Abstract
Gynostemma pentaphyllum is widely used in Asia as a herbal medicine to treat type 2 diabetes, dyslipidemia, and inflammation. Here, we investigated the anti-obesity effect and underlying mechanism of G. pentaphyllum extract (GPE) enriched in gypenoside L, gypenoside LI, and ginsenoside Rg3 and obtained using a novel extraction method. Five-week-old male C57BL/6N mice were fed a control diet (CD), high-fat diet (HFD), HFD + 100 mg/kg body weight (BW)/day GPE (GPE 100), HFD + 300 mg/kg BW/day GPE (GPE 300), or HFD + 30 mg/kg BW/day Orlistat (Orlistat 30) for 8 weeks. The HFD-fed mice showed significant increases in body weight, fat mass, white adipose tissue, and adipocyte hypertrophy compared to the CD group; but GPE inhibited those increases. GPE reduced serum levels of triglyceride, total cholesterol, and LDL-cholesterol, without affecting HDL-cholesterol. GPE significantly increased AMPK activation and suppressed adipogenesis by decreasing the mRNA expression of CCAAT/enhancer binding protein-α (C/EBPα), peroxisome proliferator-activated receptor-γ (PPARγ), sterol regulatory element-binding protein-1c (SREBP1c), PPARγ coactivator-1α, fatty acid synthase (FAS), adipocyte protein 2 (AP2), and sirtuin 1 (SIRT1) and by increasing that of carnitine palmitoyltransferase (CPT1) and hormone- sensitive lipase (HSL). This study demonstrated the ameliorative effect of GPE on obesity and elucidated the underlying molecular mechanism.
1. Introduction
The prevalence of obesity has increased worldwide over the past 50 years and has reached pandemic levels. Once considered a problem only in high-income countries, obesity is now dramatically on the rise in low- and middle-income countries. Obesity is a major life-threatening health problem because it substantially increases the risk of various chronic diseases, such as diabetes, cardiovascular diseases, and cancers, thus contributing to a decline in both quality of life and life expectancy [1,2,3]. Therefore, the prevention and treatment of obesity are extremely important. Several anti-obesity drugs have been marketed in the last few years but have been successively withdrawn because of serious adverse effects. Only a few drugs are currently prescribed for treating obesity [4,5,6]. Herbal remedies are recognized as an important component of human health care as they have few side effects [7]. Therefore, there is a growing interest in herbal remedies and functional foods [4,5,6]. However, low bioavailability is a concern, as it may limit or even interfere with the effectiveness of herbal remedies and remains a major challenge in the development of clinically useful functional foods and medicines. The development of appropriate extraction methods to increase bioavailability and increase the contents of effective compounds may offer a solution.
Gynostemma pentaphyllum (Thunb.) Makino is an herbaceous climbing vine of the family Cucurbitaceae that is widely distributed in South and East Asia. Its leaves have been traditionally used in Korea, China, and Japan as a herbal medicine or tea. G. pentaphyllum is also widely used as a health supplement in beverages, biscuits, noodles, face washes, and bath oils [8]. Because of its health benefits, G. pentaphyllum has recently attracted increasing attention. Phytochemical studies have revealed that G. pentaphyllum contains saponins, flavonoids, polysaccharides, amino acids, vitamins, and some essential elements [9,10,11,12]. In vitro and in vivo studies have suggested that extracts of G. pentaphyllum exert various beneficial bioactivities, including antimicrobial [13], antioxidant [14,15], anticancer [16,17], anti-inflammatory [17,18], antidiabetic [19,20,21], antilipidemic [22], neuroprotective [23], and anti-obesity [24,25] activities. The major bioactive phytochemicals of G. pentaphyllum are dammarane-type triterpene saponins called gypenosides [26,27]. Around 180 gypenosides have been identified in G. pentaphyllum to date [28] and have been reported to have numerous beneficial activities, such as antioxidant [29], anticancer [30], hypoglycemic [31], hypolipidemic [32], and hepatoprotective [29,32] activities. These beneficial activities are related to the levels and characteristics of the gypenosides, including molecular weight, monosaccharide composition, and chemical structure, which are influenced by the extraction and purification methods used to obtain specific gypenoside-enriched products [33]. Therefore, to produce and apply G. pentaphyllum extract to functional foods and nutraceuticals, standardization to control the amount and characteristics of gypenosides is required.
In Vivo studies have indicated that G. pentaphyllum is a safe substance that does not cause side effects, even when ingested over a long period. No toxicity or mortality was observed upon long-term administration of up to 750 mg/kg G. pentaphyllum in rats [34]. Standardized water extract of G. pentaphyllum showed no toxicity in rats [35]. Therefore, G. pentaphyllum is expected to be safe for use as a chemotherapeutic agent. Recently, we developed a G. pentaphyllum extract (GPE) with much higher contents of gypenoside L (1.8%, w/w), gypenoside LI (1.4%, w/w), and ginsenoside Rg3 (0.15%, w/w) than extracts produced by conventional methods [36]. In an in vitro study using L6 skeletal muscle cells, we found that GPE stimulated glucose uptake via activation of AMP-activated protein kinase (AMPK) [36]. The current study aimed to evaluate the in vivo anti-obesity efficacy of GPE. To this end, dietary obesity was induced in C57BL/6N mice by feeding with a high-fat diet (HFD). The effects of GPE at various concentrations on body weight, fat mass, blood lipid profile, and adipogenic transcription factors and their target genes were examined.
2. Materials and Methods
2.1. Preparation of GPE
GPE was prepared at BTC Corporation according to a previously described method [36]. In brief, the leaves of G. pentaphyllum were dried using an electric heat controller (i.e., electric fan). Extracts were prepared from 1 kg of leaves with hot water (20 L) and 50% EtOH aqueous solution (15 L). Supernatants of the two extractions were collected and combined, and then filtered. The filtrate was vacuum-evaporated to obtain GPE. Gypenoside L, gypenoside LI, and gynsenoside Rg3 were quantified by high-performance liquid chromatography. GPE contains gypenoside L (1.8%, w/w), gypenoside LI (1.4%, w/w), and ginsenoside Rg3 (0.15%, w/w).
2.2. Animals
Four-week-old male C57BL/6N mice were purchased from Doo Yeol Biotech Co. Ltd. (Seoul, Korea) and housed in controlled standard conditions (23 ± 3 °C, 50 ± 10% relative humidity, and a 12-h light/dark cycle). Mice were acclimatized for one week before use and had free access to a standard non-purified rodent diet (Cargill Agri Purina, Inc., Seongnam, Korea) and water. All animal experiments were conducted according to the protocols ratified by the Institutional Animal Care and Use Committee of Hallym University (approval number: Hallym 2018-31).
2.3. Experimental Design and Treatment
After one week of acclimation, C57BL/6N mice were randomly divided into five groups (n = 10 per group) fed the following diets: (1) control diet (CD); (2) HFD; (3) HFD + 100 mg/kg body weight (BW)/day GPE (GPE 100); (4) HFD + 300 mg/kg BW/day GPE (GPE 300); (5) HFD + 30 mg/kg BW/day Orlistat (positive control material) (Orlistat 30). The CD (containing 10 kcal% as fat, Cat No. D12450B) and HFD (containing kcal% as fat, Cat No. D12452) were purchased from Research Diets, Inc. (New Brunswick, NJ, USA). The mice were given feed and water ad libitum throughout the study period. GPE or Orlistat dissolved in saline solution was administered daily by oral gavage for eight weeks. Mice in the CD and HFD groups were given an equal volume of saline solution by oral gavage at the same time. Body weight was measured once a week and food intake monitored daily throughout the experimental period.
At the end of the experiment, all mice were fasted for 16 h and anesthetized with tribromoethanol diluted in tertiary amyl alcohol. Whole body composition (lean body mass percentage and fat mass percentage) was measured using dual-energy X-ray absorptiometry (PIXImusTM; GE Lunar, Madison, WI, USA). Then, blood was collected from the orbital vein and serum was obtained by centrifugation at 3,000 rpm at 4 °C for 20 min. Adipose tissues were rapidly excised, rinsed, and weighed.
2.4. Biochemical Analyses of Serum
Serum levels of glucose, triglyceride, total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol, as well as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured using a blood chemistry autoanalyzer (KoneLab 20XT, Thermo Fisher Scientific, Vantaa, Finland).
2.5. Histological Analysis
Epididymal adipose tissues were fixed in 4% paraformaldehyde solution, embedded in paraffin, and cut into 5-μm sections. Tissue sections were stained with hematoxylin and eosin (H&E) and examined and imaged under a light microscope (AxioImager; Carl Zeiss, Jena, Germany). Adipocyte number and size were quantified using the AxioVision Imaging System (Carl Zeiss) with a magnifying power of 200×. Slides were examined in a blinded manner.
2.6. Quantitative Reverse Transcription PCR (RT-qPCR)
Total RNA was extracted from the epididymal adipose tissues with Trizol (Cat No. 15596018, Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA content and purity were measured using a micro-volume spectrophotometer (BioSpec-nano, Shimadzu, Kyoto, Japan). RT-qPCR was conducted using a Rotor-Gene 3000 instrument (Corbett Research, Mortlake, Australia) and Rotor-GeneTM SYBR Green kit (Cat No. 204074, Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction. The sequences of the primers used in this study are listed in Table 1. The primers used in this study were ordered and synthesized in Bioneer Co. (Daejeon, Korea). Thermal cycles were: 94 °C for 3 minutes, 40 cycles of 95 °C for 10 seconds, 60 °C for 15 seconds, and 72 °C for 20 seconds. The results were analyzed with Rotor-Gene 6000 Series System Software program, version 6 (Corbett Research), and target mRNA levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (Gapdh).

Table 1.
Sequences of primers used in this study.
2.7. Western Blotting Analysis
Epididymal adipose tissue samples were homogenized with a polytron tissue homogenizer in ice-cold lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 100 mM NaF, 10 mM sodium pyrophosphate, and 1% Triton X-100) containing 5 mM iodoacetic acid and protease inhibitor (iNtRON Biotechnology, Inc., Seongnam, Korea), then solubilized at 4 °C for 40 min. Insoluble material was removed by centrifugation at 14,800× g for 10 min and the supernatant was collected and used for Western blot analyses. The protein contents of the lysates were measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Western blot analyses were conducted as described previously [37]. The antibodies against p-AMPKα (T172) (Cat No. 2535), AMPKα (Cat No. 2532), and β-actin (Cat No. 3700) were obtained from Cell Signal Technology (Beverly, MA, USA). Anti-rabbit IgG HRP-linked antibody (Cat No. 7074) and anti-mouse IgG HRP-linked antibody (7076) were obtained from Cell Signal Technology. Blots were detected with LuminataTM Forte Western HRP Substrate (Cat No. WBLUF0500, Millipore, Billerica, MA, USA). The relative protein abundances were determined using the ImageQuantTM LAS 500 imaging system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Protein expression levels were normalized to that of β-actin.
2.8. Statistical Analysis
All data are expressed as the mean ± SD. Student’s t-test was used to test differences between the CD and HFD groups. Analysis of variance (ANOVA) followed by Duncan’s multiple comparison test was used to compare means between the HFD, GPE 100, GPE 300, and Orlistat 30 groups. P < 0.05 was considered significant.
3. Results
3.1. Effects of GPE on Body Weight and Fat Mass in HFD-Induced Obese C57BL/6N Mice
Consumption of HFD for eight weeks caused significant increases in body weight (Table 2), but mean body weight gain and fat mass percentage were significantly lower in the GPE-treated groups than in the HFD group. Orlistat, a commonly used weight loss agent, has a positive effect on HFD-induced obesity in murine models [38]. Notably, a greater anti-obesity effect (in terms of weight gain) was observed in the GPE 300 group than in the Orlistat 30 group. Lean body mass was significantly decreased by HFD (P < 0.001), but this decrease tended to be suppressed by GPE treatment. Food intake was significantly lower in the HFD group than in the CD group (P < 0.001). The GPE 100 and GPE 300 groups had significantly lower food intake than the HFD group. The food efficiency ratio was significantly higher in the HFD group than in the CD group and was lowered dose-dependently by GPE treatment (P < 0.001).

Table 2.
Effect of Gynostemma pentaphyllum extract (GPE) treatment on body weight, body composition, and food intake in high-fat diet (HFD)-fed C57BL/6N mice.
3.2. Effects of GPE on Serum Glucose, Lipid, ALT, and AST Levels
Serum glucose was significantly increased in the HFD group compared to the CD group (P < 0.001). Treatment with GPE dose-dependently attenuated the increase in serum glucose in HFD-fed mice (Table 3). Serum triglyceride, total cholesterol, and LDL-cholesterol levels were also significantly increased by HFD feeding (P < 0.001, P < 0.01, P < 0.01, respectively). Serum triglyceride was significantly reduced in both the GPE 100 and GPE 300 groups compared to the HFD group. Serum total cholesterol was decreased in the GPE 300 group compared to the HFD group. LDL-cholesterol level was significantly decreased by GPE treatment. On the other hand, serum HDL-cholesterol was significantly increased in the HFD group compared to the CD group (P < 0.05), and GPE or Orlistat treatment had no effect on serum HDL-cholesterol (Table 3). ALT and AST activity levels were measured to determine liver toxicity by GPE treatment. ALT and AST activities were significantly increased by HFD feeding (P < 0.001, P < 0.01, respectively), and the increases were significantly suppressed by GPE treatment (Table 3).

Table 3.
Effect of GPE treatment on serum glucose and lipid levels and serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in HFD-fed C57BL/6N mice.
3.3. Effects of GPE on Adipose Tissue Accumulation
To investigate the effect of GPE on adipose tissue weight, the weight of white adipose tissue (epididymal, retroperitoneal, mesenteric, and inguinal fat) was monitored. As shown in Table 4, all white adipose tissues were significantly increased (albeit to different levels) in the HFD group compared to the CD group (P < 0.001). When GPE was given, the increase in weight of each adipose was dose-dependently suppressed. Figure 1A shows that the adipocyte size in epididymal adipose tissues was larger in the HFD group than in the CD group, and GPE treatment suppressed this adipocyte enlargement. The number of adipocytes was significantly higher in the HFD group than in the CD group (P < 0.001). GPE treatment significantly lowered the number of epididymal adipocytes with a diameter >120 µm (Figure 1B).

Table 4.
Effect of GPE treatment on adipose tissue weights in HFD-fed C57BL/6N mice.
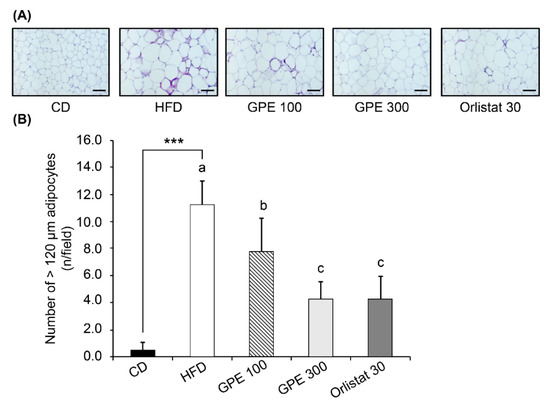
Figure 1.
Effect of GPE treatment on morphological changes in epididymal adipose tissues in HFD-fed C57BL/6N mice. GPE was administrated by oral gavage for 8 weeks to mice fed an HFD. The epididymal adipose tissue was excised, fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. Tissue sections were stained with H&E. Adipocyte number and size were quantified. (A) Representative H&E-stained epididymal adipose tissue sections (n = 10). 200× magnification, Scale bar = 100 μm. (B) The number of adipocytes over 120 μm was counted. Each bar represents the mean ± SD (n = 10). *** P < 0.001 significantly different from the CD group. Different letters indicate significant differences among the HFD, GPE 100, GPE 300, and Orlistat 30 groups at P < 0.05. CD: control diet; HFD: high-fat diet; GPE: G. pentaphyllum extract; GPE 100: HFD + 100 mg/kg body weight/day; GPE 300: HFD + 300 mg/kg body weight/day GPE; Orlistat 30: HFD + 30 mg/kg body weight/day Orlistat.
3.4. Effects of GPE on the Expression of Key Factors in Adipogenesis and Fat Oxidation
To investigate the molecular basis for the anti-obesity effect of GPE, we measured the expression of adipogenic transcription factors, including CCAAT/enhancer binding proteinα (C/EBPα), peroxisome proliferator-activated receptor-γ (PPARγ), and sterol regulatory element-binding protein-1c (SREBP-1c) as well as their target genes in epididymal adipose tissues. Compared to the CD group, the HFD group exhibited dramatically increased Cebpa, Pparg, and Srebp1c mRNA expression (P < 0.001). GPE treatment noticeably suppressed the elevated mRNA expression of these three transcription factors by HFD (Figure 2). As for fatty acid biosynthesis, the mRNA levels of fatty acid synthase (Fas) and adipocyte protein 2 (Ap2) were higher in the HFD group than in the CD group, whereas they were significantly reduced in the GPE 100 and GPE 300 groups compared to the HFD group. As for fatty acid oxidation, carnitine palmitoyltransferase 1 (Cpt1) mRNA expression was higher in the HFD group than in the CD group. Cpt1 mRNA expression in the GPE 100 group was not different from that in the HFD group, but it was significantly increased in the GPE 300 group when compared with the HFD and GPE 100 groups. Hormone sensitive lipase (Hsl) mRNA expression was significantly decreased in the HFD group compared with the CD group but was dose- dependently increased by GPE treatment (Figure 3).
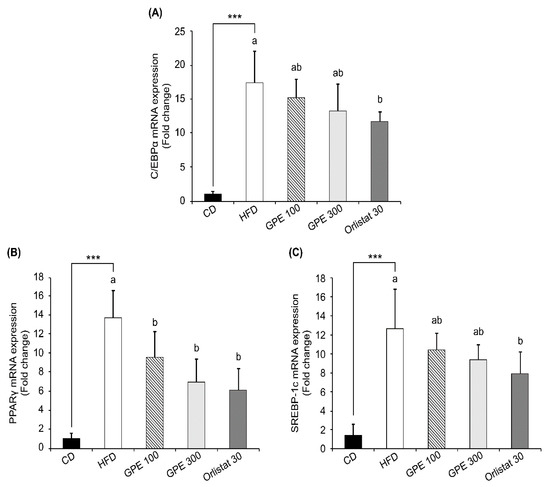
Figure 2.
Effect of GPE treatment on the expression of adipogenic transcription factors in epididymal adipose tissues of HFD-fed C57BL/6N mice. GPE was administrated by oral gavage for 8 weeks to mice fed an HFD. Total RNA was isolated from epididymal adipose tissues, reverse transcribed, and used for qRT-PCR. Relative mRNA expression levels of Cebpa (A), Pparg (B), and Srebp1c (C) in adipose tissues. Target mRNA expression was normalized to that of Gapdh and is presented relative to the CD group. Each bar represents the mean ± SD (n = 10). *** P < 0.001 significantly different from the CD group. Different letters indicate significant differences among the HFD, GPE 100, GPE 300, and Orlistat 30 groups at P < 0.05. CD: control diet; HFD: high-fat diet; GPE: G. pentaphyllum extract; GPE 100: HFD + 100 mg/kg body weight/day; GPE 300: HFD + 300 mg/kg body weight/day GPE; Orlistat 30: HFD + 30 mg/kg body weight/day Orlistat.
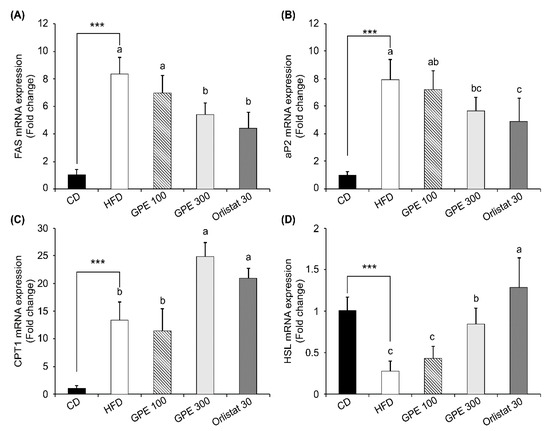
Figure 3.
Effect of GPE treatment on the expression of adipogenesis- and lipolysis-related genes in epididymal adipose tissues of HFD-fed C57BL/6N mice. GPE was administrated by oral gavage for 8 weeks to mice fed an HFD. Total RNA was isolated from epididymal adipose tissues, reverse transcribed, and used for qRT-PCR. Relative mRNA expression levels of Fas (A), Ap2 (B), Cpt1 (C), and Hsl (D) in adipose tissues. Target mRNA expression was normalized to that of Gapdh and is presented relative to the CD group. Each bar represents the mean ± SD (n = 10). *** P < 0.001 significantly different from the CD group. Different letters indicate significant differences among the HFD, GPE 100, GPE 300, and Orlistat 30 groups at P < 0.05. CD: control diet; HFD: high-fat diet; GPE: G. pentaphyllum extract; GPE 100: HFD + 100 mg/kg body weight/day; GPE 300: HFD + 300 mg/kg body weight/day GPE; Orlistat 30: HFD + 30 mg/kg body weight/day Orlistat.
3.5. Effects of GPE on SIRT1 Expression and AMPK Activation
Sirtuin 1 (SIRT1), an epigenetic enzyme involved in protein deacetylation, is the most conserved mammalian NAD+-dependent protein deacetylase. It acts primarily as a metabolic sensor that responds to changing energy status by deacetylating crucial transcription factors and cofactors. SIRT1 regulates diverse biological functions, including biogenesis, inflammation, apoptosis, oxidative stress, mitochondrial function, and cellular senescence [39]. We analyzed Sirt1 expression to determine whether the changes in expression of adipogenic transcription factors and their target genes by GPE treatment were regulated by SIRT1. As shown in Figure 4, SIRT1 mRNA expression was significantly increased in the HFD group compared to the CD group (P < 0.05), and in the GPE100 and GPE300 groups compared to the HFD group (P < 0.05).
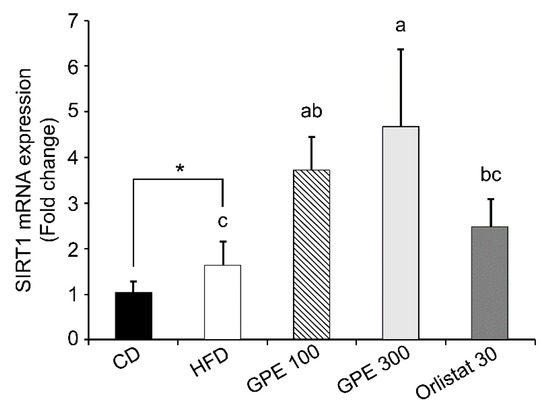
Figure 4.
Effect of GPE treatment on the expression of Sirt1 in the epididymal adipose tissues of HFD-fed C57BL/6N mice. GPE was administrated by oral gavage for 8 weeks to mice fed an HFD. Total RNA was isolated from epididymal adipose tissues, reverse transcribed, and used for RT-qPCR. Target mRNA expression was normalized to that of Gapdh and is presented relative to the CD group. Each bar represents the mean ± SD (n = 10). *** P < 0.001 significantly different from the CD group. Different letters indicate significant differences among the HFD, GPE 100, GPE 300, and Orlistat 30 groups at P < 0.05. CD: control diet; HFD: high-fat diet; GPE: G. pentaphyllum extract; GPE 100: HFD + 100 mg/kg body weight/day; GPE 300: HFD + 300 mg/kg body weight/day GPE; Orlistat 30: HFD + 30 mg/kg body weight/day Orlistat.
AMPK protein expression was significantly decreased in the HFD group compared to the CD group and was not affected by GPE treatment. However, AMPK phosphorylation (p-AMPK) was significantly reduced in the HFD group compared to the CD group, whereas GPE treatment increased the p-AMPK levels (Figure 5A). Thus, the p-AMPK/AMPK ratio was decreased by HFD feeding, but dose-dependently increased by GPE treatment (Figure 5B).
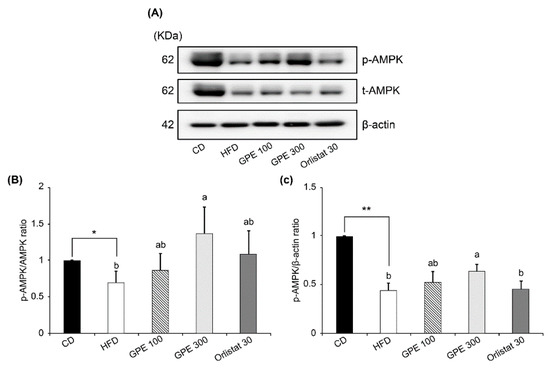
Figure 5.
Effect of GPE treatment on the phosphorylation of AMPK in the epididymal adipose tissues of HFD-fed C57BL/6N mice. GPE was administrated by oral gavage for 8 weeks to mice fed an HFD. Total lysates of epididymal adipose tissues were prepared and analyzed by Western blotting with the indicated antibodies. (A) Photographs of Western blots representative of three independent experiments are shown. (B, C) Quantitative analysis of Western blot data. Protein abundances were normalized to β-actin and are expressed relative to CD levels. Each bar represents the mean ± SD (n = 5). *** P < 0.001 significantly different from the CD group. Different letters indicate significant differences among the HFD, GPE 100, GPE 300, and Orlistat 30 groups at P < 0.05. CD: control diet; HFD: high-fat diet; GPE: G. pentaphyllum extract; GPE 100: HFD + 100 mg/kg body weight/day; GPE 300: HFD + 300 mg/kg body weight/day GPE; Orlistat 30: HFD + 30 mg/kg body weight/day Orlistat.
4. Discussion
The present study demonstrated that GPE treatment reduced body weight, body weight gain, fat mass, white adipose tissue weight, food intake, and food efficiency ratio in C57BL/6N mice with HFD-induced obesity. These results clearly showed that GPE has a potent anti-obesity effect.
In our study, blood glucose was increased by HFD feeding, which was dramatically reduced by GPE treatment. These hypoglycemic effects of G. pentaphyllum have been reported in other studies [24,40,41]. Wang et al. reported that polysaccharides extracted from G. pentaphyllum decreased α-glucosidase activity and increased GLUT2 protein expression in diabetic mice [41]. Gao et al. reported that G. pentaphyllum exerted hypoglycemic and hypolipidemic effects through NFE2-related factor 2 signaling in streptozotocin-induced diabetic rats [31]. Gauhar et al. found that a G. pentaphyllum extract named actiponin obtained by a modified method combining autoclaving and ethanol extraction contained large amounts of damulin A and damulin B and induced a hypoglycemic effect by increasing AMPK and ACC phosphorylation [24]. In our study, the increases in the serum levels of triglyceride, total cholesterol, and LDL-cholesterol induced by HFD also were remarkably suppressed by GPE treatment. G. pentaphyllum has been reported to improve blood lipid levels in Zucker fatty rats [42] and obese humans [25]. Further, our study showed that the increases in serum ALT and AST activities induced by HFD feeding were significantly suppressed by GPE treatment. These observations indicate that our GPE, which is enriched in gypenoside L, gypenoside LI, and ginsenoside Rg3, ameliorates hyperglycemic and hyperlipidemic symptoms without being toxic in HFD-induced obese C57BL/6N mice.
Adipogenesis-related transcription factors such as C/EBPα, PPARγ, and SREBP-1c act as key regulators of glucose and energy metabolism as well as of preadipocyte differentiation and lipid biosynthesis [43,44,45]. In our study, GPE treatment noticeably suppressed the increases in the mRNA expression of Cebpa, Pparg, and Srebp1c induced by HFD feeding. In the present study, GPE treatment suppressed HFD-induced increases in the mRNA expression of Fas and Ap2, involved in fatty acid biosynthesis, but increased the HFD-induced reduction in the mRNA expression of Cpt1 and Hsl, involved in fatty acid oxidation and lipolysis, in adipose tissue. FAS is a key enzyme in de novo lipogenesis, and, when activated, it increases fatty acid synthesis and insulin resistance in adipose tissues [46]. aP2, also called fatty acid binding protein 4, is used as an early biomarker of metabolic syndrome. Blocking this protein may provide a treatment for various chronic diseases, including heart disease, diabetes, and obesity [47,48,49]. CPT1 is an essential enzyme in fatty acid β-oxidation and is associated with diabetes and obesity [50]. HSL is an intracellular neutral lipase capable of hydrolyzing a variety of esters. In adipose tissue, HSL hydrolyzes stored triglycerides to free fatty acids to mobilize stored fats [51]. Taken together, our results indicate that the downregulation of transcription factors such as C/EBPα, PPARγ, and SREBP-1c, followed by the downregulation of FAS and aP2 and the upregulation of CPT1 and HSL, resulting from GPE treatment are part of the mechanism by which GPE exhibits anti-obesity effects.
AMPK is an important intracellular sensor that controls the energy balance in the whole body and cells by phosphorylating key target proteins related to lipid metabolism, fatty acid oxidation, and glucose uptake in response to energy supply and demand. When AMPK is activated, the ATP-generating pathway is promoted through increases in fatty acid and glucose oxidation, whereas the ATP-consuming pathway is suppressed through decreases in lipid and cholesterol synthesis [52,53,54]. Owing to its effect on glucose and lipid metabolism, AMPK activation has been studied for the treatment of metabolic syndrome [25,54,55]. Metformin [56,57], 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside [58,59], A-769662 (a thienopyridone AMPK activator) [55], and damulin [25] reportedly improve diabetes, dyslipidemia, and fatty liver. The commonality of these substances is that they activate AMPK. Total extract or total saponins of G. pentaphyllum have been reported to have diverse effects downstream of AMPK activation [24]. In our previous in vitro study, GPE and gypenoside L, gypenoside LI, and ginsenoside Rg3 were found to activate AMPK [36]. The present study confirmed that GPE treatment activates AMPK in HFD-induced obese C57BL/6N mice. Among the components of G. pentaphyllum and damulin A and B are major AMPK activators [24,25,60]. Our study showed that gypenoside L-, gypenoside LI-, and ginsenoside Rg3-enriched GPE also acts as an effective AMPK activator.
SIRT1 is an NAD+-dependent protein deacetylase that has emerged as a key metabolic regulator in various metabolic tissues. SIRT1 couples the deacetylation of various transcription factors and co- factors to the cleavage of NAD+, an indicator of cellular metabolic status, playing a vital role in metabolism, including fat storage, gluconeogenesis, fatty acid oxidation, lipogenesis, insulin secretion, food intake, circadian rhythm, and inflammation [40]. SIRT1 promotes fat mobilization in white adipocytes by repressing PPARγ, thereby suppressing the expression of adipose tissue markers, such as aP2 [61]. Genetic ablation of Sirt1 in adipose tissues leads to increased adiposity and insulin resistance [62]. Resveratrol, which activates SIRT1, inhibits HFD-induced obesity. AMPK activation is required for SIRT1 action [63], and AMPK regulates energy expenditure through the regulation of NAD+ metabolism and SIRT1 activity [64]. We analyzed Sirt1 mRNA expression in the epididymal adipose tissue to determine whether the anti-obesity effect of GPE was due to a change in SIRT1 expression. We found that Sirt1 was significantly increased in the HFD group compared to the CD group and was dose-dependently increased by GPE treatment.
5. Conclusions
In conclusion, the GPE produced in this study, enriched in gypenoside L, gypenoside LI, and ginsenoside Rg3, has potent anti-obesity activity. Our results suggest that the underlying mechanism of action involves AMPK phosphorylation, which leads to increased SIRT1 expression, which in turn reduces PPARγ expression, ultimately affecting targets of PPARγ, including FAS, aP2, HSL, and CPT1. Therefore, GPE is a potential candidate for the development of therapies for obesity and related diseases.
Author Contributions
Conceptualization, J.K.L., Y.H.K., J.M.M., and T.Y.K.; Methodology, J.K.L., Y.H.K., J.M.M., and T.Y.K.; Formal analysis, H.S.L., S.M.L., J.I.J., S.M.K., K.M.C., T.K.O., and E.J.K.; Investigation, H.S.L., S.-M.L., J.I.J., S.M.K., and E.J.K.; Original Draft Preparation, H.S.L. and E.J.K.; Writing—Review and Editing, H.S.L. and E.J.K.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the BTC Corporation for providing the GPE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Baretic, M. Obesity drug therapy. Minerva Endocrinolog. 2013, 38, 245–254. [Google Scholar]
- Halford, J.C. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs 2006, 7, 312–318. [Google Scholar] [PubMed]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009, 8, 399–416. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.W.; Lee, C.; Jin, Q.; Lee, M.K.; Lee, C.K.; Hwang, B.Y. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch. Pharmacal. Res. 2016, 39, 1232–1236. [Google Scholar] [CrossRef]
- Nookabkaew, S.; Rangkadilok, N.; Satayavivad, J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J. Agric. Food Chem. 2006, 54, 6939–6944. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L.L. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J. Composition analysis and dominance test of three kinds of raw variety of Gynostemma pentaphyllum. Zhongguo Zhongyao Zazhi 2004, 29, 317–319. [Google Scholar] [PubMed]
- Srichana, D.; Taengtip, R.; Kondo, S. Antimicrobial activity of Gynostemma pentaphyllum extracts against fungi producing aflatoxin and fumonisin and bacteria causing diarrheal disease. Southeast Asian J. Trop Med. Public Health 2011, 42, 704–710. [Google Scholar] [PubMed]
- Muller, C.; Gardemann, A.; Keilhoff, G.; Peter, D.; Wiswedel, I.; Schild, L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine 2012, 19, 395–401. [Google Scholar] [CrossRef]
- Schild, L.; Roth, A.; Keilhoff, G.; Gardemann, A.; Brodemann, R. Protection of hippocampal slices against hypoxia/hypoglycemia injury by a Gynostemma pentaphyllum extract. Phytomedicine 2009, 16, 734–743. [Google Scholar] [CrossRef]
- Schild, L.; Chen, B.H.; Makarov, P.; Kattengell, K.; Heinitz, K.; Keilhoff, G. Selective induction of apoptosis in glioma tumour cells by a Gynostemma pentaphyllum extract. Phytomedicine 2010, 17, 589–597. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, W.; Huang, H.; Slavin, M.; Zhao, Y.; Whent, M.; Blackford, J.; Lutterodt, H.; Zhou, H.; Chen, P.; et al. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J. Agric. Food Chem. 2010, 58, 11243–11249. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lee, M.M.; Chan, B.D.; Ma, V.W.; Zhang, W.; Yip, T.T.; Wong, W.T.; Tai, W.C. Gynostemma pentaphyllum saponins attenuate inflammation in vitro and in vivo by inhibition of NF-κB and STAT3 signaling. Oncotarget 2017, 8, 87401–87414. [Google Scholar] [CrossRef]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Ky, P.T.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effects of add-on Gynostemma pentaphyllum extract therapy with sulfonylureas in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef]
- Wang, J.; Ha, T.K.Q.; Shi, Y.P.; Oh, W.K.; Yang, J.L. Hypoglycemic triterpenes from Gynostemma pentaphyllum. Phytochemistry 2018, 155, 171–181. [Google Scholar] [CrossRef]
- Yeo, J.; Kang, Y.J.; Jeon, S.M.; Jung, U.J.; Lee, M.K.; Song, H.; Choi, M.S. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J. Med. Food 2008, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- La Cour, B.; Molgaard, P.; Yi, Z. Traditional Chinese medicine in treatment of hyperlipidaemia. J. Ethnopharmacol. 1995, 46, 125–129. [Google Scholar] [CrossRef]
- Keilhoff, G.; Esser, T.; Titze, M.; Ebmeyer, U.; Schild, L. Gynostemma pentaphyllum is neuroprotective in a rat model of cardiopulmonary resuscitation. Exp. Ther. Med. 2017, 14, 6034–6046. [Google Scholar] [PubMed]
- Gauhar, R.; Hwang, S.L.; Jeong, S.S.; Kim, J.E.; Song, H.; Park, D.C.; Song, K.S.; Kim, T.Y.; Oh, W.K.; Huh, T.L. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol. Lett. 2012, 34, 1607–1616. [Google Scholar] [CrossRef]
- Park, S.H.; Huh, T.L.; Kim, S.Y.; Oh, M.R.; Pichiah, P.B.T.; Chae, S.W.; Cha, Y.S. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): A randomized, double-blind, placebo-controlled trial. Obesity 2014, 22, 63–71. [Google Scholar] [CrossRef]
- Hu, Y.; Ip, F.C.; Fu, G.; Pang, H.; Ye, W.; Ip, N.Y. Dammarane saponins from Gynostemma pentaphyllum. Phytochemistry 2010, 71, 1149–1157. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, Y.N. Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry 2011, 72, 1453–1459. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.L.; Zhou, P.P.; Meng, X.H.; Shi, Y.P. Further new gypenosides from jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2017, 65, 5926–5934. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, P.C.; Lin, J.M. Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am. J. Chin. Med. 2000, 28, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Ren, Y.; Gao, Y.; Kang, L.; Qiao, Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 69, 1–4. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, M.; Qi, X.; Liu, Y.; Li, N.; Liu, Z.; Bian, Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch. Pharmacal. Res. 2016, 39, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.H.; Huang, H.F.; Chen, X.Y.; Liu, J.; He, M.; Ma, Y.Y.; Zhao, X.N.; Zhang, Y.; Ni, J.M. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J. Chin. Med. Assoc. 2016, 79, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Y.; Guo, X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A review. BioMed. Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef] [PubMed]
- Attawish, A.; Chivapat, S.; Phadungpat, S.; Bansiddhi, J.; Techadamrongsin, Y.; Mitrijit, O.; Chaorai, B.; Chavalittumrong, P. Chronic toxicity of Gynostemma pentaphyllum. Fitoterapia 2004, 75, 539–551. [Google Scholar] [CrossRef]
- Chiranthanut, N.; Teekachunhatean, S.; Panthong, A.; Khonsung, P.; Kanjanapothi, D.; Lertprasertsuk, N. Toxicity evaluation of standardized extract of Gynostemma pentaphyllum Makino. J. Ethnopharmacol. 2013, 149, 228–234. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.M.; Lee, J.K.; Jo, S.K.; Kim, H.J.; Cha, K.M.; Lim, C.Y.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Efficacy of Gynostemma pentaphyllum extract in anti-obesity therapy. Rec. Nat. Prod. 2019, in press. [Google Scholar]
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G996–G1005. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Zhi, S.; Feng, Z.; Lei, C.; Zhou, Y. Wide linearity range and highly sensitive MEMS-based micro-fluxgate sensor with double-layer magnetic core made of Fe-Co-B amorphous alloy. Micromachines 2017, 8, 352. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar] [PubMed]
- Sahagun, D.O.; Marquez-Aguirre, A.L.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Rojas-Mayorquin, A.E. Modulation of PPAR-gamma by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Res. 2012, 2012, 318613. [Google Scholar]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Ann. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Latasa, M.J.; Griffin, M.J.; Moon, Y.S.; Kang, C.; Sul, H.S. Occupancy and function of the −150 sterol regulatory element and −65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell. Biol. 2003, 23, 5896–5907. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Tuncman, G.; Gorgun, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005, 1, 107–119. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-sensitive lipase: Control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002, 43, 1585–1594. [Google Scholar] [CrossRef]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Biophys. Acta 2010, 1804, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef]
- Krakoff, J.; Clark, J.M.; Crandall, J.P.; Wilson, C.; Molitch, M.E.; Brancati, F.L.; Edelstein, S.L.; Knowler, W.C. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity 2010, 18, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorg. Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; de Oliveira, R.M.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).