Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Clarified Açaí (Euterpe Oleracea, Mart.) Juice for Human Consumption
2.3. Analysis of the Juice Composition
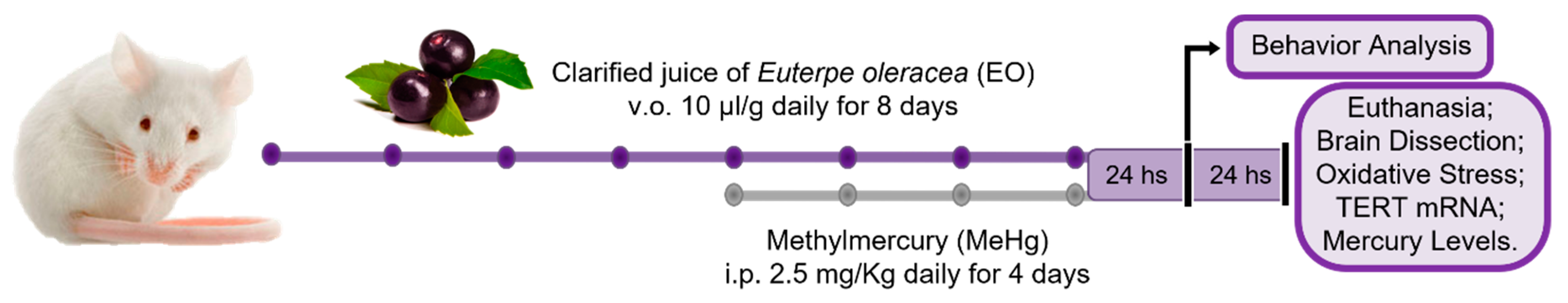
2.4. Treatments and Samples Collection
2.5. Open Field Test
2.6. Rotarod Test
2.7. Pole Test
2.8. Quantitation of the Total Protein Content
2.9. Assay of Lipid Peroxidation
2.10. Quantitation of Nitrite Levels
2.11. Telomerase Reverse Transcriptase (TERT) mRNA Expression in the Brain
2.12. Assay of Mercury Content in the Brain
2.13. Statistical Analysis
3. Results
3.1. Composition of the Euterpe Oleracea Juice for Human Consumption
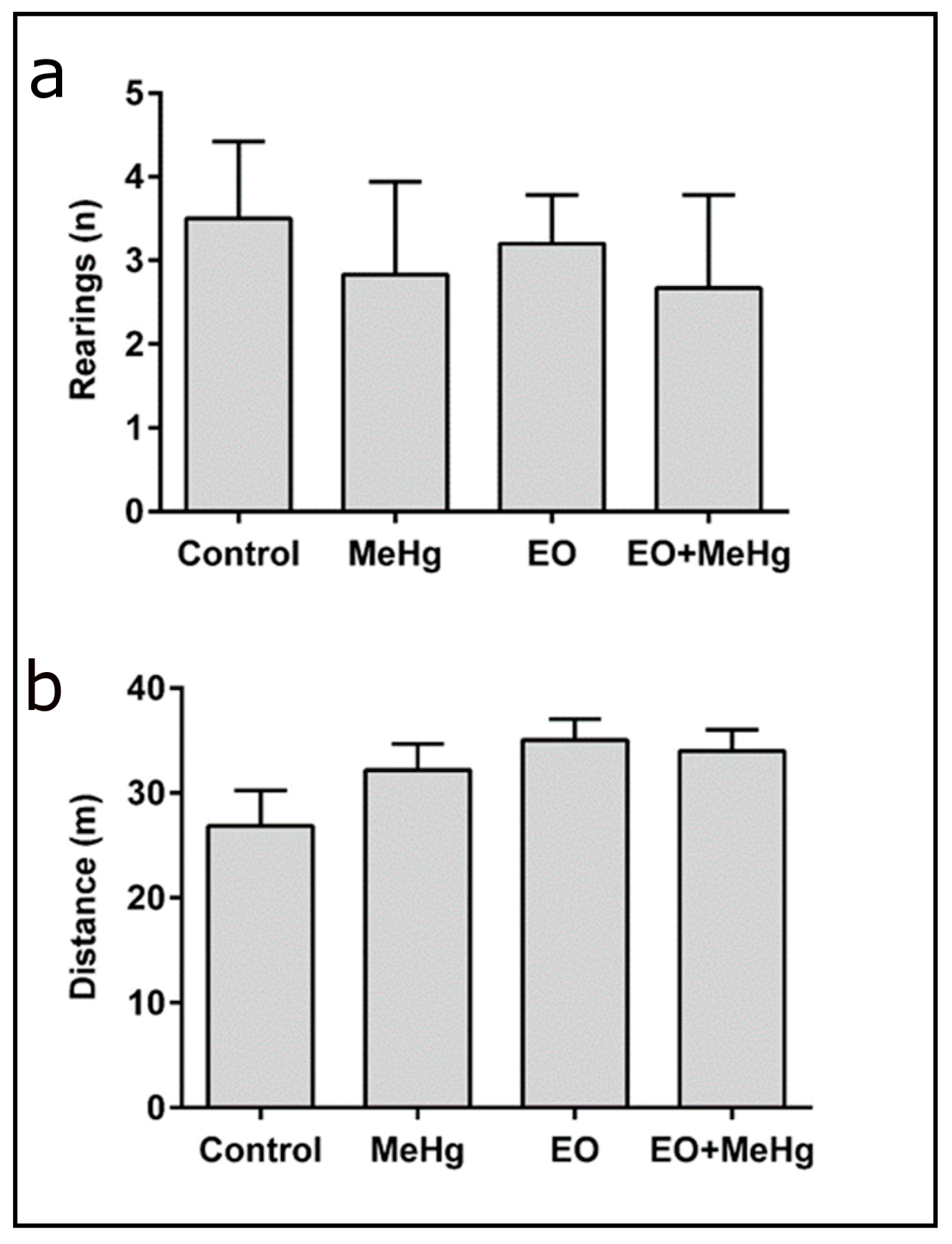
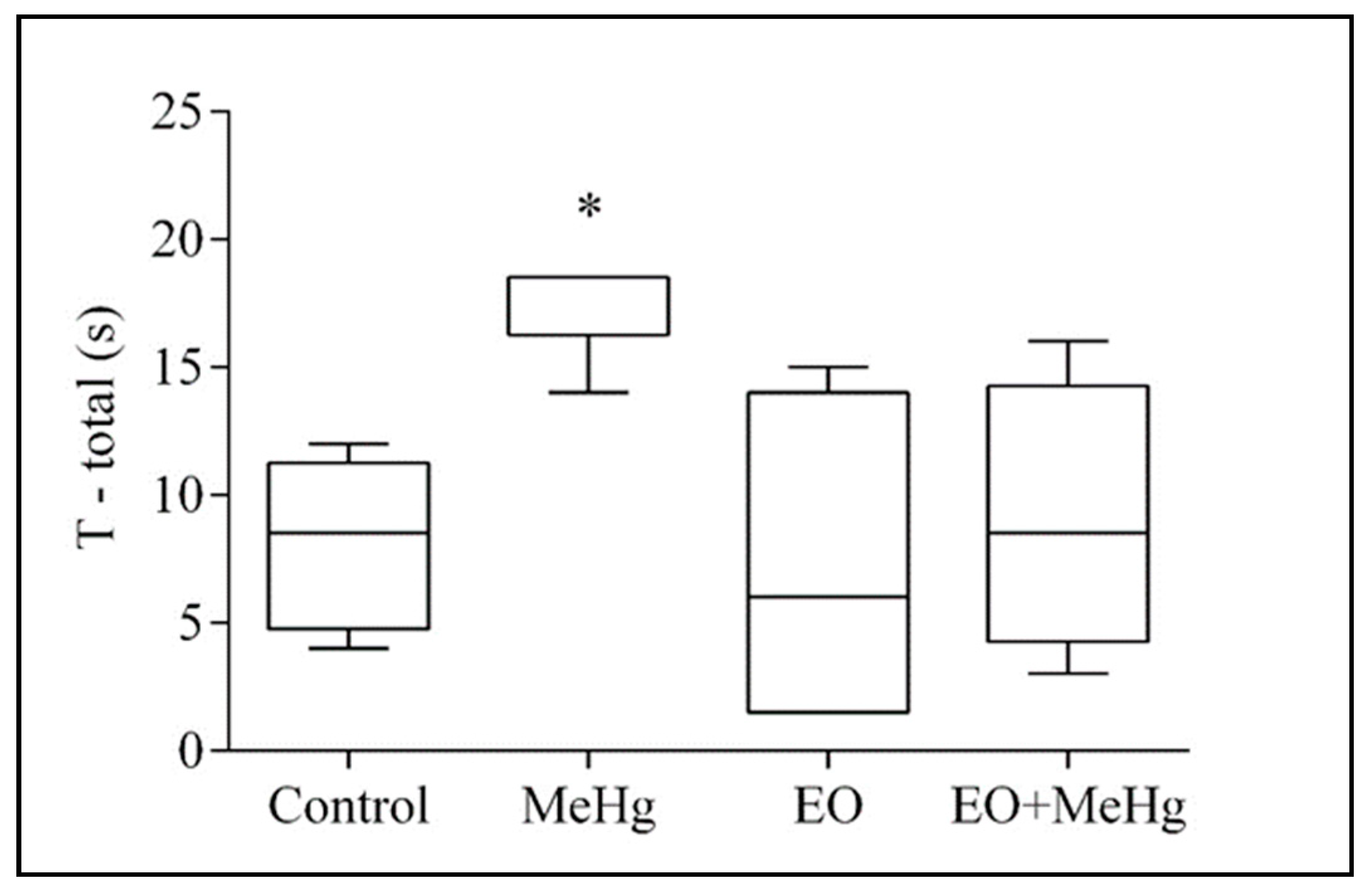
3.2. Behavioral Analysis
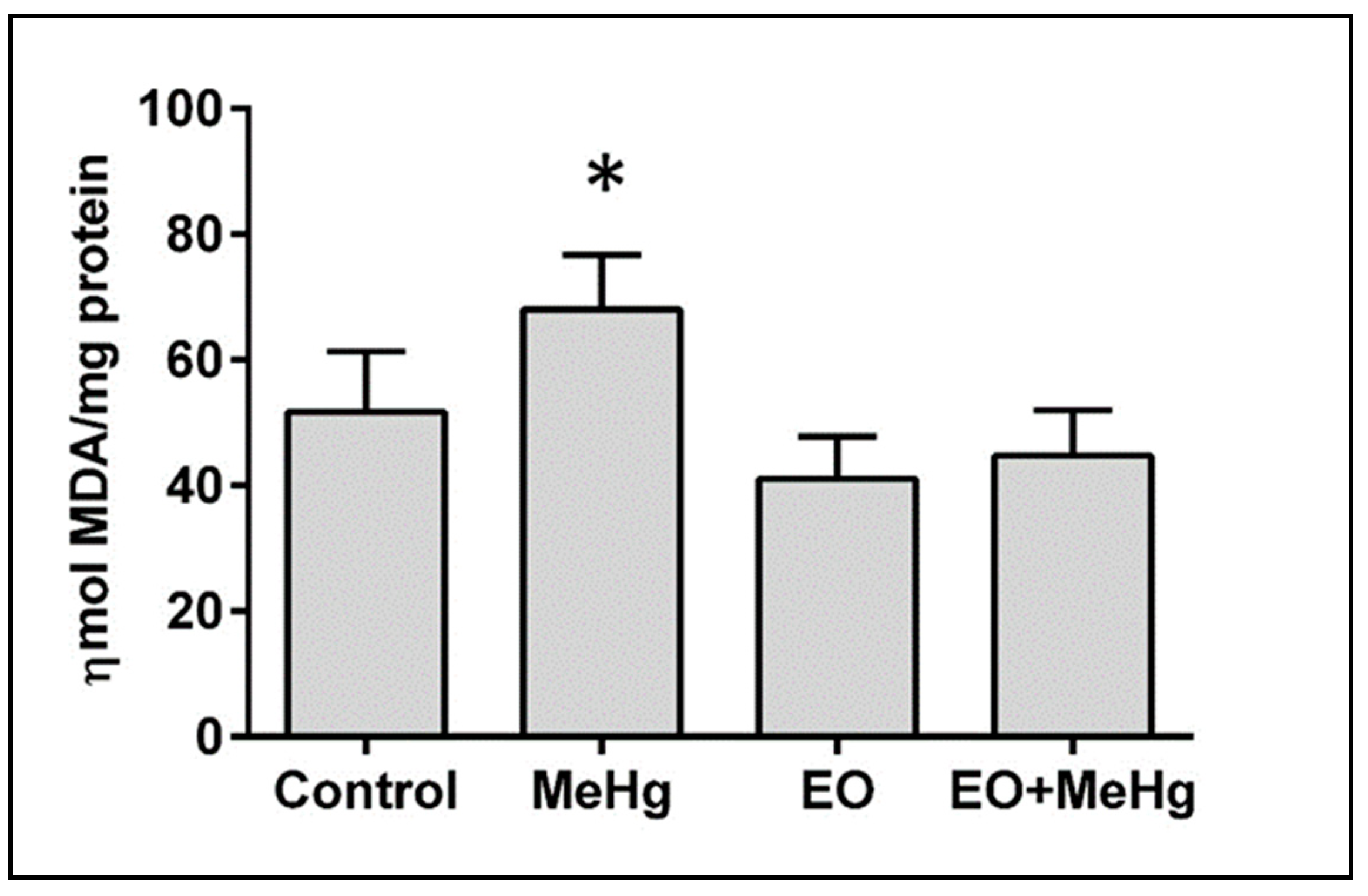
3.3. Analysis of Oxidative Stress
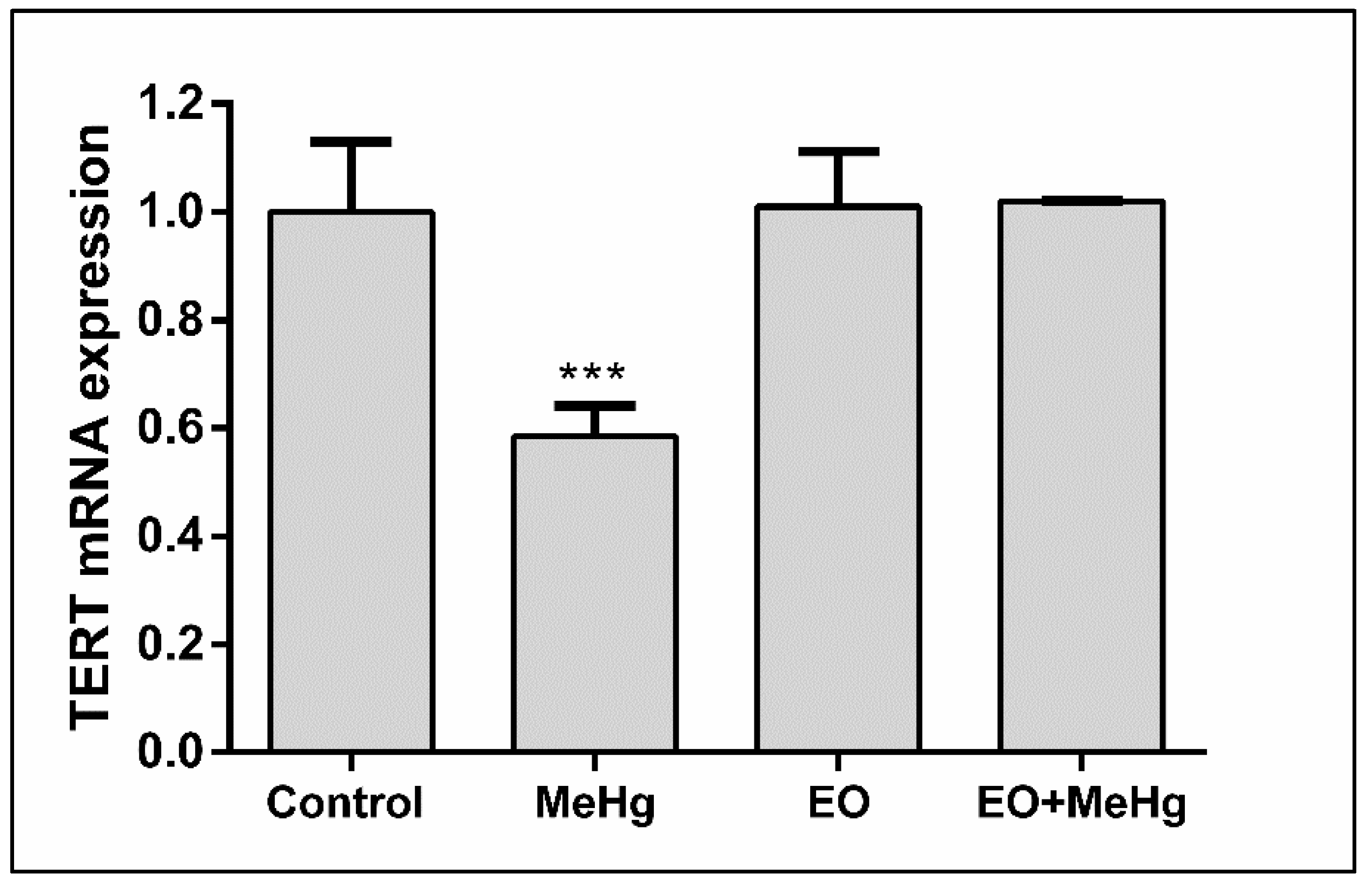
3.4. Telomerase Reverse Transcriptase (TERT) mRNA Expression in the Brain
3.5. Analysis of Mercury Content in the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crespo-Lopez, M.E.; Herculano, A.M.; Corvelo, T.C.; Do Nascimento, J.L. Mercury and neurotoxicity. Rev. Neurol. 2005, 40, 441–447. [Google Scholar]
- Nevado, J.B.; Martín-Doimeadios, R.R.; Bernardo, F.G.; Moreno, M.J.; Herculano, A.M.; Do Nascimento, J.L.M.; Crespo-López, M.E. Mercury in the tapajos river basin, brazilian amazon: A review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef]
- Roulet, M.; Lucotte, M.; Saint-Aubin, A.; Tran, S.; Rheault, I.; Farella, N.; Dezencourt, J.; Passos, C.J.S.; Soares, G.S.; Guimaraes, J.R.; et al. The geochemistry of mercury in central amazonian soils developed on the alter-do-chao formation of the lower tapajos river valley, para state, brazil. Sci. Total Environ. 1998, 223, 1–24. [Google Scholar] [CrossRef]
- Wasserman, J.C.; Hacon, S.; Wasserman, M.A. Biogeochemistry of mercury in the amazonian environment. Ambio 2003, 32, 336–342. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Ramirez-Mateos, V.; da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the tucurui dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Martín-Doimeadios, R.R.; Nevado, J.B.; Bernardo, F.G.; Moreno, M.J.; Arrifano, G.P.F.; Herculano, A.M.; do Nascimento, J.L.M.; Crespo-López, M.E. Comparative study of mercury speciation in commercial fishes of the brazilian amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Fernandez-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Alvarez-Leite, J.I.; do Nascimento, J.L.M.; Amador, M.T.; et al. Genetic susceptibility to neurodegeneration in amazon: Apolipoprotein e genotyping in vulnerable populations exposed to mercury. Front. Genet. 2018, 9, 285. [Google Scholar] [CrossRef]
- Arrifano, G.D.P.F.; Martin-Doimeadios, R.D.C.R.; Jiménez-Moreno, M.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Paraense, R.; Machado, C.R.; Farina, M.; Macchi, B.; do Nascimento, J.L.M.; et al. Assessing mercury intoxication in isolated/remote populations: Increased s100b mrna in blood in exposed riverine inhabitants of the amazon. Neurotoxicology 2018, 68, 151–158. [Google Scholar] [CrossRef]
- Rice, G.E.; Hammitt, J.K.; Evans, J.S. A probabilistic characterization of the health benefits of reducing methyl mercury intake in the united states. Environ. Sci. Technol. 2010, 44, 5216–5224. [Google Scholar] [CrossRef]
- Allen, B.C.; Hack, C.E.; Clewell, H.J. Use of markov chain monte carlo analysis with a physiologically-based pharmacokinetic model of methylmercury to estimate exposures in us women of childbearing age. Risk Anal. 2007, 27, 947–959. [Google Scholar] [CrossRef]
- Stern, A.H. A revised probabilistic estimate of the maternal methyl mercury intake dose corresponding to a measured cord blood mercury concentration. Environ. Health Perspect. 2005, 113, 155–163. [Google Scholar] [CrossRef]
- Vandewater, L.J.; Racz, W.J.; Norris, A.R.; Buncel, E. Methylmercury distribution, metabolism, and neurotoxicity in the mouse brain. Can. J. Physiol. Pharmacol. 1983, 61, 1487–1493. [Google Scholar] [CrossRef]
- Takahashi, T.; Fujimura, M.; Koyama, M.; Kanazawa, M.; Usuki, F.; Nishizawa, M.; Shimohata, T. Methylmercury causes blood-brain barrier damage in rats via upregulation of vascular endothelial growth factor expression. PLoS ONE 2017, 12, e0170623. [Google Scholar] [CrossRef]
- Carta, P.; Flore, C.; Alinovi, R.; Ibba, A.; Tocco, M.G.; Aru, G.; Carta, R.; Girei, E.; Mutti, A.; Lucchini, R.; et al. Sub-clinical neurobehavioral abnormalities associated with low level of mercury exposure through fish consumption. Neurotoxicology 2003, 24, 617–623. [Google Scholar] [CrossRef]
- Auger, N.; Kofman, O.; Kosatsky, T.; Armstrong, B. Low-level methylmercury exposure as a risk factor for neurologic abnormalities in adults. Neurotoxicology 2005, 26, 149–157. [Google Scholar] [CrossRef]
- Costa, J.M.F.J.; Lima, A.; Rodrigues, D.J.; Khoury, E.D.T.; Souza, G.D.S.; Silveira, L.C.L.; Pinheiro, M. Emotional and motor symptoms in riverside dwellers exposed to mercury in the amazon. Rev. Bra. Epidemiol. = Braz. J. Epidemiol. 2017, 20, 212–224. [Google Scholar]
- Crespo-Lopez, M.E.; Macedo, G.L.; Pereira, S.I.; Arrifano, G.P.; Picanco-Diniz, D.L.; do Nascimento, J.L.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M.; Rocha, J.B. Oxidative stress in mehg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef]
- Passos, C.J.; Mergler, D.; Fillion, M.; Lemire, M.; Mertens, F.; Guimaraes, J.R.; Philibert, A. Epidemiologic confirmation that fruit consumption influences mercury exposure in riparian communities in the brazilian amazon. Environ. Res. 2007, 105, 183–193. [Google Scholar] [CrossRef]
- Arantes, L.P.; Peres, T.V.; Chen, P.; Caito, S.; Aschner, M.; Soares, F.A. Guarana (paullinia cupana mart.) attenuates methylmercury-induced toxicity in caenorhabditis elegans. Toxicol. Res. (Camb.) 2016, 5, 1629–1638. [Google Scholar] [CrossRef]
- Algarve, T.D.; Assmann, C.E.; Cadona, F.C.; Machado, A.K.; Manica-Cattani, M.F.; Sato-Miyata, Y.; Asano, T.; Duarte, M.; Ribeiro, E.E.; Aigaki, T.; et al. Guarana improves behavior and inflammatory alterations triggered by methylmercury exposure: An in vivo fruit fly and in vitro neural cells study. Environ. Sci. Pollut. Res. Int. 2019, 26, 15069–15083. [Google Scholar] [CrossRef]
- Frenedoso da Silva, R.; Missassi, G.; dos Santos Borges, C.; Silva de Paula, E.; Hornos Carneiro, M.F.; Grotto, D.; Barbosa Junior, F.; De Grava Kempinas, W. Phytoremediation potential of mana-cubiu (solanum sessiliflorum dunal) for the deleterious effects of methylmercury on the reproductive system of rats. Biomed. Res. Int 2014, 2014, 309631. [Google Scholar] [CrossRef]
- Leao, L.K.R.; Herculano, A.M.; Maximino, C.; Brasil Costa, A.; Gouveia, A., Jr.; Batista, E.O.; Rocha, F.F.; Crespo-Lopez, M.E.; Borges, R.; Oliveira, K. Mauritia flexuosa l. Protects against deficits in memory acquisition and oxidative stress in rat hippocampus induced by methylmercury exposure. Nutr. Neurosci. 2017, 20, 297–304. [Google Scholar] [CrossRef]
- Brasil, A.; Rocha, F.A.F.; Gomes, B.D.; Oliveira, K.R.M.; de Carvalho, T.S.; Batista, E.J.O.; Borges, R.D.S.; Kremers, J.; Herculano, A.M. Diet enriched with the amazon fruit acai (euterpe oleracea) prevents electrophysiological deficits and oxidative stress induced by methyl-mercury in the rat retina. Nutr. Neurosci. 2017, 20, 265–272. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Hamoy, M.; Santana-Coelho, D.; Arrifano, G.P.; Paraense, R.S.; Costa-Malaquias, A.; Mendonca, J.R.; da Silva, R.F.; Monteiro, W.S.; Rogez, H.; et al. Anticonvulsant properties of euterpe oleracea in mice. Neurochem. Int. 2015, 90, 20–27. [Google Scholar] [CrossRef]
- Bichara, C.M.G.; Rogez, H. Açai to citrus. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Amsterdam, The Netherlands, 2011; Volume 2, pp. 1–26. [Google Scholar]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.; Mello, B.S.F.; Custodio, C.S.; Macedo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and antiaging effects of acai (euterpe oleracea mart.) in mice. Oxid. Med. Cell. Longev. 2019, 2019, 3614960. [Google Scholar] [CrossRef]
- Meramat, A.; Rajab, N.F.; Shahar, S.; Sharif, R. Cognitive impairment, genomic instability and trace elements. J. Nutr. Health Aging 2015, 19, 48–57. [Google Scholar] [CrossRef]
- Dias, A.L.; Rozet, E.; Chataigne, G.; Oliveira, A.C.; Rabelo, C.A.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. A rapid validated uhplc-pda method for anthocyanins quantification from euterpe oleracea fruits. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 907, 108–116. [Google Scholar] [CrossRef]
- Dias, A.L.; Rozet, E.; Larondelle, Y.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. Development and validation of an uhplc-ltq-orbitrap ms method for non-anthocyanin flavonoids quantification in euterpe oleracea juice. Anal. Bioanal. Chem. 2013, 405, 9235–9249. [Google Scholar] [CrossRef]
- Yee, S.; Choi, B.H. Methylmercury poisoning induces oxidative stress in the mouse brain. Exp. Mol. Pathol. 1994, 60, 188–196. [Google Scholar] [CrossRef]
- Fontes-Junior, E.A.; Maia, C.S.; Fernandes, L.M.; Gomes-Leal, W.; Costa-Malaquias, A.; Lima, R.R.; Prediger, R.D.; Crespo-Lopez, M.E. Chronic alcohol intoxication and cortical ischemia: Study of their comorbidity and the protective effects of minocycline. Oxid. Med. Cell. Longev. 2016, 2016, 1341453. [Google Scholar] [CrossRef]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in mptp-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50, 435–441. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Akagi, H. Mercury Analysis Manual; M.O.T., Ed.; Ministry of the Environment: Tokyo, Japan, 2004; p. 105.
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Branco, V.; Caito, S.; Farina, M.; Teixeira da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 119–154. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Serpeloni, J.M.; Batista, B.L.; Souza, S.S.; Barbosa, F., Jr. Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010, 84, 891–896. [Google Scholar] [CrossRef]
- Burbacher, T.M.; Shen, D.D.; Liberato, N.; Grant, K.S.; Cernichiari, E.; Clarkson, T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ. Health Perspect. 2005, 113, 1015–1021. [Google Scholar] [CrossRef]
- Nevado, J.J.B.; Martin-Doimeadios, R.C.R.; Moreno, M.J.; do Nascimento, J.L.M.; Herculano, A.M.; Crespo-Lopez, M.E. Mercury speciation analysis on cell lines of the human central nervous system to explain genotoxic effects. Microchem. J. 2009, 93, 12–16. [Google Scholar] [CrossRef]
- Bellum, S.; Thuett, K.A.; Bawa, B.; Abbott, L.C. The effect of methylmercury exposure on behavior and cerebellar granule cell physiology in aged mice. J. Appl. Toxicol. JAT 2013, 33, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Bourdineaud, J.P.; Laclau, M.; Maury-Brachet, R.; Gonzalez, P.; Baudrimont, M.; Mesmer-Dudons, N.; Fujimura, M.; Marighetto, A.; Godefroy, D.; Rostene, W.; et al. Effects of methylmercury contained in a diet mimicking the wayana amerindians contamination through fish consumption: Mercury accumulation, metallothionein induction, gene expression variations, and role of the chemokine ccl2. Int. J. Mol. Sci. 2012, 13, 7710–7738. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K.V.; Sahani, M.; Churchland, M.M. Cortical control of arm movements: A dynamical systems perspective. Annu. Rev. Neurosci. 2013, 36, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Belem-Filho, I.J.A.; Ribera, P.C.; Nascimento, A.L.; Gomes, A.R.Q.; Lima, R.R.; Crespo-Lopez, M.E.; Monteiro, M.C.; Fontes-Junior, E.A.; Lima, M.O.; Maia, C.S.F. Low doses of methylmercury intoxication solely or associated to ethanol binge drinking induce psychiatric-like disorders in adolescent female rats. Environ. Toxicol. Pharmacol. 2018, 60, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.; Pinheiro, A.M.; Belem-Filho, I.J.A.; Fernandes, L.M.P.; Cartagenes, S.C.; Ribera, P.C.; Fontes-Junior, E.A.; Crespo-Lopez, M.E.; Monteiro, M.C.; Lima, M.O.; et al. Unravelling motor behaviour hallmarks in intoxicated adolescents: Methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 21937–21948. [Google Scholar] [CrossRef]
- Matsuura, K.; Kabuto, H.; Makino, H.; Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods 1997, 73, 45–48. [Google Scholar] [CrossRef]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Rozas, G.; Guerra, M.J.; Labandeira-Garcia, J.L. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res. Protoc. 1997, 2, 75–84. [Google Scholar] [CrossRef]
- Karl, T.; Pabst, R.; von Horsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp.Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef]
- Lin, C.Y.; Liou, S.H.; Hsiech, C.M.; Ku, M.C.; Tsai, S.Y. Dose-response relationship between cumulative mercury exposure index and specific uptake ratio in the striatum on tc-99m trodat spect. Clin. Nucl. Med. 2011, 36, 689–693. [Google Scholar] [CrossRef]
- Olczak, M.; Duszczyk, M.; Mierzejewski, P.; Meyza, K.; Majewska, M.D. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav. Brain Res. 2011, 223, 107–118. [Google Scholar] [CrossRef]
- Coccini, T.; Roda, E.; Castoldi, A.F.; Poli, D.; Goldoni, M.; Vettori, M.V.; Mutti, A.; Manzo, L. Developmental exposure to methylmercury and 2,2′,4,4′,5,5′-hexachlorobiphenyl (pcb153) affects cerebral dopamine d1-like and d2-like receptors of weanling and pubertal rats. Arch. Toxicol. 2011, 85, 1281–1294. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Costa-Malaquias, A.; Oliveira, E.H.; Miranda, M.S.; Arrifano, G.P.; Souza-Monteiro, J.R.; Sagica, F.E.; Fontes-Junior, E.A.; Maia, C.S.; Macchi, B.M.; et al. Is low non-lethal concentration of methylmercury really safe? A report on genotoxicity with delayed cell proliferation. PLoS ONE 2016, 11, e162822. [Google Scholar] [CrossRef]
- Costa-Malaquias, A.; Almeida, M.B.; Souza Monteiro, J.R.; Macchi Bde, M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Morphine protects against methylmercury intoxication: A role for opioid receptors in oxidative stress? PLoS ONE 2014, 9, e110815. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Puty, B.; Charone, S.; Aragao, W.A.B.; Farias-Junior, P.M.; Silva, M.C.F.; Crespo-Lopez, M.E.; Leite, A.L.; Buzalaf, M.A.R.; Lima, R.R. Oxidative biochemistry disbalance and changes on proteomic profile in salivary glands of rats induced by chronic exposure to methylmercury. Oxid. Med. Cell. Longev. 2017, 2017, 5653291. [Google Scholar] [CrossRef]
- Huang, C.F.; Liu, S.H.; Hsu, C.J.; Lin-Shiau, S.Y. Neurotoxicological effects of low-dose methylmercury and mercuric chloride in developing offspring mice. Toxicol. Lett. 2011, 201, 196–204. [Google Scholar] [CrossRef]
- Maues, L.A.; Macchi, B.M.; Crespo-Lopez, M.E.; Nasciutti, L.E.; Picanco-Diniz, D.L.; Antunes-Rodrigues, J.; Nascimento, J.L. Methylmercury inhibits prolactin release in a cell line of pituitary origin. Braz. J. Med. Biol. Res. 2015, 48, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Caito, S.; Zeng, H.; Aschner, J.L.; Aschner, M. Methylmercury alters the activities of hsp90 client proteins, prostaglandin e synthase/p23 (pges/23) and nnos. PLoS ONE 2014, 9, e98161. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Macedo, G.L.; Arrifano, G.P.; Pinheiro Mda, C.; do Nascimento, J.L.; Herculano, A.M. Genotoxicity of mercury: Contributing for the analysis of amazonian populations. Environ. Int. 2011, 37, 136–141. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Lima de Sa, A.; Herculano, A.M.; Rodriguez Burbano, R.; Martins do Nascimento, J.L. Methylmercury genotoxicity: A novel effect in human cell lines of the central nervous system. Environ. Int. 2007, 33, 141–146. [Google Scholar] [CrossRef]
- Cebulska-Wasilewska, A.; Panek, A.; Zabinski, Z.; Moszczynski, P.; Au, W.W. Occupational exposure to mercury vapour on genotoxicity and DNA repair. Mutat. Res. 2005, 586, 102–114. [Google Scholar] [CrossRef]
- Xu, X.; Liao, W.; Lin, Y.; Dai, Y.; Shi, Z.; Huo, X. Blood concentrations of lead, cadmium, mercury and their association with biomarkers of DNA oxidative damage in preschool children living in an e-waste recycling area. Environ. Geochem. Health 2018, 40, 1481–1494. [Google Scholar] [CrossRef]
- Korashy, H.M.; Attafi, I.M.; Famulski, K.S.; Bakheet, S.A.; Hafez, M.M.; Alsaad, A.M.S.; Al-Ghadeer, A.R.M. Gene expression profiling to identify the toxicities and potentially relevant human disease outcomes associated with environmental heavy metal exposure. Environ. Pollut. 2017, 221, 64–74. [Google Scholar] [CrossRef]
- Al Bakheet, S.A.; Attafi, I.M.; Maayah, Z.H.; Abd-Allah, A.R.; Asiri, Y.A.; Korashy, H.M. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ. Pollut. 2013, 181, 226–232. [Google Scholar] [CrossRef]
- Vriens, A.; Nawrot, T.S.; Janssen, B.G.; Baeyens, W.; Bruckers, L.; Covaci, A.; De Craemer, S.; De Henauw, S.; Den Hond, E.; Loots, I.; et al. Exposure to environmental pollutants and their association with biomarkers of aging: A multipollutant approach. Environ. Sci. Technol. 2019, 53, 5966–5976. [Google Scholar] [CrossRef]
- Yeates, A.J.; Thurston, S.W.; Li, H.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Shamlaye, C.F.; Strain, J.J.; Myers, G.J.; Davidson, P.W.; et al. Pufa status and methylmercury exposure are not associated with leukocyte telomere length in mothers or their children in the seychelles child development study. J. Nutr. 2017, 147, 2018–2024. [Google Scholar] [CrossRef]
- Koedrith, P.; Seo, Y.R. Advances in carcinogenic metal toxicity and potential molecular markers. Int. J. Mol. Sci. 2011, 12, 9576–9595. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Alvarez-Leite, J.I.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.; Macchi, B.M.; Pinto, A.; Oria, R.B.; do Nascimento, J.L.M.; Crespo-Lopez, M.E. In the heart of the amazon: Noncommunicable diseases and apolipoprotein e4 genotype in the riverine population. Int. J. Environ. Res. Public Health 2018, 15, 1957. [Google Scholar] [CrossRef]
- Sumathi, T.; Christinal, J. Neuroprotective effect of portulaca oleraceae ethanolic extract ameliorates methylmercury induced cognitive dysfunction and oxidative stress in cerebellum and cortex of rat brain. Biol. Trace Elem. Res. 2016, 172, 155–165. [Google Scholar] [CrossRef]
- Christinal, J.; Sumathi, T. Effect of bacopa monniera extract on methylmercury-induced behavioral and histopathological changes in rats. Biol. Trace Elem. Res. 2013, 155, 56–64. [Google Scholar] [CrossRef]
- Lucena, G.M.; Prediger, R.D.; Silva, M.V.; Santos, S.N.; Silva, J.F.; Santos, A.R.; Azevedo, M.S.; Ferreira, V.M. Ethanolic extract from bulbs of cipura paludosa reduced long-lasting learning and memory deficits induced by prenatal methylmercury exposure in rats. Dev. Cogn. Neurosci. 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Black, P.; Niu, L.; Sachdeva, M.; Lean, D.; Poon, R.; Bowers, W.J.; Chan, H.M.; Arnason, J.T.; Pelletier, G. Modulation of the effects of methylmercury on rat neurodevelopment by co-exposure with labrador tea (rhododendron tomentosum ssp. Subarcticum). Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2336–2342. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Oxidative stress and methylmercury-induced neurotoxicity. In Developmental Neurotoxicology Research: Principles, Models, Techniques, Strategies and Mechanisms; Wang, C., Slikker, W., Jr., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 357–385. [Google Scholar]
- Yamaguchi, K.K.; Pereira, L.F.; Lamarao, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Sumathi, T.; Shobana, C.; Christinal, J.; Anusha, C. Protective effect of bacopa monniera on methyl mercury-induced oxidative stress in cerebellum of rats. Cell. Mol. Neurobiol. 2012, 32, 979–987. [Google Scholar] [CrossRef]
- Farina, M.; Franco, J.L.; Ribas, C.M.; Meotti, F.C.; Missau, F.C.; Pizzolatti, M.G.; Dafre, A.L.; Santos, A.R. Protective effects of polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J. Pharm. Pharmacol. 2005, 57, 1503–1508. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F. In situ different antioxidative systems contribute to the site-specific methylmercury neurotoxicity in mice. Toxicology 2017, 392, 55–63. [Google Scholar] [CrossRef]
- Xiao, Q.; Qu, Z.; Zhao, Y.; Yang, L.; Gao, P. Orientin ameliorates lps-induced inflammatory responses through the inhibitory of the nf-kappab pathway and nlrp3 inflammasome. Evid.-Based Complement. Altern. Med. eCAM 2017, 2017, 2495496. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, P.; Hao, L.; Tao, L.; Jia, J.; Gao, B.; Liu, J.Y.; Zheng, L.T.; Zhen, X. Antiinflammatory effects of orientin-2"-o-galactopyranoside on lipopolysaccharide-stimulated microglia. Biol. Pharm. Bull. 2014, 37, 1282–1294. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, W.Y.; Chang, C.C.; Liou, K.T.; Sung, Y.J.; Liao, J.F.; Chen, C.F.; Chang, S.; Hou, Y.C.; Chou, Y.C.; et al. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of nf-kappa b activation. J. Biomed. Sci. 2006, 13, 127–141. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich acai (euterpe oleracea mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain bv-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Lichtenstein, M.P.; Souza-Monteiro, J.R.; Farina, M.; Rogez, H.; Carvalho, J.C.T.; Sunol, C.; Crespo-Lopez, M.E. Clarified acai (euterpe oleracea) juice as an anticonvulsant agent: In vitro mechanistic study of gabaergic targets. Oxid. Med. Cell. Longev. 2018, 2018, 2678089. [Google Scholar] [CrossRef]
- Kuruvilla, K.P.; Nandhu, M.S.; Paul, J.; Paulose, C.S. Oxidative stress mediated neuronal damage in the corpus striatum of 6-hydroxydopamine lesioned parkinson’s rats: Neuroprotection by serotonin, gaba and bone marrow cells supplementation. J. Neurol. Sci. 2013, 331, 31–37. [Google Scholar] [CrossRef]



| Phenolic Compound | Content (mg/L) | Content (%) |
|---|---|---|
| Cyanidin 3-rutinoside | 448 | 63.9% * |
| Cyanidin 3-glucoside | 184 | 26.2% * |
| Taxifolin deoxyhexose | 308 | 18.5% ** |
| Orientin | 381 | 22.9% ** |
| Homoorientin | 247 | 14.8% ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-López, M.E.; Soares, E.S.; Macchi, B.d.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araújo, A.; Paraense, R.S.d.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients 2019, 11, 2585. https://doi.org/10.3390/nu11112585
Crespo-López ME, Soares ES, Macchi BdM, Santos-Sacramento L, Takeda PY, Lopes-Araújo A, Paraense RSdO, Souza-Monteiro JR, Augusto-Oliveira M, Luz DA, et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients. 2019; 11(11):2585. https://doi.org/10.3390/nu11112585
Chicago/Turabian StyleCrespo-López, Maria Elena, Ericks Sousa Soares, Barbarella de Matos Macchi, Leticia Santos-Sacramento, Priscila Yuki Takeda, Amanda Lopes-Araújo, Ricardo Sousa de Oliveira Paraense, José Rogério Souza-Monteiro, Marcus Augusto-Oliveira, Diandra Araújo Luz, and et al. 2019. "Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption" Nutrients 11, no. 11: 2585. https://doi.org/10.3390/nu11112585








