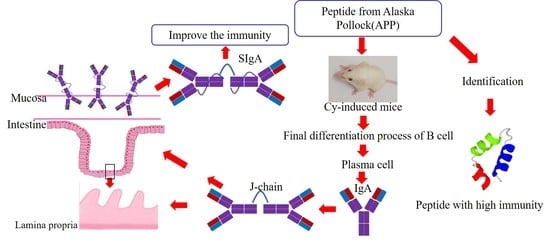
Effect of Peptides from Alaska Pollock on Intestinal Mucosal Immunity Function and Purification of Active Fragments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alaska Pollock Peptide (APP)
2.3. Amino Acid Composition of APP
2.4. Identification of Fingerprint Mapping of APP
2.5. Animal Experiments
2.6. Thymus Index and Spleen Index of Mice
2.7. H&E Staining
2.8. Elisa Analysis
2.9. Quantification of mRNA Level Using RT-qPCR
2.10. Purification and Identification of Peptide with High Immune Activity
2.11. Determination of Proliferation Activity of Spleen Lymphocytes
2.12. Statistical Analysis
3. Results and Discussion
3.1. Amino Acid Composition of APP
3.2. Characteristic Fragment of APP
3.3. Changes of Thymus Index and Spleen Index in Mice
3.4. Histological Assessment of the Intestinal Mucosa
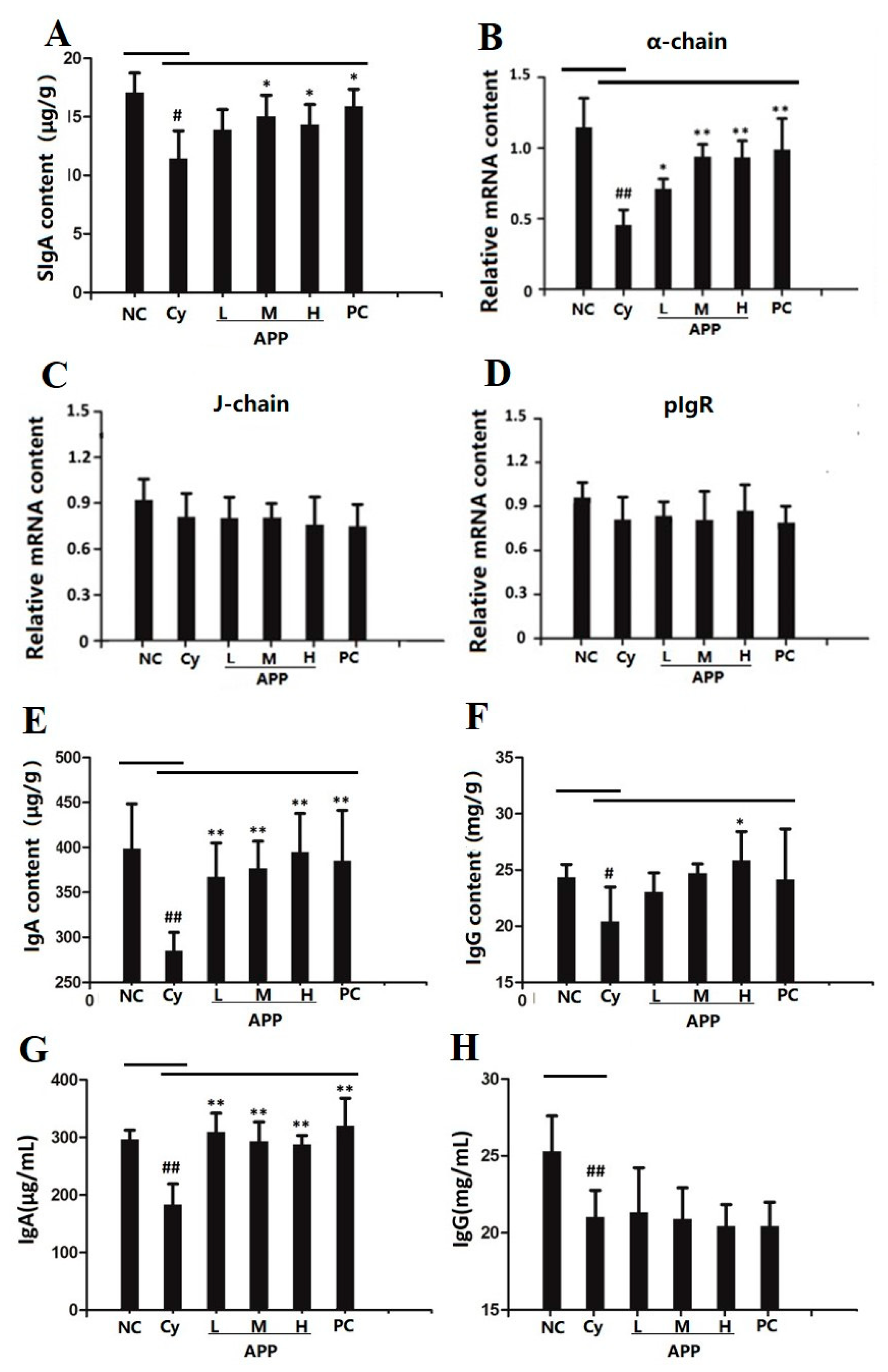
3.5. Effect of APP on the Secretion of SIgA in Small Intestinal Mucosa
3.6. Effect of APP on J Chain, pIgR, and α-chain on mRNA Level
3.7. Effect of APP on IgA and IgG in Small Intestine and Serum
3.8. Effects of APP on the Factors of Differentiation of IgA+ B Cell
3.8.1. Effects of APP on the Factors of Early Differentiation of IgA+ B Cell
3.8.2. Effects of APP on the Factors of Medium-Term Differentiation of IgA+ B Cell
3.8.3. Effects of APP on the Factors of Final Differentiation of IgA+ B Cell
3.9. Effects of APP on the Differentiation of Plasma Cell
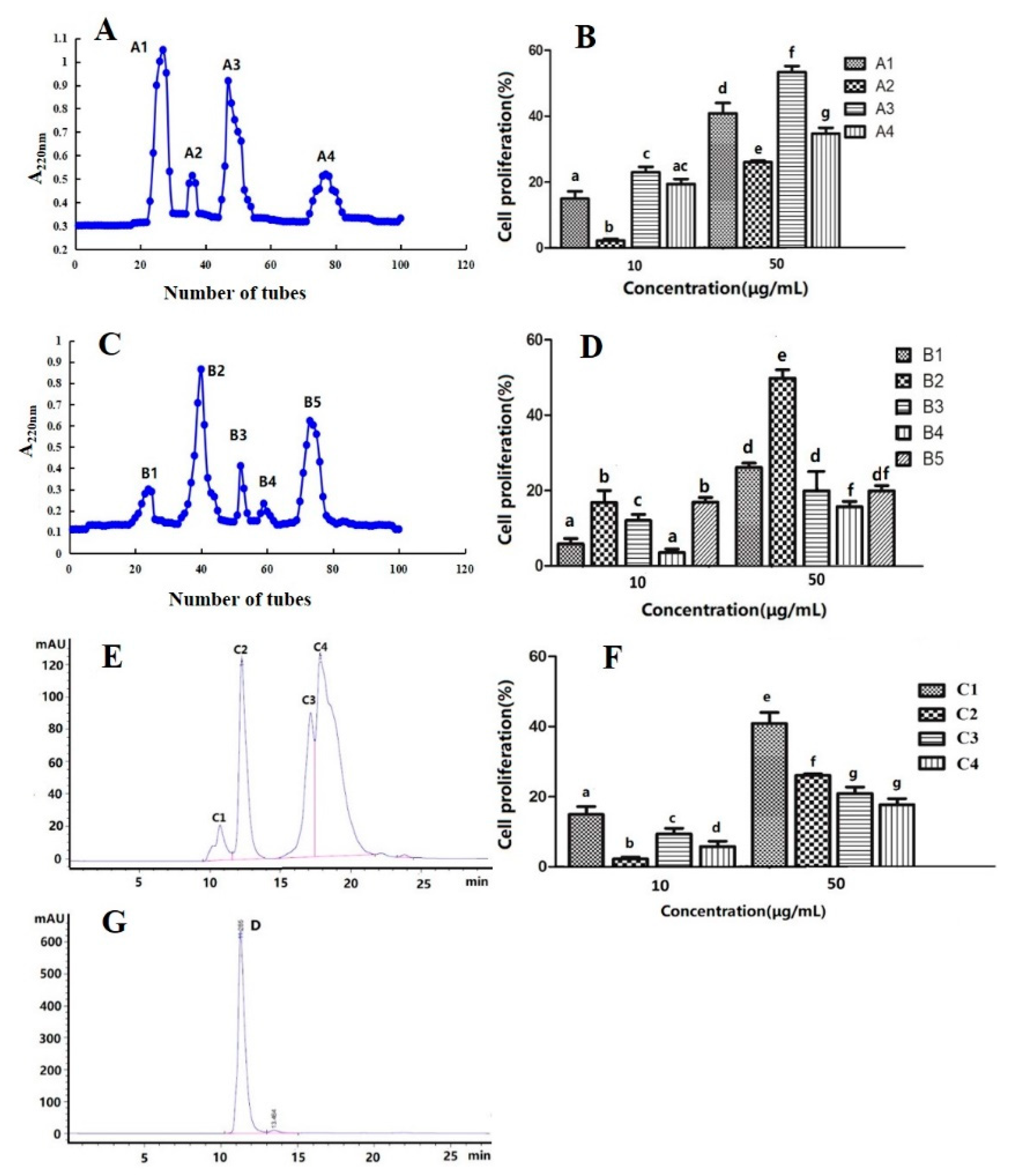
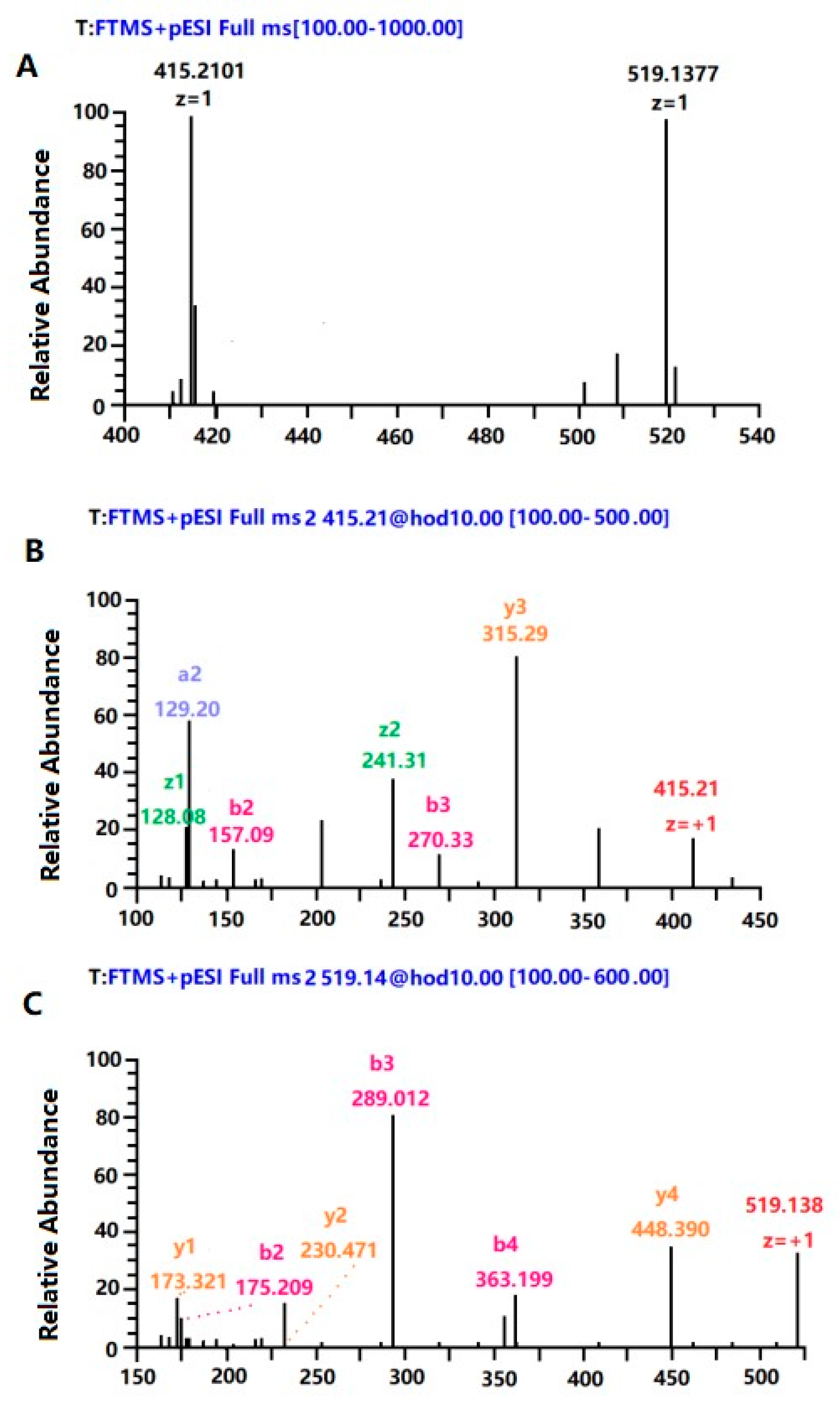
3.10. Purification and Identification of the Peptide Fraction with High Immunological Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cianci, R.; Pagliari, D.; Piccirillo, C.A.; Fritz, J.H.; Gambassi, G. The microbiota and immune system crosstalk in health and disease. Mediat. Inflamm. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; Fiocchi, C. Inflammatory bowel disease: The role of environmental factors. Autoimmun. Rev. 2004, 3, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.R.; Hou, H.; Wang, S.K.; Zhao, X.; Li, B.F. Effects of early enteral nutrition supplemented with collagen peptides on post-burn inflammatory responses in a mouse model. Food Funct. 2017, 8, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin Effects on Gut Microbiota and Gut-Associated Lymphoid Tissue in Healthy Rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef]
- Chou, W.K.; Chen, C.H.; Vuong, C.N.; Abi-Ghanem, D.; Waghela, S.D.; Mwangi, W.; Bielke, L.R.; Hargis, B.M.; Berghman, L.R. Significant mucosal sIgA production after a single oral or parenteral administration using in vivo CD40 targeting in the chicken. Res. Vet. Sci. 2016, 108, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Fan, Y.; Wang, S.K.; Si, L.L.; Li, B.F. Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor–Artificial neural network and the identification of its active central fragment. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.F.; Xue, C.H.; Yu, G.L.; Zhang, Z.H.; Zhao, X. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012, 134, 821–828. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.F.; Xue, C.H.; Yu, G.L. Preparation of immunomodulatory hydrolysates from Alaska pollock frame. J. Sci. Food Agric. 2012, 92, 3029–3038. [Google Scholar] [CrossRef]
- Yang, R.Y.; Zhang, Z.F.; Pei, X.R.; Han, X.L.; Wang, J.B.; Wang, L.L.; Long, Z.; Shen, X.Y.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Markiewicz, L.H.; Szyc, A.; Wasilewska, E. Whey prefermented with beneficial microbes modulates immune response and lowers responsiveness to milk allergens in mouse model. J. Funct. Foods 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, X.; Zhao, E.; Shi, Y.; Shen, X.C.; Wu, J.; Pei, F.; Hu, Q.H.; Qiu, W.F. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates. Food Chem. 2019, 275, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Yimit, D.; Hoxur, P.; Amat, N.; Uchikawa, K.; Yamaguchi, N. Effects of soybean peptide on immune function, brain function, and neurochemistry in healthy volunteers. Nutrition 2012, 28, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.H.; Ma, L.Z.; Kong, B.H. Hydrolyzing condition and immunocompetence of sheep bone protein enzymatic lysates. Agr. Sci. China 2009, 8, 1332–1338. [Google Scholar] [CrossRef]
- Chen, T.J.; Hou, H.; Lu, J.H.; Zhang, K.; Li, B.F. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Yang, T.T.; Zhang, K.; Li, B.F.; Hou, H. Effects of oral administration of peptides with low molecular weight from Alaska Pollock (Theragra chalcogramma) on cutaneous wound healing. J. Funct. Foods 2018, 48, 682–691. [Google Scholar] [CrossRef]
- Dong, Z.H.; Sun, Y.Y.; Wei, G.W.; Li, S.Y.; Zhao, Z.X. Ergosterol Ameliorates Diabetic Nephropathy by Attenuating Mesangial Cell Proliferation and Extracellular Matrix Deposition via the TGF-β1/Smad2 Signaling Pathway. Nutrients 2019, 11, 483. [Google Scholar] [CrossRef]
- Xiao, J.J.; Liu, B.T.; Zhuang, Y.L. Effects of rambutan (Nephelium lappaceum) peel phenolics and Leu-Ser-Gly-Tyr-Gly-Pro on hairless mice skin photoaging induced by ultraviolet irradiation. Food Chem. Toxicol. 2019, 129, 30–37. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zhao, T.R.; Liu, B.T.; Yuan, L.; Sun, L.P.; Zhuang, Y.L. ACE inhibitory activity in vitro and antihypertensive effect in vivo of LSGYGP and its transepithelial transport by Caco-2 cell monolayer. J. Funct. Foods 2019, 61, 103488. [Google Scholar]
- Wei, W.; Pang, S.J.; Fu, X.Y.; Tan, S.W.; Wang, Q.; Wang, S.Z.; Sun, D.J. The role of PERK and IRE1 signaling pathways in excessive fluoride mediated impairment of lymphocytes in rats’ spleen in vivo and in vitro. Chemosphere 2019, 223, 1–11. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.W.; Hou, H.; Zhang, H.W.; Li, B.F. Purification and characterization of a novel calcium-biding decapeptide from Pacific cod (Gadus Macrocephalus) bone: Molecular properties and calcium chelating modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Albanese, C.; Machado, F.S.; Tanowitz, H.B. Glutamine and the tumor microenvironment. Cancer Biol. Ther. 2011, 12, 1098–1100. [Google Scholar] [CrossRef][Green Version]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Martins, E., Jr.; Santos, R.V.; Caperuto, E.C.; Rogeri, P.; Rosa, L.F.C. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition. 2002, 18, 376–379. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and Protein Requirements; WHO Technical Reports Series No. 724. Report of a Joint FAO/WHO/UNU Expert Consultation; FAO: Geneva, Switzerland, 1985. [Google Scholar]
- Liang, M.Q.; Wang, J.L.; Chang, Q.; Mai, K.S. Effects of different levels of fish protein hydrolysate in the diet on the nonspecific immunity of Japanese sea bass, Lateolabrax japonicus (Cuvieret Valenciennes, 1828). Aquac. Res. 2006, 37, 102–106. [Google Scholar] [CrossRef]
- Marchbank, T.; Limdi, J.K.; Mahmood, A.; Elia, G.; Playford, R.J. Clinical trial: Protective effect of a commercial fish protein hydrolysate against indomethacin (NSAID)-induced small intestinal injury. Aliment. Pharm. Ther. 2008, 28, 799–804. [Google Scholar] [CrossRef]
- Tzehoval, E.; Segal, S.; Stabinsky, Y.; Fridkin, M.; Spirer, Z.; Feldman, M. Tuftsin (an Ig-associated tetrapeptide) triggers the immunogenic function of macrophages: Implications for activation of programmed cells. Proc. Natl. Acad. Sci. USA 1978, 75, 3400–3404. [Google Scholar] [CrossRef]
- Suszko, A.; Szczypka, M.; Lis, M.; Kuduk-Jaworska, J.; Obmińska-Mrukowicz, B. Influence of polysaccharide fraction C isolated from Caltha palustris L. on T and B lymphocyte subsets in mice. Cent. Eur. J. Immunol. 2012, 37, 193–199. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.L.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Khavinson, V.K.; Morozov, V.G. Twenty years of study on effects of pineal peptide preparation: Epithalamin in experimental gerontology and oncology. Ann. N. Y. Acad. Sci. 1994, 719, 483–493. [Google Scholar] [CrossRef]
- Zuo, T.; Zhao, R.S.; Lu, S.Y.; Zhang, N.; Zhang, Q.; Xue, C.H. Novel dietary polysaccharide SIP promotes intestinal secretory immunoglobulin A secretion in mice under chemotherapy. J. Funct. Foods 2017, 37, 379–389. [Google Scholar] [CrossRef]
- Xie, J.H.; Fan, S.T.; Nie, S.P.; Yu, Q.; Xiong, T.; Gong, D.; Xie, M.Y. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016, 7, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Wang, B.; Saxena, V.; Miles, L.L.; Tiao, J.; Mortensen, J.E.; Nathan, J.D. The gut-liver axis: Impact of a mouse model of small-bowel bacterial overgrowth. J. Surg. Res. 2018, 221, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.R.; Gao, X.; Zhang, H.W.; Li, B.F.; Yu, G.L.; Li, B. Collagen peptides administration in early enteral nutrition intervention attenuates burn-induced intestinal barrier disruption: Effects on tight junction structure. J. Funct. Foods 2019, 55, 167–174. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Kudsk, K.A. IL-25 improves IgA levels during parenteral nutrition through the JAK-STAT pathway. Ann. Surg. 2013, 258, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Cao, C.M.; Ren, D.D.; Zhang, Y.; Kou, Y.N.; Ma, L.Y.; Feng, S.B.; Li, Y.; Wang, X.C. Effects of soybean antigen proteins on intestinal permeability, 5-hydroxytryptamine levels and secretory IgA distribution in the intestine of weaned piglets. Ital. J. Anim. Sci. 2016, 15, 174–180. [Google Scholar] [CrossRef]
- Sun, H.F.; Bi, J.C.; Lei, Q.C.; Wan, X.; Jiang, T.T.; Wu, C.; Wang, X.Y. Partial enteral nutrition increases intestinal sIgA levels in mice undergoing parenteral nutrition in a dose-dependent manner. Int. J. Surg. 2018, 49, 74–79. [Google Scholar] [CrossRef]
- Johansen, F.E.; Kaetzel, C.S. Regulation of the polymeric immunoglobulin receptor and IgA transport: New advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011, 4, 598–602. [Google Scholar] [CrossRef]
- Hui, M.K.; Wu, W.K.; Shin, V.Y.; So, W.H.; Cho, C.H. Polysaccharides from the root of Angelica sinensis protect bone marrow and gastrointestinal tissues against the cytotoxicity of cyclophosphamide in mice. Int. J. Med. Sci. 2006, 3, 1–6. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Yin, Q.Q.; Yang, Y.R. Effect of soybean peptides on growth performance, intestinal structure and mucosal immunity of broilers. J. Anim. Physiol. Anim. Nutr. 2009, 93, 754–760. [Google Scholar] [CrossRef]
- Zuo, T.; Cao, L.; Xue, C.H.; Tang, Q.J. Dietary squid ink polysaccharide induces goblet cells to protect small intestine from chemotherapy induced injury. Food Funct. 2015, 6, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, W.; Kraehenbuhl, J.P. Epithelial transcytosis of immunoglobulins. J. Mammary Gland Biol. 1998, 3, 287–302. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Capitani, N.; Codolo, G.; Vallese, F.; Minervini, G.; Grassi, A.; Cianchi, F.; Troilo, A.; Fischer, W.; Zanotti, G.; Baldari, C.T.; et al. The lipoprotein HP1454 of Helicobacter pylori regulates T-cell response by shaping T-cell receptor signalling. Cell. Microbiol. 2019, 21, e13006. [Google Scholar] [CrossRef]
- Zuo, T.; Cao, L.; Sun, X.H.; Li, X.M.; Wu, J.; Lu, S.Y.; Xue, C.H.; Tang, Q.J. Dietary squid ink polysaccharide could enhance SIgA secretion in chemotherapeutic mice. Food Funct. 2014, 5, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.J.; Wang, K.; Wang, Y.R. Polymeric immunoglobulin receptor expression is predictive of poor prognosis in glioma patients. Int. J. Clin. Exp. Med. 2014, 7, 2185–2190. [Google Scholar]
- Barone, M.; Troncone, R.; Auricchio, S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef]
- Serebrennikova, O.B.; Tsatsanis, C.; Mao, C.; Gounaris, E.; Ren, W.; Siracusa, L.D.; Eliopoulos, A.G.; Khazaie, K.; Tsichlis, P.N. Tpl2 ablation promotes intestinal inflammation and tumorigenesis in Apcmin mice by inhibiting IL-10 secretion and regulatory T-cell generation. Proc. Natl. Acad. Sci. USA 2012, 109, E1082–E1091. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Bhaskarachary, K.; Vajreswari, A.; Ramesh Kumar, R.; Dinesh kumar, B. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 2015, 31, 388–398. [Google Scholar] [CrossRef]
- Turner, C.A., Jr.; Mack, D.H.; Davis, M.M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 1994, 77, 297–306. [Google Scholar] [CrossRef]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Altk, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Medina, K.L.; Lancki, D.W.; Singh, H. IRF-4, 8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003, 17, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zhao, S.; Xin, X.D.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z.Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia Pac. Entomol. 2019, 22, 633–637. [Google Scholar] [CrossRef]
- Ren, G.Y.; Sun, H.; Li, G.; Fan, J.L.; Wu, Y.; Cui, G.T. Molecular docking and muiltple spectroscopy investigation on the binding characteristics of aloe-emodin to pepsin. J. Mol. Struct. 2019, 1195, 369–377. [Google Scholar] [CrossRef]
- Qian, B.J.; Xing, M.Z.; Cui, L.; Deng, Y.; Xu, Y.Q.; Huang, M.N.; Zhang, S.H. Antioxidant, antihypertensive, and immunomodulatory activities of peptide fractions from fermented skim milk with Lactobacillus delbrueckii ssp. bulgaricus LB340. J. Dairy Res. 2011, 78, 72–79. [Google Scholar] [CrossRef]
- Stuknyte, M.; De Noni, I.; Guglielmetti, S.; Minuzzo, M.; Mora, D. Potential immunomodulatory activity of bovine casein hydrolysates produced after digestion with proteinases of lactic acid bacteria. Int. Dairy J. 2011, 21, 763–769. [Google Scholar] [CrossRef]
- Iemura, Y.; Yamada, T.; Takahashi, T.; Furukawa, K.; Hara, S. Properties of the peptides liberated from rice protein in sokujo-moto. J. Biosci. Bioeng. 1999, 88, 276–280. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | CAGGCATTGCTGACAGGATG | TGCTGATCCACATCTGCTGG |
| pIgR | CAGAAATAGCATGGCAGACTTCAA | TGCCGACTAGGCCATGTCAG |
| J chain | TTCGTTAAGGCTGTCCTTGT | AAGCGGAGCTGGAAGATCAG |
| α-chain | TGAGAGCTGGAACAGTGGCG | TCAGCGCCAGCTCCTCCGAC |
| TNF-α | CTGTACCCCACGTCGTAGC | TTGAGATGCATGCCGTTG |
| IFN-γ | CACTGCGTCTTGGCTTTGCA | GCTGATGGGCTGATTGTCTTTC |
| IL-10 | ACTGCAGCCACGTCGTAGC | TGTCCACCTGGTCCTTTGTT |
| IL-6 | GGTACCAAACTGGATATAATCAGAA | CAAGGTAGCTATGGTACTCCAGC |
| AID | GGGAAAGTGGCAATCACCTAT | CGTTCGGCAAAGCGTCAT |
| CD79a | TCAACACTGCCAGGGAACC | GGGGAAGACAGACAGCAAGAAC |
| Blimp-1 | TTTGTGGACAGAGGCCCAGT | AAAGCGTGTTCCCTCCGGTAT |
| IRF-4 | CGGGCAAGCAGGCCTACAAT | TGGCTCCCTCCGGAACAATC |
| XBP-1 | GCTGCGGAGGAAACTGCAAA | TCCATTCCCACGCGTGTTCT |
| Amino Acid | RP a | APP | |
|---|---|---|---|
| Content | CS | ||
| Leu | 70 | 67 | 0.96 |
| Thr | 40 | 40 | 1.00 |
| Ile | 40 | 26 | 0.65 |
| Phe | 60 | 34 | 0.57 |
| Lys | 55 | 74 | 1.35 |
| Met | 35 | 32 | 0.91 |
| Val | 50 | 35 | 0.70 |
| Trp | 10 | ||
| Pro | 11 | ||
| Asp | 77 | ||
| Tyr | 13 | ||
| Ser | 53 | ||
| Glu | 119 | ||
| Ala | 91 | ||
| Cys | |||
| Arg | 44 | ||
| Gly | 259 | ||
| His | 25 | ||
| Peptide Sequence of APP | Molecular Mass (Da) | Peptide Sequence of APP | Molecular Mass (Da) |
|---|---|---|---|
| Ala–Gly–Asp–Asp–Ala–Pro–Arg | 700.32 | Leu–Asp–Phe–Glu–Asn–Glu–Met–Ala–Thr | 1068.45 |
| Ser–Leu–Ser–Asp–Leu–Asn–Pro | 745.35 | Gly–Thr–Glu–Asp–Glu–Leu–Asp–Lys–Tyr | 1068.46 |
| Gly–Ser–Leu–Glu–Gln–Glu–Lys | 789.38 | Glu–Thr–Leu–Asp–Met–Leu–Glu–Thr–Met | 1087.49 |
| Gly–Val–Glu–Glu–Asp–Leu–Met | 791.34 | Gln–Glu–Tyr–Asp–Glu–Ala–Gly–Pro–Ser–Ile | 1090.45 |
| Gly–Gly–Asp–Asp–Leu–Asp–Pro–Asn | 801.32 | Ile–Glu–Glu–Leu–Glu–Glu–Glu–Ile–Glu | 1131.52 |
| His–Leu–Asp–Asp–Ala–Val–Arg | 824.42 | Ser–Ser–Pro–Gly–Asp–Asp–Asp–Met–Ala–Asn–Lys | 1135.45 |
| Gly–Val–Glu–Asp–Asp–Ser–Val–Gln | 847.36 | His–Glu–Leu–Glu–Glu–Ala–Glu–Glu–Arg | 1140.51 |
| Ala–Leu–Thr–Asp–Ala–Glu–Thr–Lys | 847.43 | Ser–Gly–Phe–Ile–Glu–Glu–Asp–Glu–Leu–Lys | 1165.55 |
| Gln–Asn–Glu–Glu–Glu–Val–Lys | 857.38 | Val–Glu–Asp–Glu–Phe–Pro–Asp–Leu–Ser–Lys | 1177.55 |
| Leu–Glu–His–Glu–Glu–Ser–Lys | 870.46 | ln–Gly–Val–Gln–Asp–Glu–Asn–Gly–Glu–Ser–His | 1182.45 |
| Ala–Ala–Glu–Asp–Leu–Lys–Glu–Gln | 902.44 | Gly–Trp–Leu–Asp–Lys–Asn–Lys–Asp–Pro–Leu | 1184.62 |
| Thr–Glu–Asn–Gly–Glu–Phe–Gly–Arg | 909.39 | Asn–Gly–Glu–Gln–Asp–Glu–Gly–Val–Ser–His–Tyr | 1234.48 |
| Gln–Ser–Glu–Glu–Ala–Glu–Glu–Gln | 931.34 | Glu–Ala–Pro–Leu–Ala–Cys–Asn–Gly–Arg–Asn–Pro–Lys | 1286.60 |
| Ala–Gly–Asp–Ser–Gly–Asp–Asp–Gly–Ala–Ile–Gly | 933.37 | Met–Glu–Gly–Asp–Leu–Asn–Glu–Met–Glu–Ile–Gln | 1308.52 |
| Leu–Pro–Asp–Gly–Gly–Val–Ile–Leu–Gln | 943.42 | Asp–Leu–Glu–Ser–Glu–Val–Asp–Asn–Glu–Gln–Arg | 1333.57 |
| Asp–Lys–Gly–Asn–Gly–Glu–Thr–Val–Met | 950.40 | Ala–Glu–Lys–Asp–Glu–Glu–Met–Glu–Gln–Ile–Lys | 1348.62 |
| Thr–Glu–Glu–Leu–Glu–Glu–Ser–Lys | 963.44 | Ser–Ile–Asp–Asp–Lys–Glu–Glu–Leu–Asp–Ala–Thr–Asp | 1349.58 |
| Glu–Gln–Ile–Asp–Asn–Leu–Gln–Arg | 1014.51 | Ala–Ser–Glu–Gly–Asp–Asp–Asn–Leu–Asn–Ala–Glu–Glu–Arg | 1418.59 |
| Asp–Glu–Glu–Met–Glu–Gln–Ile–Lys | 1020.44 | Gln–Met–Met–Thr–Asn–His–Lys–Pro–Glu–Leu–Ile–Glu | 1453.66 |
| Ser–Ala–Asp–Gln–Val–Glu–Asp–Phe–Lys | 1037.47 | Asp–Asp–Leu–Gln–Ala–Glu–Glu–Asp–Lys–Val–Asn–Thr–Leu | 1488.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, S.; Poungchawanwong, S.; Hou, H. Effect of Peptides from Alaska Pollock on Intestinal Mucosal Immunity Function and Purification of Active Fragments. Nutrients 2019, 11, 2517. https://doi.org/10.3390/nu11102517
Li Q, Wang S, Poungchawanwong S, Hou H. Effect of Peptides from Alaska Pollock on Intestinal Mucosal Immunity Function and Purification of Active Fragments. Nutrients. 2019; 11(10):2517. https://doi.org/10.3390/nu11102517
Chicago/Turabian StyleLi, Qiqi, Shikai Wang, Supanooch Poungchawanwong, and Hu Hou. 2019. "Effect of Peptides from Alaska Pollock on Intestinal Mucosal Immunity Function and Purification of Active Fragments" Nutrients 11, no. 10: 2517. https://doi.org/10.3390/nu11102517
APA StyleLi, Q., Wang, S., Poungchawanwong, S., & Hou, H. (2019). Effect of Peptides from Alaska Pollock on Intestinal Mucosal Immunity Function and Purification of Active Fragments. Nutrients, 11(10), 2517. https://doi.org/10.3390/nu11102517