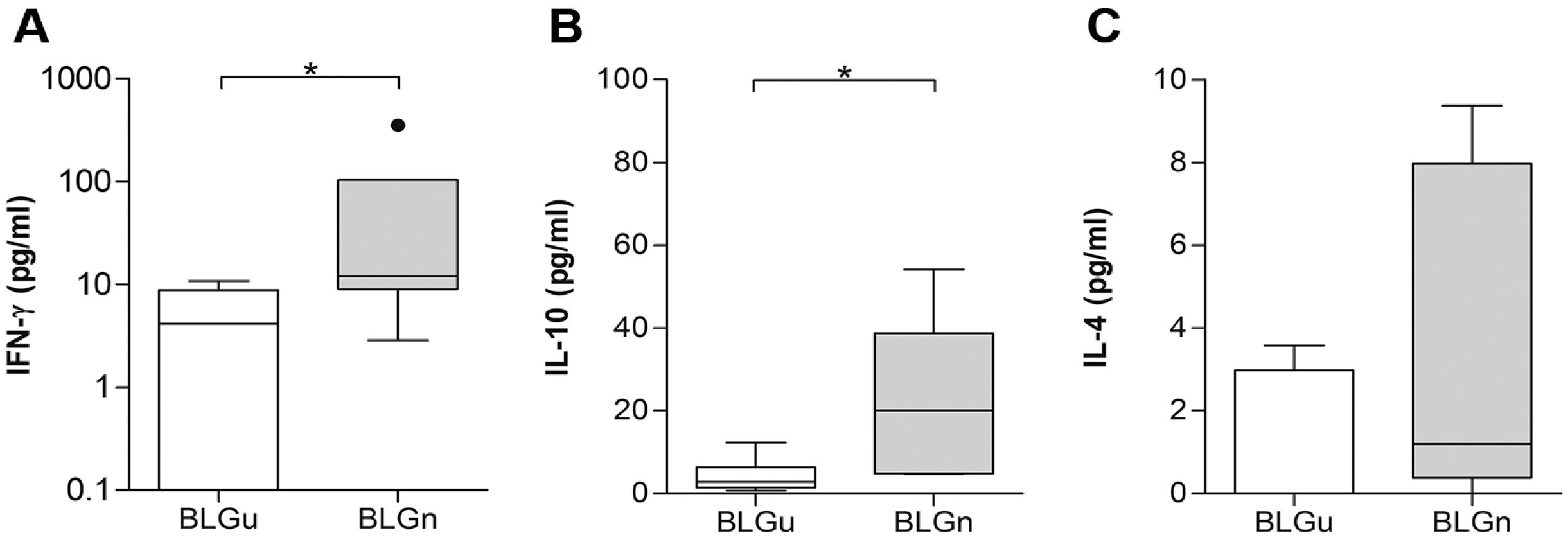

| Cytokine | BLGu Median (IQR) | BLGn Median (IQR]) | p Value |
|---|---|---|---|
| IFN-γ (pg/mL) | 59.65 (26.90–201.00) | 442.40 (128.00–781.50) | 0.03 |
| IL-10 (pg/mL) | 0.00 (0.00–8.59) | 0.00 (0.00–3.38) | 0.60 |
| IL-4 (pg/mL) | 8.75 (5.86–16.19) | 4.13 (1.86–23.81) | 0.63 |
| Immune Status | Gavage | Treg Count (Pooled Lymph Node Cells) | Treg Count (Spleens; mean ± SEM) | p Value | Tregs (%; Pooled Lymph Node Cells) | Tregs (%; Spleens; mean ± SEM) | p Value |
|---|---|---|---|---|---|---|---|
| non-allergic | BLGu | 495 | 382.0 ± 15.0 | 0.47 | 5.06 | 10.8 ± 0.46 | 0.23 |
| BLGn | 529 | 369.5 ± 6.90 | 6.59 | 11.5 ± 0.34 | |||
| allergic | BLGu | 474 | 319.3 ± 21.6 | 0.56 | 8.29 | 9.76 ± 1.05 | 0.40 |
| BLGn | 677 | 333.5 ± 23.2 | 10.1 | 10.8 ± 0.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondracek, A.S.; Heiden, D.; Oostingh, G.J.; Fuerst, E.; Fazekas-Singer, J.; Bergmayr, C.; Rohrhofer, J.; Jensen-Jarolim, E.; Duschl, A.; Untersmayr, E. Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model. Nutrients 2019, 11, 2463. https://doi.org/10.3390/nu11102463
Ondracek AS, Heiden D, Oostingh GJ, Fuerst E, Fazekas-Singer J, Bergmayr C, Rohrhofer J, Jensen-Jarolim E, Duschl A, Untersmayr E. Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model. Nutrients. 2019; 11(10):2463. https://doi.org/10.3390/nu11102463
Chicago/Turabian StyleOndracek, Anna S., Denise Heiden, Gertie J. Oostingh, Elisabeth Fuerst, Judit Fazekas-Singer, Cornelia Bergmayr, Johanna Rohrhofer, Erika Jensen-Jarolim, Albert Duschl, and Eva Untersmayr. 2019. "Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model" Nutrients 11, no. 10: 2463. https://doi.org/10.3390/nu11102463
APA StyleOndracek, A. S., Heiden, D., Oostingh, G. J., Fuerst, E., Fazekas-Singer, J., Bergmayr, C., Rohrhofer, J., Jensen-Jarolim, E., Duschl, A., & Untersmayr, E. (2019). Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model. Nutrients, 11(10), 2463. https://doi.org/10.3390/nu11102463