Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
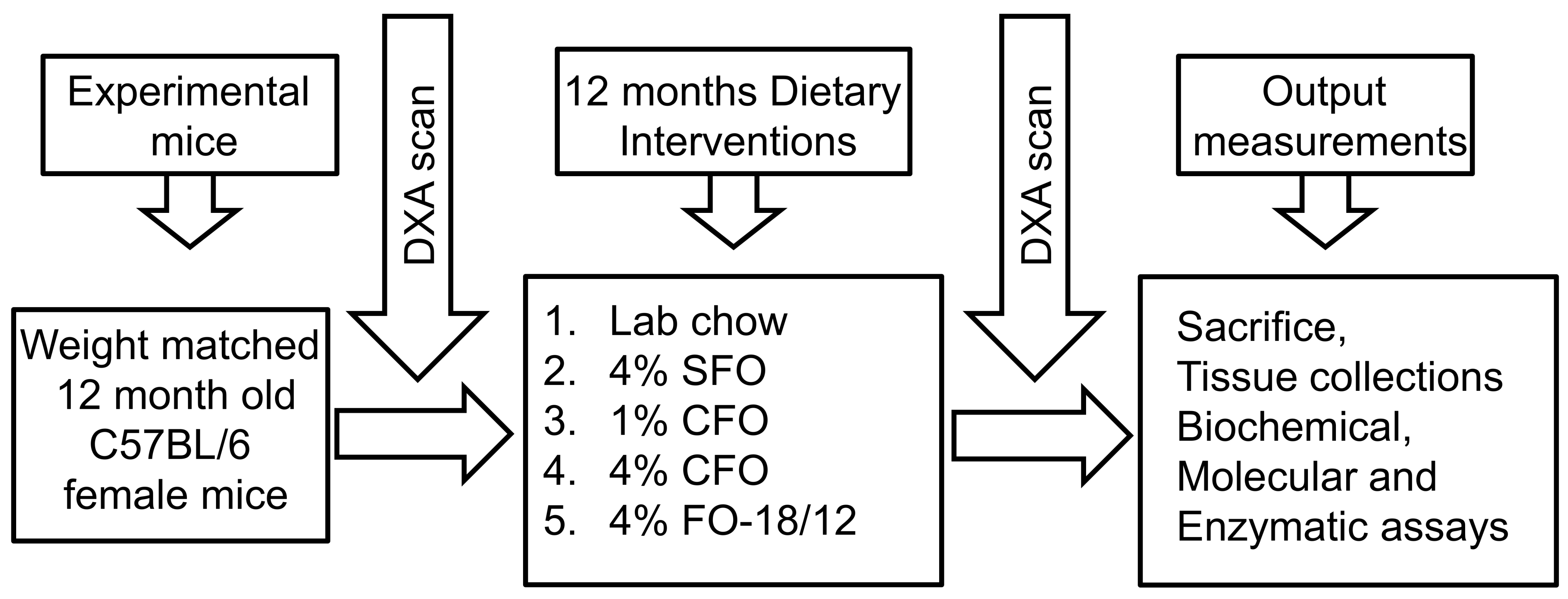
2.2. Animals and Diets
2.3. Measurement of BMD
2.3.1. Serum Bone Turnover Markers Measurement
2.3.2. Isolation of Whole Bone Marrow Cells and Culture
2.3.3. Isolation of Splenocytes and Culture
2.4. NF-κB Activation Assay
2.4.1. In-Cell Western Analysis for Phospho-JNK and Phospho-p38
2.4.2. Statistics
3. Results
3.1. Body Weight and Food Consumption
3.2. Effect of CFO on BMD During Aging
3.3. Effect of CFO on Serum Bone Turnover Markers During Aging
3.4. Effect of CFO on RANKL-Stimulated Activation of NF-κB, JNK, and p38 MAPK
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.M.; Bhattacharya, A.; Banu, J.; Fernandes, G. Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Buhr, G.; Bales, C.W. Nutritional supplements for older adults: Review and recommendations-part I. J. Nutr. Elder 2009, 28, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Bays, H. Clinical overview of Omacor: A concentrated formulation of ω-3 polyunsaturated fatty acids. Am. J. Cardiol. 2006, 98, 71i–76i. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the ω-6/ω-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Kruger, M.C.; Coetzee, M.; Haag, M.; Weiler, H. Long-chain polyunsaturated fatty acids: Selected mechanisms of action on bone. Prog. Lipid Res. 2010, 49, 438–449. [Google Scholar] [CrossRef]
- Romano, M. Inflammation resolution: Does the bone marrow have a say? Am. J. Hematol. 2008, 83, 435–436. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 Fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef]
- Fernandes, G.; Bhattacharya, A.; Rahman, M.; Zaman, K.; Banu, J. Effects of n-3 fatty acids on autoimmunity and osteoporosis. Front. Biosci. 2008, 13, 4015–4020. [Google Scholar] [CrossRef]
- Salari, P.; Rezaie, A.; Larijani, B.; Abdollahi, M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med. Sci. Monit. 2008, 14, RA37–RA44. [Google Scholar]
- Miggiano, G.A.; Gagliardi, L. Diet, nutrition and bone health. Clin. Ter. 2005, 156, 47–56. [Google Scholar]
- Rahman, M.M.; Bhattacharya, A.; Banu, J.; Kang, J.X.; Fernandes, G. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J. Cell Mol. Med. 2009, 13, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Firth, E.C.; Rogers, C.W.; Moughan, P.J.; Kruger, M.C. Specific effects of gamma-linolenic, eicosapentaenoic, and docosahexaenoic ethyl esters on bone post-ovariectomy in rats. Calcif. Tissue Int. 2007, 81, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Rahman, M.; Sun, D.; Fernandes, G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Bhattacharya, A.; Fernandes, G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J. Cell Physiol. 2008, 214, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Rahman, M.; Banu, J.; Lawrence, R.A.; McGuff, H.S.; Garrett, I.R.; Fischbach, M.; Fernandes, G. Inhibition of osteoporosis in autoimmune disease prone MRL/Mpj-Fas(lpr) mice by n-3 fatty acids. J. Am. Coll Nutr. 2005, 24, 200–209. [Google Scholar] [CrossRef]
- Weiss, L.A.; Barrett-Connor, E.; von Muhlen, D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: The Rancho Bernardo Study. Am. J. Clin. Nutr. 2005, 81, 934–938. [Google Scholar] [CrossRef]
- Kruger, G.; Huber, M.C.; Bonifer, C. The −3.9 kb DNaseI hypersensitive site of the chicken lysozyme locus harbours an enhancer with unusual chromatin reorganizing activity. Gene 1999, 236, 63–77. [Google Scholar] [CrossRef]
- Watkins, B.A.; Shen, C.L.; Allen, K.G.; Seifert, M.F. Dietary (n-3) and (n-6) polyunsaturates and acetylsalicylic acid alter ex vivo PGE2 biosynthesis, tissue IGF-I levels, and bone morphometry in chicks. J. Bone Miner. Res. 1996, 11, 1321–1332. [Google Scholar] [CrossRef]
- Watkins, B.A.; Li, Y.; Seifert, M.F. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J. Nutr. Biochem. 2006, 17, 282–289. [Google Scholar] [CrossRef]
- Maggio, M.; Artoni, A.; Lauretani, F.; Borghi, L.; Nouvenne, A.; Valenti, G.; Ceda, G.P. The impact of ω-3 fatty acids on osteoporosis. Curr. Pharm. Des. 2009, 15, 4157–4164. [Google Scholar] [CrossRef]
- Vanek, C.; Connor, W.E. Do n-3 fatty acids prevent osteoporosis? Am. J. Clin. Nutr. 2007, 85, 647–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, D.; Krishnan, A.; Zaman, K.; Lawrence, R.; Bhattacharya, A.; Fernandes, G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J. Bone Miner. Res. 2003, 18, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Li, Y.; Allen, K.G.; Hoffmann, W.E.; Seifert, M.F. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J. Nutr. 2000, 130, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Claassen, N.; Coetzer, H.; Steinmann, C.M.; Kruger, M.C. The effect of different n-6/n-3 essential fatty acid ratios on calcium balance and bone in rats. Prostaglandins Leukot. Essent. Fatty Acids 1995, 53, 13–19. [Google Scholar] [CrossRef]
- Claassen, N.; Potgieter, H.C.; Seppa, M.; Vermaak, W.J.; Coetzer, H.; Van Papendorp, D.H.; Kruger, M.C. Supplemented gamma-linolenic acid and eicosapentaenoic acid influence bone status in young male rats: Effects on free urinary collagen crosslinks, total urinary hydroxyproline, and bone calcium content. Bone 1995, 16, 385S–392S. [Google Scholar] [CrossRef]
- Green, K.H.; Wong, S.C.; Weiler, H.A. The effect of dietary n-3 long-chain polyunsaturated fatty acids on femur mineral density and biomarkers of bone metabolism in healthy, diabetic and dietary-restricted growing rats. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 121–130. [Google Scholar] [CrossRef]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef]
- Hogstrom, M.; Nordstrom, P.; Nordstrom, A. N-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 Study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [CrossRef]
- Griel, A.E.; Kris-Etherton, P.M.; Hilpert, K.F.; Zhao, G.; West, S.G.; Corwin, R.L. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr. J. 2007, 6, 2. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, T.H.; Byun, D.W.; Park, Y. Positive correlation between erythrocyte levels of n-3 polyunsaturated fatty acids and bone mass in postmenopausal Korean women with osteoporosis. Ann. Nutr. Metab. 2012, 60, 146–153. [Google Scholar] [CrossRef]
- Bassey, E.J.; Littlewood, J.J.; Rothwell, M.C.; Pye, D.W. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre- and postmenopausal women: Two randomized controlled trials of Efacal v. calcium alone. Br. J. Nutr. 2000, 83, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Ing, S.W.; Lu, B.; Belury, M.A.; Johnson, K.; Wactawski-Wende, J.; Jackson, R.D. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J. Bone Miner. Res. 2013, 28, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.M.; Reiffel, J.A.; Ellenbogen, K.A.; Naccarelli, G.V.; Kowey, P.R. Efficacy and safety of prescription ω-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: A prospective study. Am. Heart J. 2009, 158, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lavado-Garcia, J.; Roncero-Martin, R.; Moran, J.M.; Pedrera-Canal, M.; Aliaga, I.; Leal-Hernandez, O.; Rico-Martin, S.; Canal-Macias, M.L. Long-chain ω-3 polyunsaturated fatty acid dietary intake is positively associated with bone mineral density in normal and osteopenic Spanish women. PLoS ONE 2018, 13, e0190539. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kukita, A.; Kukita, T.; Shobuike, T.; Nakamura, T.; Kohashi, O. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood 2003, 101, 3451–3459. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chandrasekar, B.; Rahman, M.M.; Banu, J.; Kang, J.X.; Fernandes, G. Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochem. Biophys. Res. Commun. 2006, 349, 925–930. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef]
- Liu, D.; Yao, S.; Wise, G.E. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur. J. Oral. Sci. 2006, 114, 42–49. [Google Scholar] [CrossRef]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Kitaura, H.; Fujimura, Y.; Eguchi, T.; Kohara, H.; Morita, Y.; Yoshida, N. IL-12 inhibits TNF-alpha induced osteoclastogenesis via a T cell-independent mechanism in vivo. Bone 2009, 45, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Iotsova, V.; Caamano, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Donovan, M.G.; Doetschman, T.C.; Selmin, O.I. n-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. ω-6 vegetable oils as a driver of coronary heart disease: The oxidized linoleic acid hypothesis. Open Heart 2018, 5, e000898. [Google Scholar] [CrossRef] [PubMed]
- Reinwald, S.; Li, Y.; Moriguchi, T.; Salem, N., Jr.; Watkins, B.A. Repletion with (n-3) fatty acids reverses bone structural deficits in (n-3)-deficient rats. J. Nutr. 2004, 134, 388–394. [Google Scholar] [CrossRef]
- Weiler, H.A.; Fitzpatrick-Wong, S.C. Modulation of essential (n-6):(n-3) fatty acid ratios alters fatty acid status but not bone mass in piglets. J. Nutr. 2002, 132, 2667–2672. [Google Scholar] [CrossRef]
- Barbour, K.E.; Boudreau, R.; Danielson, M.E.; Youk, A.O.; Wactawski-Wende, J.; Greep, N.C.; LaCroix, A.Z.; Jackson, R.D.; Wallace, R.B.; Bauer, D.C.; et al. Inflammatory markers and the risk of hip fracture: The Women’s Health Initiative. J. Bone Miner. Res. 2012, 27, 1167–1176. [Google Scholar] [CrossRef]
- Stojanovic, D.; Buzkova, P.; Mukamal, K.J.; Heckbert, S.R.; Psaty, B.M.; Fink, H.A.; Cauley, J.A.; Wallace, E.; Curtis, L.H.; Hirsch, C.; et al. Soluble Inflammatory Markers and Risk of Incident Fractures in Older Adults: The Cardiovascular Health Study. J. Bone Miner. Res. 2018, 33, 221–228. [Google Scholar] [CrossRef]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Invest. 2011, 41, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Lorenzo, J.A.; Freeman, A.M.; Tomita, M.; Morham, S.G.; Raisz, L.G.; Pilbeam, C.C. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J. Clin. Invest. 2000, 105, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.L.; Rees, S.G.; Little, C.B.; Flannery, C.R.; Hughes, C.E.; Wilson, C.; Dent, C.M.; Otterness, I.G.; Harwood, J.L.; Caterson, B. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002, 46, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low ω-6/ω-3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.M.; Gomes-Filho, J.E.; Ervolino, E.; Cardoso, C.B.M.; Pipa, C.B.; Kawai, T.; Conti, L.C.; Cintra, L.T.A. ω-3 Fatty Acids Reduce Inflammation in Rat Apical Periodontitis. J. Endod. 2018, 44, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Schett, G. Pathways for bone loss in inflammatory disease. Curr. Osteoporos. Rep. 2012, 10, 101–108. [Google Scholar] [CrossRef]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef]
- Owens, J.M.; Gallagher, A.C.; Chambers, T.J. IL-10 modulates formation of osteoclasts in murine hemopoietic cultures. J. Immunol. 1996, 157, 936–940. [Google Scholar]
- Tak, P.P.; Firestein, G.S. NF-κB. A key role in inflammatory diseases. J. Clin. Invest. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Wong, B.R.; Josien, R.; Lee, S.Y.; Vologodskaia, M.; Steinman, R.M.; Choi, Y. The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J. Biol. Chem. 1998, 273, 28355–28359. [Google Scholar] [CrossRef]
- Guma, M.; Stepniak, D.; Shaked, H.; Spehlmann, M.E.; Shenouda, S.; Cheroutre, H.; Vicente-Suarez, I.; Eckmann, L.; Kagnoff, M.F.; Karin, M. Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011, 208, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Caverzasio, J.; Higgins, L.; Ammann, P. Prevention of Trabecular Bone Loss Induced by Estrogen Deficiency by a Selective p38alpha Inhibitor. J. Bone Miner. Res. 2008, 23, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Lin, J.J.; Lin, C.H.; Su, Y.; Hung, S.C. C-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by BMP2 via phosphorylation of Runx2 at Ser104. J. Bone Miner. Res. 2012, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-k B inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef]
- Lo, C.J.; Chiu, K.C.; Fu, M.; Lo, R.; Helton, S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF κB activity. J. Surg. Res. 1999, 82, 216–221. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Terra, X.; Richart, C.; Ardevol, A.; Pinent, M.; Blay, M. ω-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate NF-κB activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem. J. 2012, 441, 653–663. [Google Scholar] [CrossRef]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef]





| aDiet ingrediants | 4% Placebo | 1% Lovaza | 4% Lovaza | 4% FO-18/12 |
|---|---|---|---|---|
| Casein | 14.00 | 14.00 | 14.00 | 14.00 |
| Corn starch | 47.43 | 50.43 | 47.43 | 42.73 |
| Dextronized corn starch | 14.50 | 14.50 | 14.50 | 14.50 |
| Sucrose | 9.00 | 9.00 | 9.00 | 9.00 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| AIN-93 mineral mix | 3.50 | 3.50 | 3.50 | 3.50 |
| AIN-93 vitamin mix | 1.00 | 1.00 | 1.00 | 1.00 |
| L-cysteine | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
| TBHQ | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin E | 0.04 | 0.04 | 0.04 | 0.04 |
| Corn oil | 1.00 | 4.00 | 1.00 | 1.00 |
| SFO (Safflower oil) | 4.00 | 0.00 | 0.00 | 0.00 |
| bLovaza | 0.00 | 1.00 | 4.00 | 0.00 |
| cFish oil-18/12 | 0.00 | 0.00 | 0.00 | 4.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M.M. Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients 2019, 11, 2701. https://doi.org/10.3390/nu11112701
Abou-Saleh H, Ouhtit A, Halade GV, Rahman MM. Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients. 2019; 11(11):2701. https://doi.org/10.3390/nu11112701
Chicago/Turabian StyleAbou-Saleh, Haissam, Allal Ouhtit, Ganesh V. Halade, and Md Mizanur Rahman. 2019. "Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content" Nutrients 11, no. 11: 2701. https://doi.org/10.3390/nu11112701
APA StyleAbou-Saleh, H., Ouhtit, A., Halade, G. V., & Rahman, M. M. (2019). Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients, 11(11), 2701. https://doi.org/10.3390/nu11112701







