Abstract
There is little data on human milk oligosaccharide (HMO) composition in Sub-Saharan Africa. Iron fortificants adversely affect the infant gut microbiota, while co-provision of prebiotic galacto-oligosaccharides (GOS) mitigates most of the adverse effects. Whether variations in maternal HMO profile can influence the infant response to iron and/or GOS fortificants is unknown. The aim of this study was to determine HMO profiles and the secretor/non-secretor phenotype of lactating Kenyan mothers and investigate their effects on the maternal and infant gut microbiota, and on the infant response to a fortification intervention with 5 mg iron (2.5 mg as sodium iron ethylenediaminetetraacetate and 2.5 mg as ferrous fumarate) and 7.5 g GOS. We studied mother–infant pairs (n = 80) participating in a 4-month intervention trial in which the infants (aged 6.5–9.5 months) received daily a micronutrient powder without iron, with iron or with iron and GOS. We assessed: (1) maternal secretor status and HMO composition; (2) effects of secretor status on the maternal and infant gut microbiota in a cross-sectional analysis at baseline of the intervention trial; and (3) interactions between secretor status and intervention groups during the intervention trial on the infant gut microbiota, gut inflammation, iron status, growth and infectious morbidity. Secretor prevalence was 72% and HMOs differed between secretors and non-secretors and over time of lactation. Secretor status did not predict the baseline composition of the maternal and infant gut microbiota. There was a secretor-status-by-intervention-group interaction on Bifidobacterium (p = 0.021), Z-scores for length-for-age (p = 0.022) and weight-for-age (p = 0.018), and soluble transferrin receptor (p = 0.041). In the no iron group, longitudinal prevalence of diarrhea was higher among infants of non-secretors (23.8%) than of secretors (10.4%) (p = 0.001). In conclusion, HMO profile may modulate the infant gut microbiota response to fortificant iron; compared to infants of secretor mothers, infants of non-secretor mothers may be more vulnerable to the adverse effect of iron but also benefit more from the co-provision of GOS.
1. Introduction
Human milk oligosaccharides (HMOs) represent the third most abundant solid milk component after lipids and lactose in human breast milk, with a concentration of 5–10 g/L and more than 200 different structures [1]. The HMO composition of breast milk varies between mothers, differs across geographic regions, and changes over the course of lactation [2,3,4]. HMOs with L-fucose (Fuc) residuals in α-1-2-linkage such as 2-fucosyllactose (2′FL) and lacto-N-fucopentose I (LNFPI) are only present in breast milk of women with a functional α-1-2-fucosyltransferase (FUT2) (the secretor phenotype), but are absent in breast milk of women with a homozygous mutation in the FUT2 gene (the non-secretor phenotype) [5]. Frequency of the secretor phenotype is estimated at ~80% worldwide but varies between geographical regions [3,6]. The secretor phenotype predicts a higher risk for viral infections (e.g., norovirus [7,8], rotavirus [9,10,11] and respiratory viruses [12]), while the non-secretor phenotype predicts risk for enterotoxigenic Escherichia coli (ETEC) infection [13]. Two recent large cohort studies in the United Kingdom and Canada found no association between maternal secretor status and the overall maternal gut microbiota composition [14,15].
Most HMOs are not absorbed in the gastrointestinal tract of the breastfed infant and reach the colon intact, where they can act as prebiotics [16]. Bifidobacterium spp., with strain specific ability, and Bacteroides spp., both express enzymes for efficient use of HMOs as a carbon source [16]. The effects of HMOs on the breastfed infant gut microbiota likely depend on the breast milk HMO profile, and because there are differences in HMO composition between secretors and non-secretors [4,17], the effects may vary by maternal secretor status [18,19,20]. Abundances of Bifidobacterium were found to be higher among infants of secretor mothers [18,19,20], but not all studies agree [21,22,23]. Moreover, HMOs may act as anti-adhesive antimicrobials by operating as receptors for potential pathogenic bacteria (e.g., pathogenic E. coli, Campylobacter jejuni and Helicobacter pylori) and viruses [1,24,25,26,27,28]. High relative abundances of α-1-2-linked-fucosyloligosaccharides and a higher ratio of α-1-2-linked- to non-α-1-2-linked-fucosyloligosaccharides predict lower risk of infant diarrhea [29,30]. Recent studies suggest HMO composition might affect infant growth [31,32,33], but not all studies agree [34].
To treat or prevent iron deficiency anemia (IDA) many infants in low-income countries receive iron fortificants between 6–24 months of age [35]. However, iron is an essential micronutrient for most gut bacteria and is important for virulence and colonization of potential enteropathogens [36]. Two trials in Kenyan infants have reported lower abundances of Bifidobacteria and higher abundances of enteropathogens and enteropathogenic E. coli in infants receiving iron-containing micronutrient powders (MNPs) [37,38]. We have recently shown that co-provision of prebiotic galacto-oligosaccharides (GOS) in iron-containing MNPs mitigates most of the adverse effects of the iron on the infant gut microbiota and increases iron absorption [37,39]. As ‘natural prebiotics’, HMOs could provide similar protection from the adverse effects of iron fortificants on the infant gut microbiota, and these protective effects could depend on specific HMO composition of breast milk and maternal secretor status.
Therefore, our study aim was to: (1) determine breast milk HMO concentrations and secretor status of lactating Kenyan mothers and investigate the effect of maternal secretor status on the maternal and infant gut microbiota composition and gut inflammation, as well as on infant iron status and growth; and (2) investigate the effect of maternal secretor status on the infant response to iron fortificants with or without co-provision of GOS, in terms of effects on the infant gut microbiota, enteropathogen abundances, inflammation, iron status, growth and infectious morbidity. We hypothesized that: (1) maternal secretor status would not affect the maternal gut microbiota but would affect the infant gut microbiota, with infants of secretor mothers having higher abundances of Bifidobacterium and Bacteroides but lower abundances of enteropathogens; and (2) the adverse effect of iron on the infant gut microbiota, as well as the beneficial effects of co-provision of GOS on the infant gut microbiota and on iron absorption, would be stronger among infants of non-secretor mothers.
2. Materials and Methods
2.1. Study Design
This study was nested within a 4-month, double-masked randomized controlled intervention trial, conducted between October 2014 and January 2016 in southern coastal Kenya; its methods have been previously described in detail [37]. The intervention trial was approved by the ethics and research committees of the Kenyatta National Hospital/University of Nairobi, Kenya (P521/10/2013) and the Zurich Cantonal Ethical Commission (2014–0232); this sub-study was approved by the Kenyatta National Hospital/University of Nairobi, Kenya (P521/10/2013). The participating mothers gave informed consent for themselves and their infant by either a written signature or a fingerprint. An independent Data Safety Monitoring Board monitored the intervention study. The study was registered at ClinicalTrials.gov; identifier: NCT02118402.
In the intervention trial, we included generally healthy infants 6.5–9.5 months of age who had received no vitamin and mineral supplements 8 weeks and no antibiotics 10 weeks prior to study entry, who had a hemoglobin (Hb) >70 g/L, and Z-scores weight-for-age (WAZ) and weight-for-length (WLZ) both >−3. Enrolled infants (n = 155) were randomly assigned to three intervention groups using a computer-generated list and three color codes: (1) the control group, receiving daily for 4 months a MNP containing several minerals and vitamins but no iron and 10.5 g of maltodextrin as a carrier in the sachet, (2) the Fe group, receiving daily for 4 months the same MNP but with 2.5 mg iron as sodium iron ethylenediaminetetraacetate (NaFeEDTA) and 2.5 mg iron as ferrous fumarate, and (3) the FeGOS group, receiving daily for 4 months the alike MNP as the Fe group, except the maltodextrin was replaced with 10.5 g of 75% GOS (Vivinal GOS 75 Powder, Friesland Campina, Wageningen, The Netherlands). Table 1 shows the composition of the MNPs used in the study. Study participants and investigators were masked to group assignment. Weekly for 4 months we dispensed seven MNP sachets and 2 kg of unfortified, refined maize flour to the mothers. During the weekly visits, we assessed compliance by questioning the caregiver and collecting the previous week’s used and unused MNP sachets, and infant morbidity over the previous seven days using a forced-choice questionnaire on days affected by: diarrhea (defined as ≥ 3 loose stools in a day) and/or mucus in stool; respiratory tract infections (RTIs) (defined as cough and/or difficult or rapid breathing); fever; and/or other illness.

Table 1.
Composition of the micronutrient powder formulations used in this study.
All of the mothers of the participating infants (n = 155) were asked to join this sub-study; 80 mothers gave informed consent and were included in this sub-study. Fecal samples from all included mothers and infants were collected at baseline, after 3 weeks and after 4 months of the intervention for determination of the gut microbiota by 16S rDNA sequencing, enteropathogens by quantitative polymerase chain reaction (qPCR) and fecal calprotectin. Mothers were carefully instructed on fecal sample collection and were given plastic diapers (for collection of the infant fecal sample), spatulas and screw-cap plastic containers containing a carbon dioxide generator system to create an anaerobic atmosphere (Microbiology Anaerocult A mini, Merck, Darmstadt, Germany). The fecal samples were collected at home into the containers. The study team aliquoted and stored the fecal sample at −20 °C the same day until further analysis. Breast milk samples for analysis of HMOs were obtained by manual expression by the mother into a clean plastic container. The samples were kept cool until the study team aliquoted and stored them at −20 °C the same day until further analysis. From all mothers (n = 80) we collected a breast milk sample at one time point; the time point of this sample collection was not standardized. From a sub-group of all mothers (n = 16) we collected breast milk samples at two different time points of lactation for determination of potential changes in HMO composition over time; the time points of collection of these two samples were standardized in that the second sample was collected 3 months later in lactation than the first sample. A blood sample from all infants was collected at baseline and after 4 months of the intervention for determination of Hb, plasma ferritin (PF), soluble transferrin receptor (sTfR), C reactive protein (CRP), alpha-glycoprotein (AGP) and intestinal fatty acid binding protein (I-FABP). At baseline and after 4 months of the intervention we measured infant weight and length. At baseline we recorded demographic characteristics, brief medical history and feeding habits of the infant and mothers, including information on breastfeeding, using a questionnaire and local health records.
2.2. Laboratory Methods
Breast milk samples: HMO composition in maternal breast milk samples was quantitatively determined using high-performance anion-exchange chromatography coupled with pulse amperometric detection (HPAE-PAD) [40,41]; details are given in the Supplementary Material (Supplementary Methods and Table S1).
Fecal samples: Details on the protocol used for DNA extraction of infant and maternal fecal samples are given in the Supplementary Material (Supplementary Methods). Using a 2-step PCR, barcoded amplicons from the V3–V4 region of 16S rRNA genes were generated. For initial amplification of the V3–V4 part of the 16S rRNA we used universal primers appended with Illumina adaptor sequences. PCR products were purified, checked on a Bioanalyzer (Agilent) and quantified. Illumina MiSeq with the paired-end (2×) 300 bp protocol and indexing was used for sequencing, followed by de-multiplexing and quality control. Details of the method are given in the Supplementary Material (Supplementary Methods). Selected enteropathogenic bacteria (Clostridium difficile, Clostridium perfringens, enterohemorrhagic E. coli with shiga toxin 1 (EHEC stx1), enterohemorrhagic E. coli with shiga toxin 2 (EHEC stx2), enteropathogenic E. coli with the attaching and effacing gene (EPEC eaeA), enterotoxigenic E. coli with heat-stable enterotoxin (ETEC ST), enterotoxigenic E. coli with heat-labile enterotoxin (ETEC LT), Salmonella spp. and Staphylococcus aureus) were targeted in infant and maternal fecal DNA using qPCR. Details of the qPCR method are given in the Supplementary Material (Supplementary Methods). Primers used for qPCR [42,43,44] are given in Table S2. We measured fecal calprotectin in all infant fecal samples using the Calprest ELISA assay for fecal samples (Eurospital, Trieste, Italy).
Blood samples: We collected a venous blood sample (3 mL) from the infant at baseline and 4 months. Using a HemoCue 300 analyzer (HemoCue, Angelholm, Sweden) we measured Hb on the day of collection. After centrifugation to separate plasma, we froze the plasma on the day of collection until further analysis of PF, sTfR, CRP and AGP using a multiplex immunoassay [45]. We measured plasma I-FABP using a commercially available ELISA (Hycult Biotech, Uden, The Netherlands).
2.3. Data and Statistical Analysis
We defined total fucosylated HMOs as the sum of 2′FL, 3-fucosyllactose (3′FL), LNFPI, lacto-N-fucopentaose II (LNFPII) and lacto-N-fucopentaose III (LNFPIII); total sialylated HMOs as the sum of 6-sialyllactose (6′SL), 3-sialyllactose (3′SL), sialyllacto-N-tetraose d (LSTd), sialyllacto-N-tetraose a (LSTa) and disialyl-lacto-N-teraose (DSLNT); and total non-fucosylated and non-sialylated HMOs as the sum of lacto-N-neotetraose (LNnT), lacto-N-teraose (LNT) and lacto-N-neohexaose (LNnH). We analyzed absolute and relative concentration of HMOs; details are given in the Supplementary Material (Supplementary Methods). We defined a mother as being non-secretor if there were no detectable concentrations of α-1-2-linked fucosylated HMOs (2′FL and LNFPI) in her breast milk sample. Z-scores for length-for-age (LAZ), WAZ and WLZ were calculated using the WHO Anthro software (Version 3.2.2.1). We calculated body iron stores (BIS) from the sTfR/PF ratio using the following equation: BIS (mg/kg) = ((log(sTfR / PF) − 2.8229) / 0.1207 [46]. We calculated total BIS by multiplying BIS with infant weight.
We analyzed 16S rRNA gene sequences using a workflow based on Qiime 1.8 [47]. We performed operational taxonomic unit (OTU) clustering (open reference), taxonomic assignment and reference alignment with the pick_open_reference_otus.py workflow script of Qiime, using uclust as clustering method (97% identity) and GreenGenes v13.8 as reference database for taxonomic assignment. Reference-based chimera removal was done with Uchime [48]. The RDP classifier version 2.2 was performed for taxonomic classification [49]. We performed statistical tests as implemented in SciPy (https://www.scipy.org/), downstream of the Qiime-based workflow. We used the Fluidigm Real-Time PCR Analysis Software and Excel (Microsoft Office 2010) for processing the qPCR data, including melting curve analysis. Details of the methods are given in the Supplementary Material (Supplementary Methods).
We performed multivariate redundancy analyses (RDAs) on the infant and maternal gut microbiota composition as assessed by 16S rRNA gene sequencing in Canoco version 5.11 using default settings of the analysis type “Constrained” [50]; details of the method and comparisons are given in the Supplementary Material (Supplementary Methods). We tested for between group differences at baseline (secretor vs. non-secretor mothers and infants of secretor mothers vs. infants of non-secretor mothers, respectively) in alpha diversity and abundance of the taxa of primary interest (Bacteroidetes, Bifidobacterium, Clostridiales, Enterobacteriaceae and Lactobacillus) and of all taxa, using Wilcoxon rank-sum tests with FDR correction for multiple testing. We tested for differences in alpha diversity (PD whole tree, and for Bifidobacterium diversity analysis also Shannon index), phylogenetic distance (weighted UniFrac within individuals between baseline and 3 weeks sample, and between baseline and 4 months) and abundance of the taxa of primary interest and of all taxa, at baseline, 3 weeks and 4 months between the intervention–secretor-status groups (control group infants of secretor mothers (control-S) and of non-secretor mothers (control-NS), Fe group infants of secretor mothers (Fe-S) and of non-secretor mothers (Fe-NS), FeGOS group infants of secretor mothers (FeGOS-S) and of non-secretor mothers (FeGOS-NS)), using Kruskal–Wallis tests with Dunn’s post hoc test to adjust for multiple comparisons. Details of the comparisons are given in the Supplementary Material (Supplementary Methods).
We analyzed demographic, anthropometric and biochemical data using the R statistical programming environment (R. 3.4.1 software; R Core Team). Normally distributed data are shown as mean ± SD and non-normally distributed data as median (IQR). We tested for changes over time of lactation in HMO concentration using Wilcoxon signed-rank tests. We tested for between group differences (secretor vs. non-secretor mothers and infants of secretor mothers vs. infants of non-secretor mothers, respectively) of biochemical data at baseline using t-tests or Wilcoxon rank-sum tests. The interaction between maternal secretor status (secretor and non-secretor) and intervention groups (control, Fe and FeGOS) was investigated by fitting linear mixed-effect models using CRAN package lme. We defined the fixed effects on the variance as time, secretor status, intervention group, time-by-secretor-status, time-by-intervention-group, secretor-status-by-intervention group and time-by-secretor-status-by-intervention-group, and the random structure as the subject to control between-subject differences. Post hoc analyses were performed to investigate the effect of time within group (intervention–secretor-status groups). Details of the methods and comparisons are given in the Supplementary Material (Supplementary Methods). We evaluated infant morbidity using longitudinal prevalence ratio (LPR) [51,52]; details of the method are given in the Supplementary Material (Supplementary Methods).
In all analyses, p values < 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the Study Population
Eighty mother–infant pairs were enrolled; 5 mother–infant pairs were excluded due to inadequate fecal and/or breast milk sample collection. Data from 75 mother–infant pairs were included in the data analyses. Characteristics of the study population at baseline, overall and by maternal secretor status are given in Table 2. Median duration of exclusive breastfeeding was 6 months. At baseline, all infants were already receiving complementary foods, mainly maize porridge, and all were still partially breastfed. During the period of the intervention study, more than 50% of the mothers reported breastfeeding >5 times per day and the remaining mothers reported breastfeeding between 1–5 times per day. During the intervention study, compliance with the MNP sachets was 96%, 97% and 95% in the control, Fe and FeGOS groups, respectively.

Table 2.
Characteristics of the study population at baseline. Age, parity, gender, anthropometrics, hematological and inflammation status, fecal calprotectin and plasma I-FABP. Differences in these variables among Kenyan mothers (n = 75) and Kenyan infants (n = 75) at baseline, by maternal secretor status.
3.2. Maternal Secretor Status, HMO Composition and the Maternal Gut Microbiota
Maternal secretor status and HMO composition: A breast milk sample was collected from 75 mothers at one time point of lactation. Median (IQR) lactation stage (month postpartum) at the time of this breast milk sample collection was for all mothers 14.1 (11.2–16.5), for secretor mothers 13.8 (11.2–15.9) and for non-secretor mothers 16.1 (11.4–18.0). Fifty-four of the mothers (72%) were identified as being secretors and 21 (28%) as non-secretors. HMO concentrations among all mothers and by secretor status are given in Table S3. Mean relative HMO concentrations by secretor status are shown in Figure 1A. Only breast milk of secretor mothers contained 2′FL and LNFPI, while there was no measurable concentration of these two HMOs in breast milk of non-secretor mothers. Absolute and relative concentrations of LNFPIII and relative concentration of LNT were higher among non-secretor mothers compared to secretor mothers (p = 0.015 and p = 0.035, respectively). The higher absolute concentrations of LNFPII and LNT among non-secretor mothers compared to secretor mothers failed to reach significance (p = 0.097 and p = 0.075, respectively).
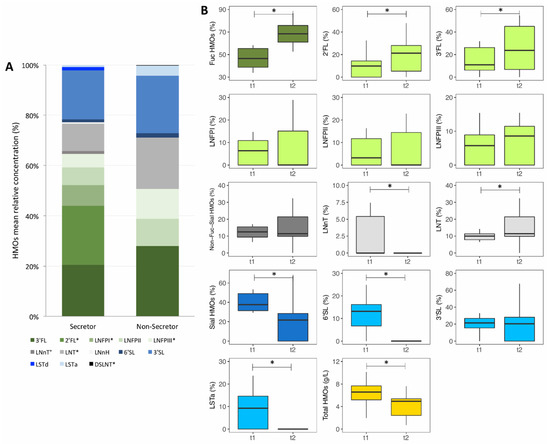
Figure 1.
Concentration of human milk oligosaccharides (HMOs) in breast milk samples of Kenyan mothers. (A) Mean relative concentration of HMOs in breast milk samples of Kenyan mothers (n = 75), by secretor status. (B) Absolute concentration of total HMOs (g/L) and relative concentration (%) of single HMOs, Sial HMOs (total sialylated HMOs), Fuc HMOs (total fucosylated HMOs), Non-Fuc-Sial HMOs (total non-fucosylated and non-sialylated HMOs) in breast milk samples of Kenyan mothers (n = 16) over 3 months of lactation. 2′FL, 2-fucosyllactose; 3′FL, 3-fucosyllactose; LNFPI, lacto-N-fucopentaose I; LNFPII, lacto-N-fucopentaose II; LNFPIII, lacto-N-fucopentaose III; LNnT, lacto-N-neotetraose; LNT, lacto-N-teraose; LNnH, lacto-N-neohexaose; 6′SL, 6-sialyllactose; 3′SL, 3-sialyllactose; LSTd, sialyllacto-N-tetraose d; LSTa, sialyllacto-N-tetraose a; and DSLNT, disialyl-lacto-N-teraose. Differences between groups and time points were tested using Wilcoxon rank-sum tests and Wilcoxon signed-rank tests, respectively; * p < 0.05, ° p < 0.10.
HMO abundances over the course of lactation: From a sub-group of all mothers (n = 16) we collected breast milk samples at two different time points of lactation. Median (IQR) lactation stage (months postpartum) when the first and second breast milk samples were collected was 7.9 (7.8–8.5) and 10.9 (10.7–11.5). Absolute and relative HMO concentrations at the two time points are given in Table S4 and are shown in Figure 1B. Absolute concentrations of LNnT, 6′SL, LSTa, total sialylated HMOs and total HMOs decreased over lactation (p = 0.022, p = 0.003, p = 0.006, p = 0.001 and p = 0.034, respectively). Absolute concentration of non-fucosylated and non-sialylated HMOs decreased among secretor mothers (p = 0.042). Relative concentrations of LNnT, 6′SL, LSTa and of total sialylated HMOs decreased over lactation (p = 0.022, p = 0.003, p = 0.006 and p = 0.001, respectively). Relative concentration of 3′SL decreased among secretor mothers (p = 0.042). In contrast, relative concentrations of 2′FL, 3′FL, LNT and of total fucosylated HMOs increased over lactation (p = 0.003, p = 0.004, p = 0.025 and p = 0.002, respectively).
Maternal gut microbiota composition by secretor status, by 16S rDNA sequencing: Per sample on average 16657 bacterial 16S rDNA sequences were analyzed by 16S rDNA sequencing. At baseline, among all mothers, among secretor mothers and among non-secretor mothers, respectively the microbiota was composed of the phylum Actinobacteria (8.9%, 9.0% and 8.7% of the 16S rDNA reads), Firmicutes (73.7%, 73.3% and 74.6%), Bacteroidetes (12.6%, 12.5% and 12.8%) and Proteobacteria (3.6%, 4.1% and 2.5%). Details on the microbiota composition are shown in Figure S1A–C. There were no significant differences in the RDA on all OTUs and in the RDA on Bifidobacterium OTUs, in phylogenetic diversity (alpha-diversity; PD whole tree), or in any individual taxa at baseline between secretor mothers and non-secretor mothers.
Maternal gut microbiota by secretor status, by qPCR: abundances and occurrence of gut pathogens: From all maternal fecal samples analyzed (n = 66), we detected C. perfringens in 67% (median log gene copies/g feces in samples with detected abundances: 4.4 (IQR: 4.0–5.1)), EPEC eaeA in 39% (5.1 (4.4–5.5)), ETEC ST in 23% (4.1 (3.9–4.5)), EHEC stx2 in 18% (4.5 (4.1–5.4)), ETEC LT in 14% (5.7 (5.0–6.3)), and EHEC stx1 in 9% (4.1 (3.9–4.3)). C. difficile was not detected. Abundance of C. perfringens was significantly higher among non-secretor mothers (detected in 70%, 4.9 (4.7–5.6)) compared to secretor mothers (65%, 4.1 (3.8–4.9)) (p = 0.028). There was no significant difference in the abundance of the sum of virulence and toxin genes (VTGs) of pathogenic E. coli (eaeA, LT, ST, stx1, stx2) between secretor mothers (61%, 5.1 (4.6–5.8)) and non-secretor mothers (50%, 4.4 (4.1–5.6)) (p = 0.125) and in the abundance of the sum of all pathogens between secretor mothers (85%, 5.1 (4.3–5.8)) and non-secretor mothers (85%, 5.3 (4.7–5.7)) (p = 0.698).
3.3. Cross-Sectional Analyses at Baseline: Comparison of Infants of Secretor Mothers and Infants of Non-Secretor Mothers
Infant gut microbiota composition by 16S rDNA sequencing: Per sample on average 24696 bacterial 16S rDNA sequences were analyzed by 16S rDNA sequencing. At baseline, among all infants, among infants of secretor mothers and among infants of non-secretor mothers, respectively the microbiota consisted of the phyla Actinobacteria (68.2%, 66.7% and 72.2% of the 16S rDNA reads, which was mostly represented by the family Bifidobacteriaceae (61.8%, 61.1% and 63.6%)), Firmicutes (27.2%, 28.7% and 23.3%; including 10.6%, 10.4% and 11.2% Clostridiales, and 3.6%, 4.0% and 2.7% Lactobacillus), Bacteroidetes (1.5%, 1.8% and 0.6%) and Proteobacteria (3.0%, 2.7% and 3.7%; with the predominant family Enterobacteriaceae (2.9%, 2.6% and 3.7%)). Details are given in Figure S2A–C. There were no significant differences in the RDA on all OTUs and in the RDA on Bifidobacterium OTUs, in overall phylogenetic diversity (alpha-diversity; PD whole tree) and Bifidobacterium OTUs specific phylogenetic diversity (PD whole tree and Shannon index), or in any individual taxa at baseline between infants of secretor mothers and infants of non-secretor mothers.
Infant gut microbiota by PCR: abundance and occurrence of gut pathogens: From all analyzed infant fecal samples (n = 75), we detected C. perfringens in 68% (median log gene copies/g feces in samples with detected abundances: 4.6 (IQR: 3.9–5.1)), EPEC eaeA in 65% (5.4 (4.8–6.5)), C. difficile in 44% (5.6 (4.7–6.0)), ETEC LT in 27% (6.6 (5.8–8.0)), EHEC stx2 in 19% (4.3 (4.0–4.7)), ETEC ST in 15% (4.4 (4.1–5.8)), S. aureus in 13% (4.5 (4.5–5.1)), EHEC stx1 in 7% (4.3 (3.9–4.7)) and Salmonella spp. in 3% (4.5 (4.4–4.6)). Comparing infants of secretor mothers to infants of non-secretor mothers, there were no significant differences in the abundance of the sum of all pathogens (p = 0.895), the abundance of the sum of VTGs of pathogenic E. coli (p = 0.763), or when analyzing all pathogens separately. Secretor status did not predict the sum of all pathogens and VTGs of pathogenic E. coli in a linear regression model.
Infant anthropometrics, hematological and inflammation status, plasma I-FABP and fecal calprotectin: There were no significant differences in anthropometrics, Hb, PF, sTfR, CRP, AGP, I-FABP, or fecal calprotectin between infants of secretor mothers and infants of non-secretor mothers (Table 2). In linear regression models, maternal secretor status did not significantly predict infant weight, length, LAZ, WAZ, WLZ, Hb, PF, sTfR, CRP, AGP, I-FABP or fecal calprotectin.
3.4. Effect of Maternal Secretor Status on the Infant Response to the Iron and GOS Intervention
Infant gut microbiota composition by 16S rDNA sequencing: There were no significant differences in the RDAs on all OTUs and in the RDAs on Bifidobacterium OTUs, in phylogenetic diversity (alpha-diversity; PD whole tree), phylogenetic diversity on Bifidobacterium OTUs (PD whole tree), and Shannon Index on Bifidobacterium OTUs, or in beta-diversity (weighted Unifrac) at baseline, 3 weeks and 4 months when comparing the intervention–secretor-status groups (control-S (n = 21), control-NS (n = 5), Fe-S (n = 16), Fe-NS (n = 8), FeGOS-S (n = 17) and FeGOS-NS (n = 8)). In the mixed-effect model on Bifidobacterium abundances there was a significant secretor-status-by-intervention-group interaction (p = 0.021). Post hoc tests for within intervention–secretor-status group differences showed a significant decrease in Bifidobacterium abundances from baseline to 4 months in the Fe-NS group (p = 0.028). Relative abundances of Bifidobacterium at baseline, 3 weeks and 4 months, by intervention–secretor-status groups are shown in Figure 2A. In the cross-sectional analyses at baseline, 3 weeks and 4 months and in the longitudinal analyses from baseline to 3 weeks and from baseline to 4 months there were no significant differences in any other taxa when comparing the intervention–secretor-status groups.
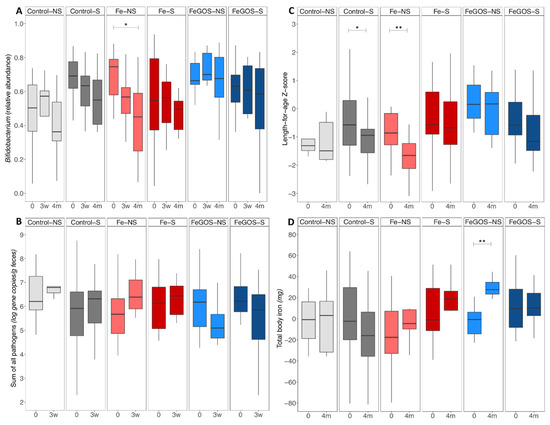
Figure 2.
Relative abundances of Bifidobacterium and of the sum of virulence and toxin genes of the 10 targeted pathogens, length-for-age Z-score and total body iron, by group. Kenyan infants (n = 75) receiving daily a micronutrient powder containing either no iron (Control–) (n = 26), containing 5 mg of iron (Fe–) (n = 24), or containing 5 mg of iron and 7.5 g of galacto-oligosaccharides (FeGOS–) (n = 25), and being breastfed by either a non-secretor mother (–NS) (n = 21) or a secretor mother (–S) (n = 54). (A) Relative abundance of Bifidobacterium by group at baseline (0), 3 weeks (3w) and 4 month (4m) of the intervention. (B) Log gene copies/g feces of the sum of all pathogens by group at baseline (0) and 3 weeks (3 w) of the intervention. (C) Length-for-age Z-score by group at baseline (0) and 4 month (4m) of the intervention. (D) Total body iron (mg) by group at baseline (0) and 4 month (4m) of the intervention. We assessed an interaction between maternal secretor status and intervention-groups by fitting linear mixed-effect models using CRAN package lme. We defined the fixed effects on the variance as time, secretor-status, intervention-group, time-by-intervention-group, time-by-secretor-status, secretor-status-by-intervention-group, and time-by-secretor-status-by-intervention-group, and the random structure was defined as the subject. There was a significant secretor-status-by-intervention-group effect on Bifidobacterium abundance (p = 0.021), a significant time-by-secretor-status-by-intervention-group effect (p = 0.023) and a significant secretor-status-by-intervention group effect (p = 0.022) on length-for-age Z-score, and a significant time-by-intervention-group (p = 0.003) and time-by-secretor-status (p = 0.016) effect on total body iron. Post hoc analyses were performed to investigate the effect of time within group. Boxes show the median and 25th and 75th percentile; whiskers extend to the furthest data point that is within 1.5 times the IQR. * p < 0.05; ** p < 0.01.
Infant gut microbiota by qPCR: abundances and occurrence of gut pathogens: There was a significant time-by-intervention-group (p = 0.007) and a secretor-status-by-intervention-group interaction (p = 0.096) on the sum of all pathogens from baseline to 3 weeks. Log gene copies/g feces of the sum of all pathogens at baseline and 3 weeks, by intervention–secretor-status groups are shown in Figure 2B. Within the Fe intervention group infants of non-secretor mothers (Fe-NS) have a greater increase in the sum of all pathogens. Within the FeGOS intervention groups the decrease in the sum of pathogens was particularly pronounced among infants of non-secretor mothers (FeGOS-NS). Post hoc tests for within group differences were not statistically significant after adjusting for multiple testing.
Fecal calprotectin and plasma I-FABP: There was a time-by-secretor-status-by-intervention-group (p = 0.086), time-by-secretor-status (p = 0.066) and time-by-intervention-group (p = 0.068) effect on fecal calprotectin from baseline to 3 weeks and a time-by-secretor-status effect from baseline to 4 months (p = 0.017). Post hoc tests on within group differences from baseline to 3 weeks and from baseline to 4 months showed a significant decrease in the Control-NS group (p = 0.008 and p = 0.014, respectively). There was no significant time-by-secretor-status-by-intervention-group, time-by-secretor-status or secretor-status-by-intervention-group effect on I-FABP.
Infant anthropometrics: There was a significant time-by-secretor-status-by-intervention-group effect (p = 0.023) and secretor-status-by-intervention-group effect (p = 0.022) on LAZ and a significant secretor-status-by-intervention-group effect (p = 0.018) on WAZ. Post hoc tests on within group differences from baseline to 4 months showed a significant decrease in LAZ in the Control-S and Fe-NS groups (p = 0.031 and p = 0.002, respectively). LAZ at baseline and 4 months of the intervention, by intervention–secretor-status groups is shown in Figure 2C.
Hemoglobin and iron status: There was a significant time-by-intervention-group effect on Hb (p = 0.036) but no significant time-by-secretor-status-by-intervention-group, time-by-secretor-status or secretor-status-by-intervention-group effect. The time-by-secretor-status effect on PF was borderline significant (p = 0.052) and there was a significant time-by-intervention-group and secretor-status-by-intervention-group effect on sTfR (p = 0.002 and p = 0.041, respectively). There was a significant time-by-intervention-group effect and time-by-secretor-status effect on BIS (p = 0.011 and p = 0.023, respectively) and on total BIS (p = 0.003 and p = 0.016, respectively). Post hoc tests on within group differences from baseline to 4 months showed a significant increase in PF, BIS and total BIS in the FeGOS-NS group (p = 0.043, p = 0.012 and p = 0.002, respectively). Total BIS at baseline and 4 months, by intervention–secretor-status groups is shown in Figure 2D.
Infant morbidity: Longitudinal prevalence (percentage of weeks with illness) of diarrhea and/or mucus in the stool was higher in the Control-NS group (23.8%) compared to the Control-S group (10.4%) (LPR = 2.28, 95% CI 1.38 to 3.77, p = 0.001) but was not different between FeGOS-NS (16.4%) and FeGOS-S (11.0%) (p = 0.132) and between Fe-NS (9.4%) and Fe-S (12.5%) (p = 0.376). Longitudinal prevalence of RTIs was higher in Fe-S (19.5%) compared to Fe-NS (10.9%) (LPR = 0.56, 95% CI 0.32 to 0.97, p = 0.040) but was not different between Control-NS (10.0%) and Control-S (14.0%) (p = 0.359) and between FeGOS-NS (18.1%) and FeGOS-S (12.5%) (p = 0.135).
4. Discussion
Our main findings are: (1) a secretor prevalence of 72%; (2) significant differences in concentration of HMOs between secretor and non-secretor mothers and over time of lactation; (3) no significant differences in the overall gut microbiota composition, phylogenetic diversity, abundances of taxa of primary interest and abundances of enteropathogens comparing secretor to non-secretor mothers and infants of secretor to infants of non-secretor mothers, respectively, with the exception of a higher abundance of C. perfringens among non-secretor compared to secretor mothers; (4) a significant secretor-status-by-intervention-group interaction on Bifidobacterium abundance, LAZ and WAZ, and sTfR; and (5) among infants in the intervention control group, a higher longitudinal prevalence of diarrhea among infants of non-secretor compared to infants of secretor mothers.
Secretor prevalence (72%) in our study is at the lower end of published prevalence data. Worldwide secretor frequency is estimated at ~80%, but varies across different geographic areas [3,6]: 67–95% in the United States [3,7,18,53,54,55], 64–87% in the United Kingdom, Spain, Finland, Sweden and Italy [2,14,17,56,57,58,59,60], 85% in India [56], 77% and 80% in China [20,61], and 51–81% in Africa (Burkina Faso, Ethiopia, Ghana, Kenya, South Africa, The Gambia and Malawi) [3,54,60,62,63]. Secretor prevalence in urban and rural Gambia is 85% and 65%, respectively, and in urban and rural Ethiopia is 78% and 65%, respectively [3]. In urban Kenya (Nakuru), secretor prevalence is 81% [3], and like the above data from The Gambia and Ethiopia, our secretor prevalence in rural Kenya is lower, at 72%.
Our findings agree with previous studies reporting higher concentration of total HMOs [2,17,64] and higher abundances of fucosylated HMOs [2,54,64] in secretor compared to non-secretor mothers, but not all studies agree [18,54]. Two studies reported higher abundances of sialylated HMOs in non-secretor compared to secretor mothers [2,54], while others (as in our study) found no difference [64]. Higher abundances of 2′FL, LNFPI and 3′FL have been reported in secretor compared to non-secretor mothers [2,17], but others found 3′FL to be higher in non-secretor mothers [4]. Our findings agree with previous studies reporting higher amounts of LNT, LNFPII and LNFPIII in non-secretor mothers [2,4,17]. Our findings also agree with previous studies reporting total HMO concentrations decrease over the course of lactation [54]. The relatively low median total HMO concentration of 4.5 g/L in our study, compared to reported values of 5–10 g/L [1], is likely explained by our sampling in the late stage of lactation. Our finding of decreases over lactation of LNnT, 6′SL, LSTa and total sialylated HMOs but increases of 3′FL, 2′FL, LNT and total fucosylated HMOs are in general agreement with previous data, although findings vary [2,4,20,54,65].
To our knowledge, our study is the first to investigate the effect of maternal secretor status among mothers and infants living in rural Kenya, an area with low hygiene conditions and a high burden of infectious diseases. Our findings show that maternal secretor status does not have a major impact on the gut microbiota of the mothers. Previous studies have linked maternal secretor status to a higher risk for viral infections, including norovirus [7,8,66], rotavirus [9,10,11,58,67,68] and respiratory viruses [12] in women. In contrast, maternal non-secretor status has been linked with symptomatic ETEC infection in women [13]. In our study, there were no differences in abundance of enteropathogens comparing secretor to non-secretor mothers, with the exception of higher C. perfringens among non-secretor mothers. Our findings agree with two cohort studies in the United Kingdom (n = 1503) and Canada (n = 1190), reporting no association between maternal secretor status and the overall gut microbiota composition, microbiota diversity and relative abundance of taxa in women [14,15], while others reported differences in the gut microbiota comparing secretor and non-secretor women [53,56,58].
Our findings also show that secretor status does not have a major impact on the infant gut microbiota in this setting. Some studies have found differences in the gut microbiota of infants of secretor mothers compared to infants of non-secretor mothers [18,19,20], but others have not [21,22,23]. Our findings agree with studies from Finland, India and the United States reporting no differences in the infant gut microbiome based on maternal secretor status [21,22,23] and with studies showing no association between infant microbiota alpha-diversity and maternal secretor status [19,20]. Studies in Australian toddlers, and from younger infants in China and the United States, reported higher abundance of Bifidobacterium among children of secretor mothers [18,19,20]; this was not confirmed in our study.
In our study, during the 4 months of intervention, among infants in the control group, longitudinal prevalence of diarrhea and/or mucus in the stool was higher among infants of non-secretor mothers compared to infants of secretor mothers. Two previous studies found lower incidence of diarrhea among infants breastfed with milk being high in relative abundance of α-1-2-linked-fucosyloligosaccharides or having a high ratio of α-1-2-linked- to non-α-1-2-linked-fucosyloligosaccharides [29,30]. Higher relative amounts of LNT and lower levels of LNFPI and LNFPII were found in milk of Gambian mothers whose infant was sick compared to milk of mothers whose infant was not sick [32].
There were no significant differences in anthropometrics at baseline between infants of secretor mothers and infants of non-secretor mothers in our study. Recent studies suggest breast milk HMO composition may affect infant growth [31,32,33], but not all studies agree [34]. Breast milk from non-secretor mothers from severely stunted infants in Malawi showed different HMO concentrations compared to breast milk from non-secretor mothers from healthy infants, with lower concentrations of sialylated and fucosylated HMOs [31].
Importantly, the differing breast milk HMO profile of secretor and non-secretor mothers appeared to modulate the impact of the iron and GOS intervention. Infants in the Fe group and of non-secretor mothers were particularly vulnerable to the decrease in abundance of Bifidobacterium and to the increase in the sum of all pathogens. However, infants of non-secretor mothers benefited the most from the co-provision of GOS (FeGOS-NS group) in maintaining high abundances of Bifidobacterium and reducing enteropathogens. Moreover, there was a secretor-status-by-intervention-group interaction on iron status: within the FeGOS intervention group, there were greater improvements in PF, sTfR and BIS among infants of non-secretor mothers, suggesting the enhancing effect of GOS on iron absorption [39] might be stronger in infants of non-secretor mothers.
Our study has several strengths: (1) the quantitative analysis of HMOs in breast milk samples including investigation of changes over duration of lactation; (2) the extensive characterization of the infant and maternal gut microbiota using both 16S rDNA sequencing and qPCR for selected enteropathogens; and (3) inclusion of mothers and infants from an area of poor hygiene with a high burden of diarrhea and other infectious diseases. Our study was limited in that we did not standardize the time of the day when the breast milk samples were collected nor on whether to collect fore or hind milk, we did not investigate individual Bifidobacterium spp., and we enrolled a relatively small sample size.
5. Conclusions
In conclusion, maternal secretor status modulated the effects of the iron and GOS intervention: compared to infants of secretor mothers, infants of non-secretor mothers may be more vulnerable to the adverse effect of fortificant iron on the gut microbiota, resulting in decreased abundances of Bifidobacterium and increased abundances of enteropathogens, but also benefit more from the co-provision of GOS in terms of beneficial effects on the gut microbiota and improving iron status. Future studies would be valuable to investigate the effect of specific HMOs on iron absorption and their effects on the gut microbiota when given with fortificant iron.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/11/2596/s1, supplementary material: Supplementary Methods and Supplementary Results including Table S1: Gradient elution program, Table S2: Primers used for quantitative polymerase chain reaction, Table S3: Absolute and relative concentration of human milk oligosaccharides (HMOs) in breast milk samples of Kenyan mothers (n = 75), all and by secretor status, Table S4: Absolute and relative concentration of human milk oligosaccharides (HMOs) in breast milk samples of Kenyan mothers (n = 16) at two time points of lactation, Figure S1: Gut microbiota composition as assessed by 16S rDNA sequencing among Kenyan mothers at baseline. (A) All mothers, (B) secretor mothers and (C) non-secretor mothers. The fraction of 16S rDNA reads (%), attributed to specific taxonomic levels is given below the taxon name, Figure S2: Gut microbiota composition as assessed by 16S rDNA sequencing among Kenyan infants at baseline. (A) All infants, (B) infants of secretor mothers and (C) infants of non-secretor mothers. The fraction of 16S rDNA reads (%) attributed to specific taxonomic levels is given below the taxon name.
Author Contributions
Conceptualization, D.P., M.A.U. and M.B.Z.; data curation, D.P., M.A.U., G.A.M.K., J.B., S.S., T.H. and M.B.Z.; formal analysis, D.P., G.A.M.K., J.B., S.S., T.H. and M.B.Z.; funding acquisition, D.P. and M.B.Z.; investigation, D.P. and M.A.U.; methodology, D.P., M.A.U. and M.B.Z.; project administration, D.P., M.A.U. and M.B.Z.; resources, G.A.M.K., J.B., T.H. and M.B.Z.; supervision, S.K., T.H. and M.B.Z.; visualization, D.P., G.A.M.K. and J.B.; writing—original draft, D.P., G.A.M.K. and M.B.Z.; writing—review and editing, D.P., M.A.U., G.A.M.K., J.B., S.S., S.K., T.H. and M.B.Z.
Funding
Funding was provided by ETH Global and the Sawiris Foundation for Social Development, ETH Zurich, Switzerland, and DSM Nutritional Products, Kaiseraugst, Switzerland. Sight and Life (Kaiseraugst, Switzerland) donated the micronutrient powders used in this study. FrieslandCampina (Wageningen, The Netherlands) donated the galacto-oligosaccharides used in this study.
Acknowledgments
We thank the families who participated in this study, the field worker and nurses from Msambweni County Referral Hospital (Msambweni, Kenya) and Kikoneni Health Center (Kikoneni, Kenya), as well as C Zeder, A Minder and S Kobel (ETH Zurich, Switzerland) and J Erhardt (Willstaett, Germany) for support of the laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hennet, T.; Weiss, A.; Borsig, L. Decoding breast milk oligosaccharides. Swiss Med Wkly. 2014, 144, w13927. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef]
- Castanys-Munoz, E.; Martin, M.J.; Prieto, P.A. 2’-fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef]
- Currier, R.L.; Payne, D.C.; Staat, M.A.; Selvarangan, R.; Shirley, S.H.; Halasa, N.; Boom, J.A.; Englund, J.A.; Szilagyi, P.G.; Harrison, C.J.; et al. Innate susceptibility to norovirus infections influenced by fut2 genotype in a united states pediatric population. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1631–1638. [Google Scholar] [CrossRef]
- Menon, V.K.; George, S.; Sarkar, R.; Giri, S.; Samuel, P.; Vivek, R.; Saravanabavan, A.; Liakath, F.B.; Ramani, S.; Iturriza-Gomara, M.; et al. Norovirus gastroenteritis in a birth cohort in southern india. PLoS ONE 2016, 11, e0157007. [Google Scholar] [CrossRef]
- Imbert-Marcille, B.M.; Barbe, L.; Dupe, M.; Le Moullac-Vaidye, B.; Besse, B.; Peltier, C.; Ruvoen-Clouet, N.; Le Pendu, J. A fut2 gene common polymorphism determines resistance to rotavirus a of the p [8] genotype. J. Infect. Dis. 2014, 209, 1227–1230. [Google Scholar] [CrossRef]
- Yang, T.A.; Hou, J.Y.; Huang, Y.C.; Chen, C.J. Genetic susceptibility to rotavirus gastroenteritis and vaccine effectiveness in taiwanese children. Sci. Rep. 2017, 7, 6412. [Google Scholar] [CrossRef]
- Payne, D.C.; Currier, R.L.; Staat, M.A.; Sahni, L.C.; Selvarangan, R.; Halasa, N.B.; Englund, J.A.; Weinberg, G.A.; Boom, J.A.; Szilagyi, P.G.; et al. Epidemiologic association between fut2 secretor status and severe rotavirus gastroenteritis in children in the united states. JAMA Pediatrics 2015, 169, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.W.; Blackwell, C.C.; Molyneaux, P.; James, V.S.; Ogilvie, M.M.; Inglis, J.M.; Weir, D.M. Association between secretor status and respiratory viral illness. BMJ 1991, 303, 815–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mottram, L.; Wiklund, G.; Larson, G.; Qadri, F.; Svennerholm, A.M. Fut2 non-secretor status is associated with altered susceptibility to symptomatic enterotoxigenic escherichia coli infection in bangladeshis. Sci. Rep. 2017, 7, 10649. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.R.; Goodrich, J.K.; Bell, J.T.; Spector, T.D.; Ley, R.E.; Clark, A.G. Abo antigen and secretor statuses are not associated with gut microbiota composition in 1,500 twins. BMC Genom. 2016, 17, 941. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Bedrani, L.; Espin-Garcia, O. Fut2 genotype and secretory status are not associated with fecal microbial composition and inferred function in healthy subjects. Gut Microbes 2018, 9, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; Garcia-Mantrana, I.; Bertua-Rios, B.; Martinez-Costa, C.; Borsch, C.; Rudloff, S. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J. Pediatric Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.S.; German, J.B.; et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef]
- Smith-Brown, P.; Morrison, M.; Krause, L.; Davies, P.S. Mothers secretor status affects development of childrens microbiota composition and function: A pilot study. PLoS ONE 2016, 11, e0161211. [Google Scholar] [CrossRef]
- Bai, Y.; Tao, J.; Zhou, J.; Fan, Q.; Liu, M.; Hu, Y.; Xu, Y.; Zhang, L.; Yuan, J.; Li, W.; et al. Fucosylated human milk oligosaccharides and n-glycans in the milk of chinese mothers regulate the gut microbiome of their breast-fed infants during different lactation stages. mSystems 2018, 3, e00206-18. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci. Rep. 2018, 8, 13757. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 2018, 9, 5010. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatric Res. 2015, 78, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2’-fucosyllactose and 6’-sialyllactose on the adhesion of escherichia coli and salmonella fyris to caco-2 cells. J. Matern. Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2’-fucosyllactose modulates cd14 expression in human enterocytes, thereby attenuating lps-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human milk oligosaccharides protect against enteropathogenic escherichia coli attachment in vitro and epec colonization in suckling mice. J. Pediatric Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef]
- Simon, P.M.; Goode, P.L.; Mobasseri, A.; Zopf, D. Inhibition of helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun. 1997, 65, 750–757. [Google Scholar]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal h(o) antigen (fuc alpha 1, 2gal beta 1, 4glcnac), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatrics 2004, 145, 297–303. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Altaye, M.; Chaturvedi, P.; Meinzen-Derr, J.; Guerrero Mde, L.; Morrow, A.L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004, 14, 253–263. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Suchdev, P.S.; Vist, G.E.; Walleser, S.; Pena-Rosas, J.P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (review). Evid. Based Child Health Cochrane Rev. J. 2013, 8, 112–201. [Google Scholar] [CrossRef]
- Kortman, G.A.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Cercamondi, C.I.; Moretti, D.; Mwasi, E.; Schwab, C.; Bechtler, S.; Mutuku, F.M.; Galetti, V.; Lacroix, C.; et al. Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron edta: A stable-isotope study in kenyan infants. Am. J. Clin. Nutr. 2017, 106, 1020–1031. [Google Scholar] [CrossRef]
- Thurl, S.; Muller-Werner, B.; Sawatzki, G. Quantification of individual oligosaccharide compounds from human milk using high-ph anion-exchange chromatography. Anal. Biochem. 1996, 235, 202–206. [Google Scholar] [CrossRef]
- Rudloff, S.; Pohlentz, G.; Diekmann, L.; Egge, H.; Kunz, C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. (Oslo Norway 1992) 1996, 85, 598–603. [Google Scholar] [CrossRef]
- Rinttilae, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16s rdna-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time pcr. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Rinttilä, T.; Lyra, A.; Krogius-Kurikka, L.; Palva, A. Real-time pcr analysis of enteric pathogens from fecal samples of irritable bowel syndrome subjects. Gut Pathog. 2011, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Tsunomori, Y.; Seki, R. Duplex real-time sybr green pcr assays for detection of 17 species of food- or waterborne pathogens in stools. J. Clin. Microbiol. 2003, 41, 5134–5146. [Google Scholar] [CrossRef]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and c-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The ribosomal database project: Improved alignments and new tools for rrna analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Braak, C.J.F.; Smilauer, P. Canoco Reference Manual Anc Canodraw for Windows User’s Guide: Software for Canonical Communiy Ordination; Microcomputer Power: Ithaca, NY, USA, 2002; p. 500. [Google Scholar]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to obtain the p value from a confidence interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef] [PubMed]
- Gampa, A.; Engen, P.A.; Shobar, R.; Mutlu, E.A. Relationships between gastrointestinal microbiota and blood group antigens. Physiol. Genom. 2017, 49, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Davis, J.C.; Goonatilleke, E.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Absolute quantitation of human milk oligosaccharides reveals phenotypic variations during lactation. J. Nutr. 2017, 147, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman-Leyer, K.M.; O’Ryan, M.L.; Ruiz-Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of human milk neutral oligosaccharides in a diverse population. J. Pediatric Gastroenterol. Nutr. 2000, 30, 181–192. [Google Scholar] [CrossRef]
- Kumbhare, S.V.; Kumar, H.; Chowdhury, S.P.; Dhotre, D.P.; Endo, A.; Matto, J.; Ouwehand, A.C.; Rautava, S.; Joshi, R.; Patil, N.P.; et al. A cross-sectional comparative study of gut bacterial community of indian and finnish children. Sci. Rep. 2017, 7, 10555. [Google Scholar] [CrossRef]
- King, J.R.; Varade, J.; Hammarstrom, L. Fucosyltransferase gene polymorphisms and lewisb-negative status are frequent in swedish newborns, with implications for infectious disease susceptibility and personalized medicine. J. Pediatric Infect. Dis. Soc. 2018. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, J.; Garcia-Mantrana, I.; Vila-Vicent, S.; Gozalbo-Rovira, R.; Buesa, J.; Monedero, V.; Collado, M.C. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci. Rep. 2017, 7, 45559. [Google Scholar] [CrossRef]
- Wacklin, P.; Makivuokko, H.; Alakulppi, N.; Nikkila, J.; Tenkanen, H.; Rabina, J.; Partanen, J.; Aranko, K.; Matto, J. Secretor genotype (fut2 gene) is strongly associated with the composition of bifidobacteria in the human intestine. PLoS ONE 2011, 6, e20113. [Google Scholar] [CrossRef]
- Musumeci, M.; Simpore, J.; D’Agata, A.; Sotgiu, S.; Musumeci, S. Oligosaccharides in colostrum of italian and burkinabe women. J. Pediatric Gastroenterol. Nutr. 2006, 43, 372–378. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human milk oligosaccharides in colostrum and mature milk of chinese mothers: Lewis positive secretor subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Armah, G.E.; Cortese, M.M.; Dennis, F.E.; Yu, Y.; Morrow, A.L.; McNeal, M.M.; Lewis, K.D.C.; Awuni, D.A.; Armachie, J.; Parashar, U.D. Rotavirus vaccine take in infants is associated with secretor status. J. Infect. Dis. 2018, 219, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, E.; Autran, C.A.; Nel, D.G.; Kirsten, G.F.; Blaauw, R.; Bode, L. Human milk oligosaccharides differ between hiv-infected and hiv-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J. Nutr. 2014, 144, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef] [PubMed]
- Nijman, R.M.; Liu, Y.; Bunyatratchata, A.; Smilowitz, J.T.; Stahl, B.; Barile, D. Characterization and quantification of oligosaccharides in human milk and infant formula. J. Agric. Food Chem. 2018, 66, 6851–6859. [Google Scholar] [CrossRef]
- Thorven, M.; Grahn, A.; Hedlund, K.O.; Johansson, H.; Wahlfrid, C.; Larson, G.; Svensson, L. A homozygous nonsense mutation (428g-->a) in the human secretor (fut2) gene provides resistance to symptomatic norovirus (ggii) infections. J. Virol. 2005, 79, 15351–15355. [Google Scholar] [CrossRef]
- Gunaydin, G.; Nordgren, J.; Sharma, S.; Hammarstrom, L. Association of elevated rotavirus-specific antibody titers with hbga secretor status in swedish individuals: The fut2 gene as a putative susceptibility determinant for infection. Virus Res. 2016, 211, 64–68. [Google Scholar] [CrossRef]
- Nordgren, J.; Sharma, S.; Bucardo, F.; Nasir, W.; Gunaydin, G.; Ouermi, D.; Nitiema, L.W.; Becker-Dreps, S.; Simpore, J.; Hammarstrom, L.; et al. Both lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1567–1573. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).