Utility of Novel Hypochromia and Microcythemia Markers in Classifying Hematological and Iron Status in Male Athletes
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Blood Analysis
2.3. Statistical Analysis
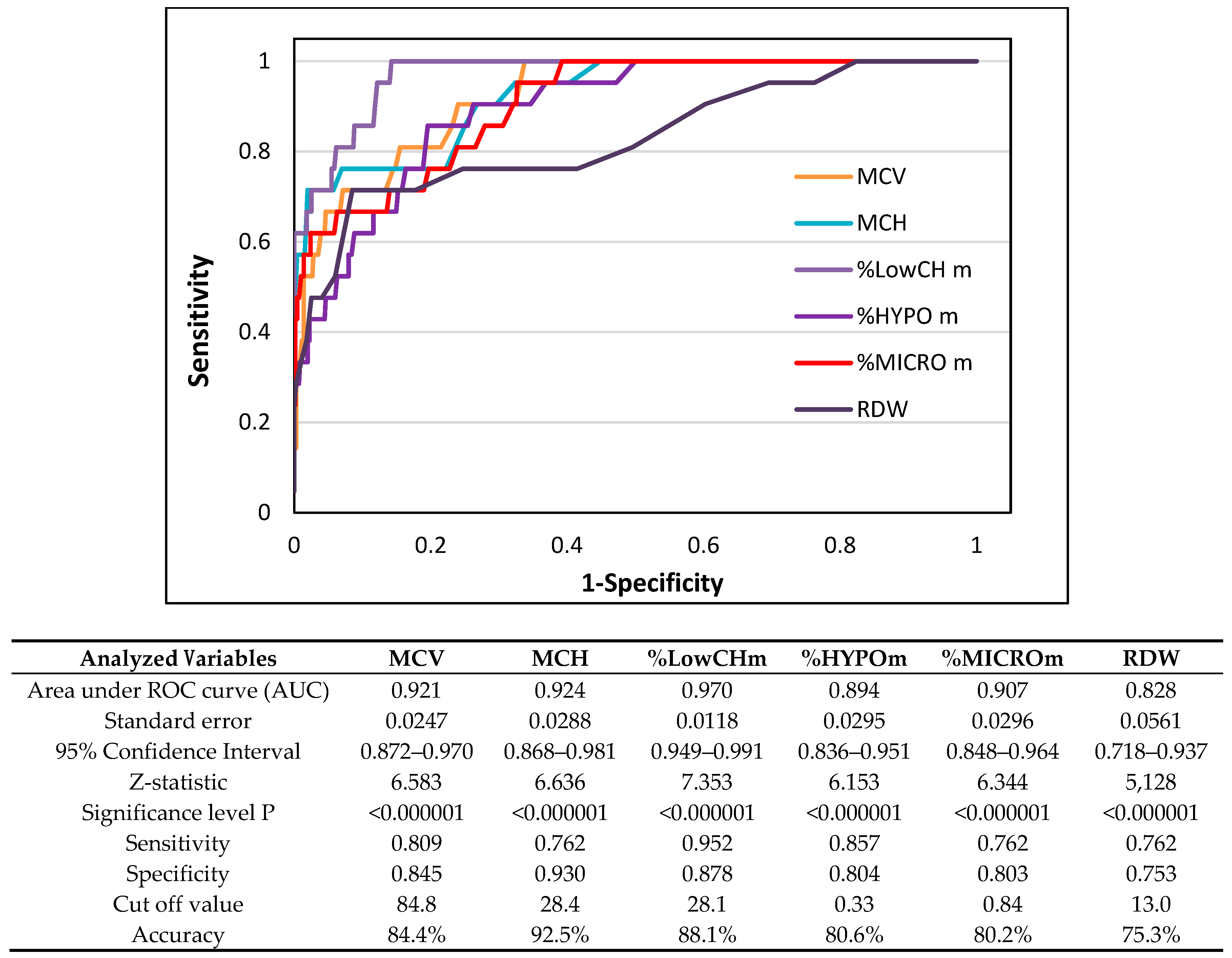
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Urrechaga, E.; Borque, L.; Escanero, J. New laboratory parameters for the assessment of iron status and erythropoiesis. In Advances in Medicine and Biology; Berhard, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 185–207. [Google Scholar]
- Hinton, P.S. Iron and the endurance athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P. Exercise as a mediator of hepcidin activity in athletes. Eur. J. Appl. Physiol. 2010, 110, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Sanford, T.C.; Davidson, M.M.; Yakushko, O.F.; Beck, N.C. Nutrient intakes and dietary behaviors of male and female collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 389–405. [Google Scholar] [CrossRef]
- Bruinvels, G.; Burden, R.; Brown, N.; Richards, T.; Pedlar, C. The prevalence and impact of heavy menstrual bleeding (Menorrhagia) in elite and non-elite athletes. PLoS ONE 2016, 11, e0149881. [Google Scholar] [CrossRef] [PubMed]
- Souglis, A.; Bogdanis, G.C.; Giannopoulou, I.; Papadopoulos, C.; Apostolidis, N. Comparison of inflammatory responses and muscle damage indices following a soccer, basketball, volleyball and handball game at an elite competitive level. Res. Sports Med. 2015, 23, 59–72. [Google Scholar] [CrossRef]
- Souglis, A.G.; Papapanagiotou, A.; Bogdanis, G.C.; Travlos, A.K.; Apostolidis, N.G.; Geladas, N.D. Comparison of inflammatory responses to a soccer match between elite male and female players. J. Strength Cond. Res. 2015, 29, 1227–1233. [Google Scholar] [CrossRef]
- Gore, C.J.; Morkeberg, J.; Schmidt, W.; Garvican, L.A.; Fellman, N. Plasma volume shifts during multiday racing. Clin. Chem. Lab. Med. 2013, 51, e107–e109. [Google Scholar] [CrossRef]
- Voss, S.C.; Alsayrafi, M.; Bourdon, P.C.; Klodt, F.; Nonis, D.; Hopkins, W.G.; Schumacher, Y.O. Variability of serum markers of erythropoiesis during 6 days of racing in highly trained cyclists. Int. J. Sports Med. 2014, 35, 89–94. [Google Scholar] [CrossRef]
- Cai, J.; Wu, M.; Ren, J.; Du, Y.; Long, Z.; Li, G.; Han, B.; Yang, L. Evaluation of the efficiency of the Reticulocyte hemoglobin content on diagnosis for iron deficiency anemia in chinese adults. Nutrients 2017, 9, 450. [Google Scholar] [CrossRef]
- Ibanez-Alcalde, M.M.; Vazquez-Lopez, M.A.; Ruiz-Sanchez, A.M.; Lendinez-Molinos, F.J.; Galera-Martinez, R.; Bonillo-Perales, A.; Parron-Carreno, T. Reference values of reticulocyte hemoglobin content in healthy adolescents. J. Pediatr. Hematol. Oncol. 2018, 40, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Baart, A.M.; Balvers, M.G.J.; Hopman, M.T.E.; Eijsvogels, T.M.H.; Klein Gunnewiek, J.M.T.; van Kampen, C.A. Reticulocyte hemoglobin content in a large sample of the general Dutch population and its relation to conventional iron status parameters. Clin. Chim. Acta. 2018, 483, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Urrechaga, I.E.; Hoffmann, J.J.M.L.; Izquierdo-Alvarez, S.; Escanero, J.F. Reticulocyte hemoglobin content (MCHr) in the detection of iron deficiency. J. Trace Elem. Med. Biol. 2017, 43, 29–32. [Google Scholar] [CrossRef]
- Vazquez-Lopez, M.A.; Lopez-Ruzafa, E.; Ibanez-Alcalde, M.; Martin-Gonzalez, M.; Bonillo-Perales, A.; Lendinez-Molinos, F. The usefulness of reticulocyte haemoglobin content, serum transferrin receptor and the sTfR-ferritin index to identify iron deficiency in healthy children aged 1-16 years. Eur. J. Pediatr. 2019, 178, 41–49. [Google Scholar] [CrossRef]
- Torino, A.B.B.; Gilberti, M.D.F.P.; da Costa, E.; de Lima, G.A.F.; Grotto, H.Z.W. Evaluation of erythrocyte and reticulocyte parameters as indicative of iron deficiency in patients with anemia of chronic disease. Rev. Bras. Hematol. Hemoter. 2015, 37, 77–81. [Google Scholar] [CrossRef]
- Leers, M.P.G.; Keuren, J.F.W.; Oosterhuis, W.P. The value of the Thomas-plot in the diagnostic work up of anemic patients referred by general practitioners. Int. J. Lab. Hematol. 2010, 32, 572–581. [Google Scholar] [CrossRef]
- Lorenz, L.; Arand, J.; Buchner, K.; Wacker-Gussmann, A.; Peter, A.; Poets, C.F.; Franz, A.R. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F198–F202. [Google Scholar] [CrossRef]
- Urrechaga, E.; Borque, L.; Escanero, J.F. Clinical value of hypochromia markers in the detection of latent iron deficiency in nonanemic premenopausal women. J. Clin. Lab. Anal. 2016, 30, 623–627. [Google Scholar] [CrossRef]
- Mast, A.E.; Blinder, M.A.; Dietzen, D.J. Reticulocyte hemoglobin content. Am. J. Hematol. 2008, 83, 307–310. [Google Scholar] [CrossRef]
- Nalado, A.M.; Mahlangu, J.N.; Duarte, R.; Paget, G.; Olorunfemi, G.; Jacobson, B.F.; Naicker, S. Utility of reticulocyte haemoglobin content and percentage hypochromic red cells as markers of iron deficiency anaemia among black CKD patients in South Africa. PLoS ONE 2018, 13, e0204899. [Google Scholar] [CrossRef]
- Piva, E. Comment on: Evaluation of erythrocyte and reticulocyte parameters as indicative of iron deficiency in patients with anemia of chronic disease. Rev. Bras. Hematol. Hemoter. 2015, 37, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.E.; Steele, W.R.; Wright, D.J.; Mast, A.E.; Carey, P.M.; Murphy, E.L.; Gottschall, J.L.; Simon, T.L.; Cable, R.G. Laboratory variables for assessing iron deficiency in REDS-II iron Status evaluation (RISE) blood donors. Transfusion 2013, 53, 2766–2775. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Nemeth, E.; Ganz, T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010, 116, 4754–4761. [Google Scholar] [CrossRef] [PubMed]
- Malczewska-Lenczowska, J.; Orysiak, J.; Szczepańska, B.; Turowski, D.; Burkhard-Jagodzińska, K.; Gajewski, J. Reticulocyte and erythrocyte hypochromia markers in detection of iron deficiency in adolescent female athletes. Biol. Sport 2017, 34, 111–118. [Google Scholar] [CrossRef]
- Ashenden, M.J.; Pyne, D.B.; Parisotto, R.; Dobson, G.P.; Hahn, A.G. Can reticulocyte parameters be of use in detecting iron deficient erythropoiesis in female athletes? J. Sports Med. Phys. Fit. 1999, 39, 140–146. [Google Scholar]
- Voss, S.C.; Varamenti, E.; Elzain Elgingo, M.; Bourdon, P.C. New parameters and reference values for monitoring iron status in Middle Eastern adolescent male athletes. J. Sports Med. Phys. Fit. 2014, 54, 179–185. [Google Scholar]
- Malczewska, J.; Szczepanska, B.; Stupnicki, R.; Sendecki, W. The assessment of frequency of iron deficiency in athletes from the transferrin receptor-ferritin index. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 42–52. [Google Scholar] [CrossRef]
- Malczewska, J.; Raczyński, G. Iron status in male endurance athletes and in non athletes. Biol. Sport 1997, 14, 259–273. [Google Scholar]
- Sinclair, L.M.; Hinton, P.S. Prevalence of iron deficiency with and without anemia in recreationally active men and women. J. Am. Diet. Assoc. 2005, 105, 975–978. [Google Scholar] [CrossRef]
- Telford, R.D.; Cunningham, R.B. Sex, sport, and body-size dependency of hematology in highly trained athletes. Med. Sci. Sports Exerc. 1991, 23, 788–794. [Google Scholar] [CrossRef]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Munoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Rao Baikady, R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Clenin, G. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med. Wkly. 2017, 147, w14434. [Google Scholar] [PubMed]
- Clenin, G.; Cordes, M.; Huber, A.; Schumacher, Y.O.; Noack, P.; Scales, J.; Kriemler, S. Iron deficiency in sports—definition, influence on performance and therapy. Swiss Med. Wkly. 2015, 145, w14196. [Google Scholar] [CrossRef] [Green Version]
- Schrier, S.L.; Auerbach, M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. Up to date. Available online: https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults (accessed on 13 August 2018).
- Rosenthal, R.; Rubin, D.B. r equivalent: A simple effect size indicator. Psychol. Methods 2003, 8, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988; pp. 20–27. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Malczewska, J.; Blach, W.; Stupnicki, R. The effects of physical exercise on the concentrations of ferritin and transferrin receptor in plasma of female judoists. Int. J. Sports Med. 2000, 21, 175–179. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Bejder, J.; Andersen, A.B.; Goetze, J.P.; Aachmann-Andersen, N.J.; Nordsborg, N.B. Plasma volume reduction and hematological fluctuations in high-level athletes after an increased training load. Scand. J. Med. Sci. Sports 2017, 27, 1605–1615. [Google Scholar] [CrossRef]
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Markovic, M.; Majkic-Singh, N.; Ignjatovic, S.; Singh, S. Reticulocyte haemoglobin content vs. soluble transferrin receptor and ferritin index in iron deficiency anaemia accompanied with inflammation. Int. J. Lab. Hematol. 2007, 29, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Schapkaitz, E.; Buldeo, S.; Mahlangu, J.N. Diagnosis of iron deficiency anaemia in hospital patients: Use of the reticulocyte haemoglobin content to differentiate iron deficiency anaemia from anaemia of chronic disease. S. Afr. Med. J. 2015, 106, 53–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Thomas, L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin. Chem. 2002, 48, 1066–1076. [Google Scholar] [PubMed]
- Ceylan, C.; Miskioglu, M.; Colak, H.; Kiliccioglu, B.; Ozdemir, E. Evaluation of reticulocyte parameters in iron deficiency, vitamin B(12) deficiency and beta-thalassemia minor patients. Int. J. Lab. Hematol. 2007, 29, 327–334. [Google Scholar] [CrossRef]
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S.; et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int. J. Hematol. 2017, 106, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Franck, S.; Messinger, M.; Linssen, J.; Thome, M.; Thomas, C. Reticulocyte hemoglobin measurement--comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin. Chem. Lab. Med. 2005, 43, 1193–1202. [Google Scholar] [CrossRef]
- Kotisaari, S.; Romppanen, J.; Penttila, I.; Punnonen, K. The Advia 120 red blood cells and reticulocyte indices are useful in diagnosis of iron-deficiency anemia. Eur. J. Haematol. 2002, 68, 150–156. [Google Scholar] [CrossRef]
- Asberg, A.E.; Mikkelsen, G.; Aune, M.W.; Asberg, A. Empty iron stores in children and young adults—The diagnostic accuracy of MCV, MCH, and MCHC. Int. J. Lab. Hematol. 2014, 36, 98–104. [Google Scholar] [CrossRef]
- Higgins, J.M. Red blood cell population dynamics. Clin. Lab. Med. 2015, 35, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Koehler, K.; Braun, H.; Achtzehn, S.; Hildebrand, U.; Predel, H.-G.; Mester, J.; Schanzer, W. Iron status in elite young athletes: Gender-dependent influences of diet and exercise. Eur. J. Appl. Physiol. 2012, 112, 513–523. [Google Scholar] [CrossRef]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron deficiency and anemia among collegiate athletes: A retrospective chart review. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Merkel, D.; Huerta, M.; Grotto, I.; Blum, D.; Tal, O.; Rachmilewitz, E.; Fibach, E.; Epstein, Y.; Shpilberg, O. Prevalence of iron deficiency and anemia among strenuously trained adolescents. J. Adolesc. Health 2005, 37, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Dubnov, G.; Constantini, N.W. Prevalence of iron depletion and anemia in top-level basketball players. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 30–37. [Google Scholar] [CrossRef]
- Fallon, K.E. Utility of hematological and iron-related screening in elite athletes. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2004, 14, 145–152. [Google Scholar] [CrossRef]
- Fallon, K.E. Screening for haematological and iron-related abnormalities in elite athletes-analysis of 576 cases. J. Sci. Med. Sport 2008, 11, 329–336. [Google Scholar] [CrossRef]
- Reinke, S.; Taylor, W.R.; Duda, G.N.; von Haehling, S.; Reinke, P.; Volk, H.-D.; Anker, S.D.; Doehner, W. Absolute and functional iron deficiency in professional athletes during training and recovery. Int. J. Cardiol. 2012, 156, 186–191. [Google Scholar] [CrossRef]
- Hallberg, L.; Magnusson, B. The etiology of “sports anemia”. A physiological adaptation of the oxygen-dissociation curve of hemoglobin to an unphysiological exercise load. Acta Med. Scand. 1984, 216, 147–148. [Google Scholar]
- Weight, L.M.; Darge, B.L.; Jacobs, P. Athletes’ pseudoanaemia. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 358–362. [Google Scholar] [CrossRef]
- Eichner, E.R. Sports medicine pearls and pitfalls: nature’s anticoagulant. Curr. Sports Med. Rep. 2009, 8, 2–3. [Google Scholar] [CrossRef]
- Sawka, M.N.; Montain, S.J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 2000, 72, 564S–572S. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-H.; Lai, J.-I.; Wang, S.-H.; How, C.-K.; Li, L.-H.; Kao, W.-F.; Yang, C.-C.; Chen, R.-J. Early changes of the anemia phenomenon in male 100-km ultramarathoners. J. Chin. Med. Assoc. 2015, 78, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Orysiak, J.; Pokrywka, A.; Szygula, Z. Total haemoglobin mass, blood volume and morphological indices among athletes from different sport disciplines. Arch. Med. Sci. 2013, 9, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Otto, J.M.; Plumb, J.O.M.; Clissold, E.; Kumar, S.B.; Wakeham, D.J.; Schmidt, W.; Grocott, M.P.W.; Richards, T.; Montgomery, H.E. Hemoglobin concentration, total hemoglobin mass and plasma volume in patients: Implications for anemia. Haematologica 2017, 102, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Brugnara, C.; Spolaore, F.; Plebani, M. Clinical utility of reticulocyte parameters. Clin. Lab. Med. 2015, 35, 133–163. [Google Scholar] [CrossRef]


| Variable | Mean Value, SD and (Range) | ||
|---|---|---|---|
| Age | (years) | 18.7 ± 3.5 | (13.0–35.1) |
| Body mass | (kg) | 75.4 ± 11.5 | (36.2–129.0) |
| Height | (cm) | 182.5 ± 8.2 | (149.5–209.7) |
| Athletic experience | (years) | 7.7 ± 3.5 | (1.0–22.0) |
| Variable | Healthy Subjects | Iron Deficiency b | ||
|---|---|---|---|---|
| Normal Iron Status | Sport Anemia a | Iron Depletion | IDE | |
| (n = 659) | (n = 57) | (n = 191) | (n = 21) | |
| Ferritin (µg/L) | 64.1 ± 32.0 | 61.0 ± 22.8 | 22.6 ± 4.9*** | 13.5 ± 7.3*** |
| sTfR (mg/L) | 4.9 ± 1.2 | 4.98 ± 1.13 | 5.56 ± 1.29*** | 9.35 ± 2.84***^^^ |
| TIBC (µg/dL) | 319.6 ± 32.9 | 314.4 ± 27.7 | 347.4 ± 35.8** | 394.6 ± 41.9***^^ |
| Iron (µg/dL) | 102.3 ± 39.9 | 89.4 ± 35.5* | 89.7 ± 37.5*** | 63.6 ± 32.0***^ |
| RBC (×1012/L) | 5.18 ± 0.25 | 4.62 ± 0.16*** | 5.20 ± 0.30 | 5.39 ± 0.27**^ |
| Hct (%) | 45.4 ± 2.0 | 40.8 ± 1.29*** | 45.0 ± 2.3* | 44.3 ± 1.9* |
| Hb (g/L) | 154.9 ± 7.4 | 140.7 ± 4.4*** | 152.0 ± 8.4*** | 147.5 ± 6.9*** |
| MCH (pg) | 29.9 ± 1.11 | 30.5 ± 1.29** | 29.3 ± 1.24*** | 27.4 ± 1.41***^^^ |
| MCHC (g/L) | 341.3 ± 10.5 | 345.3 ± 13.5* | 338.4 ± 10.8*** | 333.1 ± 10.6*** |
| MCV (fL) | 87.8 ± 2.8 | 88.4 ± 3.8 | 86.6 ± 3.4*** | 82.2 ± 2.9***^^^ |
| CH (pg) | 29.9 ± 0.99 | 30.3 ± 1.09* | 29.2 ± 1.11*** | 27.3 ± 1.55***^^^ |
| RDW (%) | 12.7 ± 0.43 | 12.7 ± 0.45 | 13.0 ± 0.49*** | 13.5 ± 0.82*** |
| HYPOm (%) | 0.24 ± 0.34 | 0.23 ± 0.26 | 0.55 ± 0.73*** | 1.72 ± 2.28***^^ |
| Low CHm (%) | 18.5 ± 7.3 | 16.8 ± 7.1 | 25.8 ± 10.1*** | 44.7 ± 14.5***^^^ |
| MICROm (%) | 0.61 ± 0.33 | 0.57 ± 0.33 | 0.83 ± 0.47*** | 1.99 ± 1.41**^^^ |
| Reticulocytes (%) | 1.46 ± 0.34 | 1.44 ± 0.37 | 1.42 ± 0.35 | 1.41 ± 0.26 |
| Reticulocytes (109/L) | 75.5 ± 18.4 | 66.6 ± 17.3*** | 73.6 ± 18.3 | 76.1 ± 15.7 |
| MCVr (fl) | 100.8 ± 2.7 | 101.7 ± 3.2 | 100.6 ± 2.8 | 97.7 ± 2.76***^^^ |
| CHCMr (g/dL) | 32.1 ± 0.99 | 32.1 ± 0.90 | 31.3 ± 1.11*** | 30.0 ± 1.18***^^^ |
| CHr (pg) | 32.3 ± 1.07 | 32.4 ± 1.09 | 31.4 ± 1.22*** | 29.2 ±1.48***^^^ |
| Low CHr (%) | 5.9 ± 3.7 | 5.7 ± 3.9 | 9.8 ± 6.1*** | 26.4 ± 16.3***^^^ |
| HYPOr (%) | 5.7 ± 4.7 | 5.6 ± 3.7 | 11.1 ± 8.7*** | 25.8 ± 17.4***^^^ |
| Body Iron (mg/kg) | 7.55 ± 1.86 | 7.40 ± 1.63 | 3.57 ± 1.44*** | −0.67 ± 3.11*** |
| Iron depletion | IDE | |||||
|---|---|---|---|---|---|---|
| Analyzed Variables | AUC of ROC | (95% CI) | p-value | AUC of ROC | (95% CI) | p-value |
| Hb | 0.564 | 0.518–0.610 | 0.006366 | 0.718 | 0.616–0.820 | 0.00065 |
| RBC | 0.558 | 0.512–0.605 | 0.012606 | 0.741 | 0.644–0.838 | 0.000161 |
| Hct | 0.517 | 0.470–0.563 | 0.470684 | 0.615 | 0.502–0.729 | 0.070867 |
| MCHC | 0.582 | 0.537–0.628 | 0.0005 | 0.719 | 0.602–0.837 | <0.0006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malczewska-Lenczowska, J.; Surała, O.; Orysiak, J.; Turowski, D.; Szczepańska, B.; Tomaszewski, P. Utility of Novel Hypochromia and Microcythemia Markers in Classifying Hematological and Iron Status in Male Athletes. Nutrients 2019, 11, 2767. https://doi.org/10.3390/nu11112767
Malczewska-Lenczowska J, Surała O, Orysiak J, Turowski D, Szczepańska B, Tomaszewski P. Utility of Novel Hypochromia and Microcythemia Markers in Classifying Hematological and Iron Status in Male Athletes. Nutrients. 2019; 11(11):2767. https://doi.org/10.3390/nu11112767
Chicago/Turabian StyleMalczewska-Lenczowska, Jadwiga, Olga Surała, Joanna Orysiak, Dariusz Turowski, Beata Szczepańska, and Paweł Tomaszewski. 2019. "Utility of Novel Hypochromia and Microcythemia Markers in Classifying Hematological and Iron Status in Male Athletes" Nutrients 11, no. 11: 2767. https://doi.org/10.3390/nu11112767
APA StyleMalczewska-Lenczowska, J., Surała, O., Orysiak, J., Turowski, D., Szczepańska, B., & Tomaszewski, P. (2019). Utility of Novel Hypochromia and Microcythemia Markers in Classifying Hematological and Iron Status in Male Athletes. Nutrients, 11(11), 2767. https://doi.org/10.3390/nu11112767





