Increasing Energy Flux to Maintain Diet-Induced Weight Loss
Abstract
1. Introduction
2. How is Energy Flux Defined and Measured?
3. Does Human Evolutionary Biology Interacting with the Current Obesogenic Environment Necessitate a High Energy Flux?
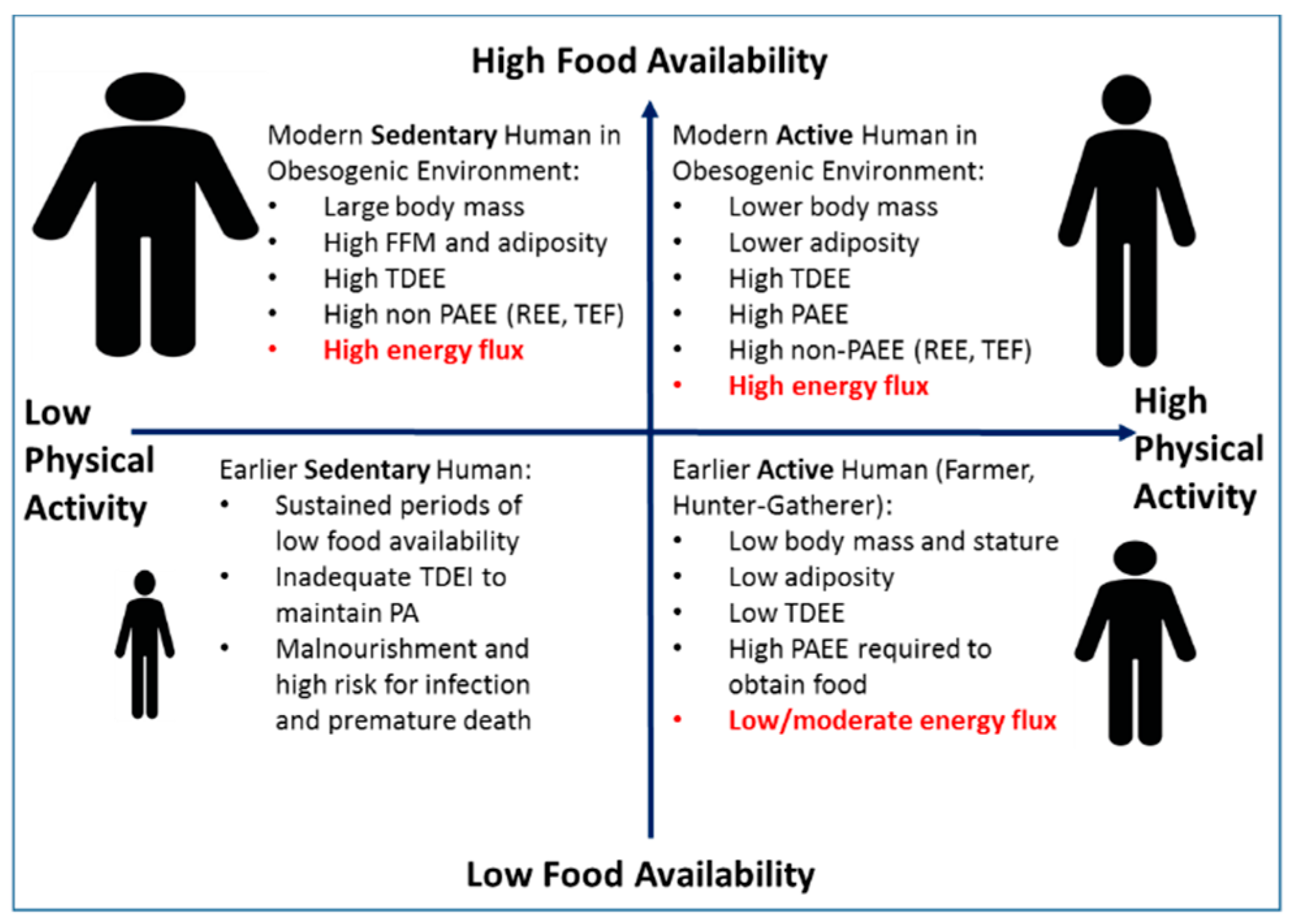
Proposed Relationships among Physical Activity, Energy Flux, and Body Size
4. Energy Flux: Does It Matter How It Is Attained?
4.1. Metabolic Advantages of Achieving High Flux through High Levels of Physical Activity
Appetite Regulation
4.2. Metabolic Disadvantages of Achieving High Flux through Increased Body Mass
Appetite Dysregulation
5. How Does Physical Activity Influence Energy Flux?
5.1. Physical Activity/Exercise Effects on TDEE
5.2. Acute Endurance Type Exercise and Energy Flux
5.3. Impact of Acute High Intensity Interval Training (HIIT) on Energy Flux
5.4. Impact of Chronic Endurance-Type Exercise on Energy Flux
5.5. Impact of Resistance Exercise on Energy Flux
5.6. Impact of Exercise on the Thermic Effect of Feeding
6. Is Energy Flux Important for Weight Loss Maintenance and if so, How?
6.1. Physiology of the Weight Reduced State
6.2. The Role of High Flux in Maintenance of Lost Weight
7. What are the Important Research Questions That Should be Addressed?
8. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phelan, S.; Wing, R.R. Prevalence of successful weight loss. Arch. Int. Med. 2005, 165, 2430. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Day, D.S.; Jones, P.P.; Christou, D.D.; Petitt, D.S.; Osterberg, K.; Melby, C.L.; Seals, D.R. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J. Clin. Endocrinol. Metab. 2004, 89, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Calles-Escandon, J.; Poehlman, E.T.; O’Connell, M.; Danforth, E., Jr. Effects of increased energy intake and/or physical activity on energy expenditure in young healthy men. J. Appl. Physiol. 1994, 77, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bullough, R.C.; Gillette, C.A.; Harris, M.A.; Melby, C.L. Interaction of acute changes in exercise energy expenditure and energy intake on resting metabolic rate. Am. J. Clin. Nutr. 1995, 61, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hagele, F.A.; Busing, F.; Nas, A.; Hasler, M.; Muller, M.J.; Blundell, J.E.; Bosy-Westphal, A. Appetite Control Is Improved by Acute Increases in Energy Turnover at Different Levels of Energy Balance. J. Clin. Endocrinol. Metab. 2019, 104, 4481–4491. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Lo, S.K.; Westerterp, K.R.; Rush, E.C.; Rosenbaum, M.; Luke, A.; Schoeller, D.A.; DeLany, J.P.; Butte, N.F.; et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am. J. Clin. Nutr. 2009, 89, 1723–1728. [Google Scholar] [CrossRef]
- Hume, D.J.; Yokum, S.; Stice, E. Low energy intake plus low energy expenditure (low energy flux), not energy surfeit, predicts future body fat gain. Am. J. Clin. Nutr. 2016, 103, 1389–1396. [Google Scholar] [CrossRef]
- Hand, G.A.; Shook, R.P.; Hill, J.O.; Giacobbi, P.R.; Blair, S.N. Energy flux: Staying in energy balance at a high level is necessary to prevent weight gain for most people. Expert Rev. Endocrinol. Metab. 2015, 10, 599–605. [Google Scholar] [CrossRef]
- Melby, C.L.; Paris, H.L.; Foright, R.M.; Peth, J. Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? Nutrients 2017, 9, 486. [Google Scholar] [CrossRef]
- Paris, H.L.; Foright, R.M.; Werth, K.A.; Larson, L.C.; Beals, J.W.; Cox-York, K.; Bell, C.; Melby, C.L. Increasing energy flux to decrease the biological drive toward weight regain after weight loss—A proof-of-concept pilot study. Clin. Nutr. ESPEN 2016, 11, 12–20. [Google Scholar] [CrossRef]
- Schoeller, D.A. Misdefined energy flux and increased fatness. Am. J. Clin. Nutr. 2016, 104, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Thurber, C.; Dugas, L.R.; Ocobock, C.; Carlson, B.; Speakman, J.R.; Pontzer, H. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv. 2019, 5, 341. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. If body fatness is under physiological regulation, then how come we have an obesity epidemic? Physiology (Bethesda) 2014, 29, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. The evolution of body fatness: Trading off disease and predation risk. J. Exp. Biol. 2018, 221, 167254. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Kaprio, J.; Korkeila, M.; Koskenvuo, M.; Neale, M.C.; Hayakawa, K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 501–506. [Google Scholar] [PubMed]
- Stunkard, A.J.; Foch, T.T.; Hrubec, Z. A twin study of human obesity. JAMA 1986, 256, 51–54. [Google Scholar] [CrossRef]
- Bouchard, C.; Tremblay, A.; Despres, J.P.; Nadeau, A.; Lupien, P.J.; Theriault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, J.H.; Luan, J.; Luben, R.N.; Rodwell, S.A.; Khaw, K.T.; Ong, K.K.; Wareham, N.J.; Loos, R.J. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am. J. Clin. Nutr. 2010, 91, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Willer, C.J.; Speliotes, E.K.; Loos, R.J.; Li, S.; Lindgren, C.M.; Heid, I.M.; Berndt, S.I.; Elliott, A.L.; Jackson, A.U.; Lamina, C.; et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009, 41, 25–34. [Google Scholar]
- Muller, M.J.; Geisler, C.; Blundell, J.; Dulloo, A.; Schutz, Y.; Krawczak, M.; Bosy-Westphal, A.; Enderle, J.; Heymsfield, S.B. The case of GWAS of obesity: Does body weight control play by the rules? Int. J. Obes. (Lond.) 2018, 42, 1395–1405. [Google Scholar] [CrossRef]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Raichlen, D.A.; Gordon, A.D.; Schroepfer-Walker, K.K.; Hare, B.; O’Neill, M.C.; Muldoon, K.M.; Dunsworth, H.M.; Wood, B.M.; Isler, K.; et al. Primate energy expenditure and life history. Proc. Natl. Acad. Sci. USA 2014, 111, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exerc. Sport Sci. Rev. 2015, 43, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Energy Constraint as a Novel Mechanism Linking Exercise and Health. Physiology (Bethesda) 2018, 33, 384–393. [Google Scholar]
- Pettee Gabriel, K.; Sidney, S.; Jacobs, D.R., Jr.; Whitaker, K.M.; Carnethon, M.R.; Lewis, C.E.; Schreiner, P.J.; Malkani, R.I.; Shikany, J.M.; Reis, J.P.; et al. Ten-Year Changes in Accelerometer-Based Physical Activity and Sedentary Time During Midlife: The CARDIA Study. Am. J. Epidemiol. 2018, 187, 2145–2150. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Hopkins, M.; Blundell, J.E. Energy balance, body composition, sedentariness and appetite regulation: Pathways to obesity. Clin. Sci. (Lond.) 2016, 130, 1615–1628. [Google Scholar] [CrossRef]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef]
- Schoeller, D.A. The effect of holiday weight gain on body weight. Physiol. Behav. 2014, 134, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Horgan, G.W.; King, N.; Blundell, J.E. A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. Am. J. Clin. Nutr. 2004, 79, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Shook, R.P.; Hand, G.A.; Drenowatz, C.; Hebert, J.R.; Paluch, A.E.; Blundell, J.E.; Hill, J.O.; Katzmarzyk, P.T.; Church, T.S.; Blair, S.N. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. Am. J. Clin. Nutr. 2015, 102, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.M.; Bullough, R.C.; Melby, C.L. Resting metabolic rate and postprandial thermogenesis by level of aerobic fitness in young women. Eur. J. Clin. Nutr. 1993, 47, 575–585. [Google Scholar] [PubMed]
- Ostendorf, D.M.; Caldwell, A.E.; Creasy, S.A.; Pan, Z.; Lyden, K.; Bergouignan, A.; MacLean, P.S.; Wyatt, H.R.; Hill, J.O.; Melanson, E.L.; et al. Physical Activity Energy Expenditure and Total Daily Energy Expenditure in Successful Weight Loss Maintainers. Obesity 2019, 27, 496–504. [Google Scholar] [CrossRef]
- Phelain, J.F.; Reinke, E.; Harris, M.A.; Melby, C.L. Postexercise energy expenditure and substrate oxidation in young women resulting from exercise bouts of different intensity. J. Am. Coll. Nutr. 1997, 16, 140–146. [Google Scholar] [CrossRef]
- Sedlock, D.A.; Fissinger, J.A.; Melby, C.L. Effect of exercise intensity and duration on postexercise energy expenditure. Med. Sci. Sports Exerc. 1989, 21, 662–666. [Google Scholar] [CrossRef]
- Bahr, R. Excess postexercise oxygen consumption--magnitude, mechanisms and practical implications. Acta Physiol. Scand. Suppl. 1992, 605, 1–70. [Google Scholar]
- Hunter, G.R.; Moellering, D.R.; Carter, S.J.; Gower, B.A.; Bamman, M.M.; Hornbuckle, L.M.; Plaisance, E.P.; Fisher, G. Potential Causes of Elevated REE after High-Intensity Exercise. Med. Sci. Sports Exerc. 2017, 49, 2414–2421. [Google Scholar] [CrossRef]
- Bahr, R.; Hansson, P.; Sejersted, O.M. Triglyceride/fatty acid cycling is increased after exercise. Metabolism 1990, 39, 993–999. [Google Scholar] [CrossRef]
- Cocks, M.; Shaw, C.S.; Shepherd, S.O.; Fisher, J.P.; Ranasinghe, A.M.; Barker, T.A.; Tipton, K.D.; Wagenmakers, A.J. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J. Physiol. 2013, 591, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Little, J.P.; Punthakee, Z.; Tarnopolsky, M.A.; Riddell, M.C.; Gibala, M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Sevits, K.J.; Melanson, E.L.; Swibas, T.; Binns, S.E.; Klochak, A.L.; Lonac, M.C.; Peltonen, G.L.; Scalzo, R.L.; Schweder, M.M.; Smith, A.M.; et al. Total daily energy expenditure is increased following a single bout of sprint interval training. Physiol. Rep. 2013, 1, 131. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.C.; Johnson, T.K.; Kuzma, J.N.; Lonac, M.C.; Schweder, M.M.; Voyles, W.F.; Bell, C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J. Physiol. 2010, 588, 2961–2972. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Melby, C.L.; Badylak, S.F.; Calles, J. Aerobic fitness and resting energy expenditure in young adult males. Metabolism 1989, 38, 85–90. [Google Scholar] [CrossRef]
- Broeder, C.E.; Burrhus, K.A.; Svanevik, L.S.; Wilmore, J.H. The effects of aerobic fitness on resting metabolic rate. Am. J. Clin. Nutr. 1992, 55, 795–801. [Google Scholar] [CrossRef]
- Monroe, M.B.; Seals, D.R.; Shapiro, L.F.; Bell, C.; Johnson, D.; Parker Jones, P. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 740–744. [Google Scholar] [CrossRef]
- Bell, C.; Seals, D.R.; Monroe, M.B.; Day, D.S.; Shapiro, L.F.; Johnson, D.G.; Jones, P.P. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J. Clin. Endocrinol. Metab. 2001, 86, 4440–4444. [Google Scholar] [CrossRef]
- Alvarez, G.E.; Halliwill, J.R.; Ballard, T.P.; Beske, S.D.; Davy, K.P. Sympathetic neural regulation in endurance-trained humans: Fitness vs. fatness. J. Appl. Physiol. 2005, 98, 498–502. [Google Scholar] [CrossRef]
- Seals, D.R.; Bell, C. Chronic sympathetic activation: Consequence and cause of age-associated obesity? Diabetes 2004, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.P.; Davy, K.P.; Seals, D.R. Relations of total and abdominal adiposity to muscle sympathetic nerve activity in healthy older males. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.B.; Van Pelt, R.E.; Schiller, B.C.; Seals, D.R.; Jones, P.P. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Stob, N.R.; Seals, D.R. Thermogenic responsiveness to beta-adrenergic stimulation is augmented in exercising versus sedentary adults: Role of oxidative stress. J. Physiol. 2006, 570, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.A.; Richards, J.C.; Johnson, T.K.; Kuzma, J.N.; Lonac, M.C.; Paxton, R.J.; Rynn, G.M.; Voyles, W.F.; Bell, C. Short-term sympathoadrenal inhibition augments the thermogenic response to beta-adrenergic receptor stimulation. J. Endocrinol. 2010, 206, 307–315. [Google Scholar] [CrossRef]
- Seals, D.R.; Dinenno, F.A. Collateral damage: Cardiovascular consequences of chronic sympathetic activation with human aging. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 1895–1905. [Google Scholar] [CrossRef]
- Gullestad, L.; Hallen, J.; Medbo, J.I.; Gronnerod, O.; Holme, I.; Sejersted, O.M. The effect of acute vs chronic treatment with beta-adrenoceptor blockade on exercise performance, haemodynamic and metabolic parameters in healthy men and women. Br. J. Clin. Pharmacol. 1996, 41, 57–67. [Google Scholar] [CrossRef]
- Martin, N.B.; Broeder, C.E.; Thomas, E.L.; Wambsgans, K.C.; Scruggs, K.D.; Jesek, J.K.; Hofman, Z.; Wilmore, J.H. Comparison of the effects of pindolol and propranolol on exercise performance in young men with systemic hypertension. Am. J. Cardiol. 1989, 64, 343–347. [Google Scholar] [CrossRef]
- Proctor, D.N.; Luck, J.C.; Maman, S.R.; Leuenberger, U.A.; Muller, M.D. Esmolol acutely alters oxygen supply-demand balance in exercising muscles of healthy humans. Physiol. Rep. 2018, 6, 13673. [Google Scholar] [CrossRef]
- Wolfel, E.E.; Selland, M.A.; Cymerman, A.; Brooks, G.A.; Butterfield, G.E.; Mazzeo, R.S.; Grover, R.F.; Reeves, J.T. O2 extraction maintains O2 uptake during submaximal exercise with beta-adrenergic blockade at 4,300 m. J. Appl. Physiol. 1998, 85, 1092–1102. [Google Scholar] [CrossRef][Green Version]
- Silva, J.E.; Bianco, S.D. Thyroid-adrenergic interactions: Physiological and clinical implications. Thyroid 2008, 18, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Danforth, E., Jr. The role of thyroid hormones and insulin in the regulation of energy metabolism. Am. J. Clin. Nutr. 1983, 38, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Serog, P.; Apfelbaum, M.; Autissier, N.; Baigts, F.; Brigant, L.; Ktorza, A. Effects of slimming and composition of diets on VO2 and thyroid hormones in healthy subjects. Am. J. Clin. Nutr. 1982, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Melby, C.; Scholl, C.; Edwards, G.; Bullough, R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J. Appl. Physiol. 1993, 75, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, K.L.; Melby, C.L. Effect of acute resistance exercise on postexercise oxygen consumption and resting metabolic rate in young women. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Heller, M.; Later, W.; Heymsfield, S.B.; Muller, M.J. Evaluation of specific metabolic rates of major organs and tissues: Comparison between men and women. Am. J. Hum. Biol. 2011, 23, 333–338. [Google Scholar] [CrossRef]
- Blundell, J.E.; Finlayson, G.; Gibbons, C.; Caudwell, P.; Hopkins, M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol. Behav. 2015, 152, 473–478. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Jacquet, J.; Miles-Chan, J.L.; Schutz, Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur. J. Clin. Nutr. 2017, 71, 353–357. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Hopkins, M.; Finlayson, G.S.; Duarte, C.; Gibbons, C.; Blundell, J.E. Potential effects of fat mass and fat-free mass on energy intake in different states of energy balance. Eur. J. Clin. Nutr. 2018, 72, 698–709. [Google Scholar] [CrossRef]
- Reed, G.W.; Hill, J.O. Measuring the thermic effect of food. Am. J. Clin. Nutr. 1996, 63, 164–169. [Google Scholar] [CrossRef]
- Schutz, Y.; Bessard, T.; Jequier, E. Exercise and postprandial thermogenesis in obese women before and after weight loss. Am. J. Clin. Nutr. 1987, 45, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Burkhard-Jagodzinska, K.; Nazar, K.; Ladyga, M.; Starczewska-Czapowska, J.; Borkowski, L. Resting metabolic rate and thermogenic effect of glucose in trained and untrained girls age 11–15 years. Int. J. Sport Nutr. 1999, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Tagliaferro, A.R.; Kertzer, R.; Gerardo, T.; Nichols, J.; Wheeler, J. Variations of dietary-induced thermogenesis and body fatness with aerobic capacity. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.P.; Van Pelt, R.E.; Johnson, D.G.; Seals, D.R. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J. Clin. Endocrinol. Metab. 2004, 89, 5138–5144. [Google Scholar] [CrossRef]
- Stob, N.R.; Bell, C.; van Baak, M.A.; Seals, D.R. Thermic effect of food and beta-adrenergic thermogenic responsiveness in habitually exercising and sedentary healthy adult humans. J. Appl. Physiol. 2007, 103, 616–622. [Google Scholar] [CrossRef][Green Version]
- Thyfault, J.P.; Richmond, S.R.; Carper, M.J.; Potteiger, J.A.; Hulver, M.W. Postprandial metabolism in resistance-trained versus sedentary males. Med. Sci. Sports Exerc. 2004, 36, 709–716. [Google Scholar] [CrossRef]
- Witt, K.A.; Snook, J.T.; O’Dorisio, T.M.; Zivony, D.; Malarkey, W.B. Exercise training and dietary carbohydrate: Effects on selected hormones and the thermic effect of feeding. Int. J. Sport Nutr. 1993, 3, 272–289. [Google Scholar] [CrossRef]
- Matsuo, T.; Suzuki, M. Effects of dumb-bell exercise with and without energy restriction on resting metabolic rate, diet-induced thermogenesis and body composition in mildly obese women. Asia Pac. J. Clin. Nutr. 1999, 8, 136–141. [Google Scholar] [CrossRef]
- Morio, B.; Montaurier, C.; Pickering, G.; Ritz, P.; Fellmann, N.; Coudert, J.; Beaufrere, B.; Vermorel, M. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br. J. Nutr. 1998, 80, 511–519. [Google Scholar] [CrossRef]
- Luscombe, N.D.; Clifton, P.M.; Noakes, M.; Farnsworth, E.; Wittert, G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 582–590. [Google Scholar] [CrossRef]
- Nichols, J.F.; Bigelow, D.M.; Canine, K.M. Short-term weight loss and exercise training effects on glucose-induced thermogenesis in obese adolescent males during hypocaloric feeding. Int. J. Obes. 1989, 13, 683–690. [Google Scholar] [PubMed]
- Quatela, A.; Callister, R.; Patterson, A.; MacDonald-Wicks, L. The Energy Content and Composition of Meals Consumed after an Overnight Fast and Their Effects on Diet Induced Thermogenesis: A Systematic Review, Meta-Analyses and Meta-Regressions. Nutrients 2016, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.M.; Castillo, P.; Pi-Sunyer, F.X. Meal size and frequency: Effect on the thermic effect of food. Am. J. Clin. Nutr. 1991, 54, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Calame, W.; Siemensma, A.D.; Saris, W.H.; van Baak, M.A. The thermogenic and metabolic effects of protein hydrolysate with or without a carbohydrate load in healthy male subjects. Metabolism 2007, 56, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Van Baak, M.A. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 2008, 94, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Additional protein intake limits weight regain after weight loss in humans. Br. J. Nutr. 2005, 93, 281–289. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Nijs, I.; van Ooijen, M.; Kovacs, E.M. High protein intake sustains weight maintenance after body weight loss in humans. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 57–64. [Google Scholar] [CrossRef]
- Muller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef]
- Muller, M.J.; Enderle, J.; Pourhassan, M.; Braun, W.; Eggeling, B.; Lagerpusch, M.; Gluer, C.C.; Kehayias, J.J.; Kiosz, D.; Bosy-Westphal, A. Metabolic adaptation to caloric restriction and subsequent refeeding: The Minnesota Starvation Experiment revisited. Am. J. Clin. Nutr. 2015, 102, 807–819. [Google Scholar] [CrossRef]
- Hill, J.O.; Peters, J.C.; Wyatt, H.R. Using the energy gap to address obesity: A commentary. J. Am. Diet. Assoc. 2009, 109, 1848–1853. [Google Scholar] [CrossRef]
- Dulloo, A.G. Collateral fattening: When a deficit in lean body mass drives overeating. Obesity 2017, 25, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Heaner, M.; Goldsmith, R.L.; Christian Schulze, P.; Shukla, A.; Shen, W.; Shane, E.J.; Naor, E.; Leibel, R.L.; Aronne, L.J. Resistance Training Reduces Skeletal Muscle Work Efficiency in Weight-Reduced and Non-Weight-Reduced Subjects. Obesity 2018, 26, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Casanova, N.; Beaulieu, K.; Finlayson, G.; Hopkins, M. Metabolic adaptations during negative energy balance and their potential impact on appetite and food intake. Proc. Nutr. Soc. 2019, 78, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Maclean, P.S.; Bergouignan, A.; Cornier, M.A.; Jackman, M.R. Biology’s response to dieting: The impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 581–600. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Marcus, B.H.; Lang, W.; Janney, C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch. Int. Med. 2008, 168, 1550–1559. [Google Scholar] [CrossRef]
- Klem, M.L.; Wing, R.R.; McGuire, M.T.; Seagle, H.M.; Hill, J.O. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am. J. Clin. Nutr. 1997, 66, 239–246. [Google Scholar] [CrossRef]
- Schoeller, D.A.; Shay, K.; Kushner, R.F. How much physical activity is needed to minimize weight gain in previously obese women? Am. J. Clin. Nutr. 1997, 66, 551–556. [Google Scholar] [CrossRef]
- Foright, R.M.; Presby, D.M.; Sherk, V.D.; Kahn, D.; Checkley, L.A.; Giles, E.D.; Bergouignan, A.; Higgins, J.A.; Jackman, M.R.; Hill, J.O.; et al. Is regular exercise an effective strategy for weight loss maintenance? Physiol. Behav. 2018, 188, 86–93. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- MacLean, P.S.; Higgins, J.A.; Wyatt, H.R.; Melanson, E.L.; Johnson, G.C.; Jackman, M.R.; Giles, E.D.; Brown, I.E.; Hill, J.O. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Karfopoulou, E.; Mouliou, K.; Koutras, Y.; Yannakoulia, M. Behaviours associated with weight loss maintenance and regaining in a Mediterranean population sample. A qualitative study. Clin. Obes. 2013, 3, 141–149. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melby, C.L.; Paris, H.L.; Sayer, R.D.; Bell, C.; Hill, J.O. Increasing Energy Flux to Maintain Diet-Induced Weight Loss. Nutrients 2019, 11, 2533. https://doi.org/10.3390/nu11102533
Melby CL, Paris HL, Sayer RD, Bell C, Hill JO. Increasing Energy Flux to Maintain Diet-Induced Weight Loss. Nutrients. 2019; 11(10):2533. https://doi.org/10.3390/nu11102533
Chicago/Turabian StyleMelby, Christopher L., Hunter L. Paris, R. Drew Sayer, Christopher Bell, and James O. Hill. 2019. "Increasing Energy Flux to Maintain Diet-Induced Weight Loss" Nutrients 11, no. 10: 2533. https://doi.org/10.3390/nu11102533
APA StyleMelby, C. L., Paris, H. L., Sayer, R. D., Bell, C., & Hill, J. O. (2019). Increasing Energy Flux to Maintain Diet-Induced Weight Loss. Nutrients, 11(10), 2533. https://doi.org/10.3390/nu11102533





