Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Bronchoalveolar Lavage (BAL) Sampling
2.3. Flow Cytometry
2.4. Cytokine and IgE Determinations
2.5. Lung Histology and Immunohistochemistry
2.6. RNA Extraction and mRNA and miRNA Detections
2.7. Statistical Analysis
3. Results
3.1. Weight and Biochemical Characteristics of Mice Before Mating and Pregnancy Outcomes
3.2. Increased Eosinophil Accumulation in BAL of OVA-Challenged Mice born to HFD-Fed Mice
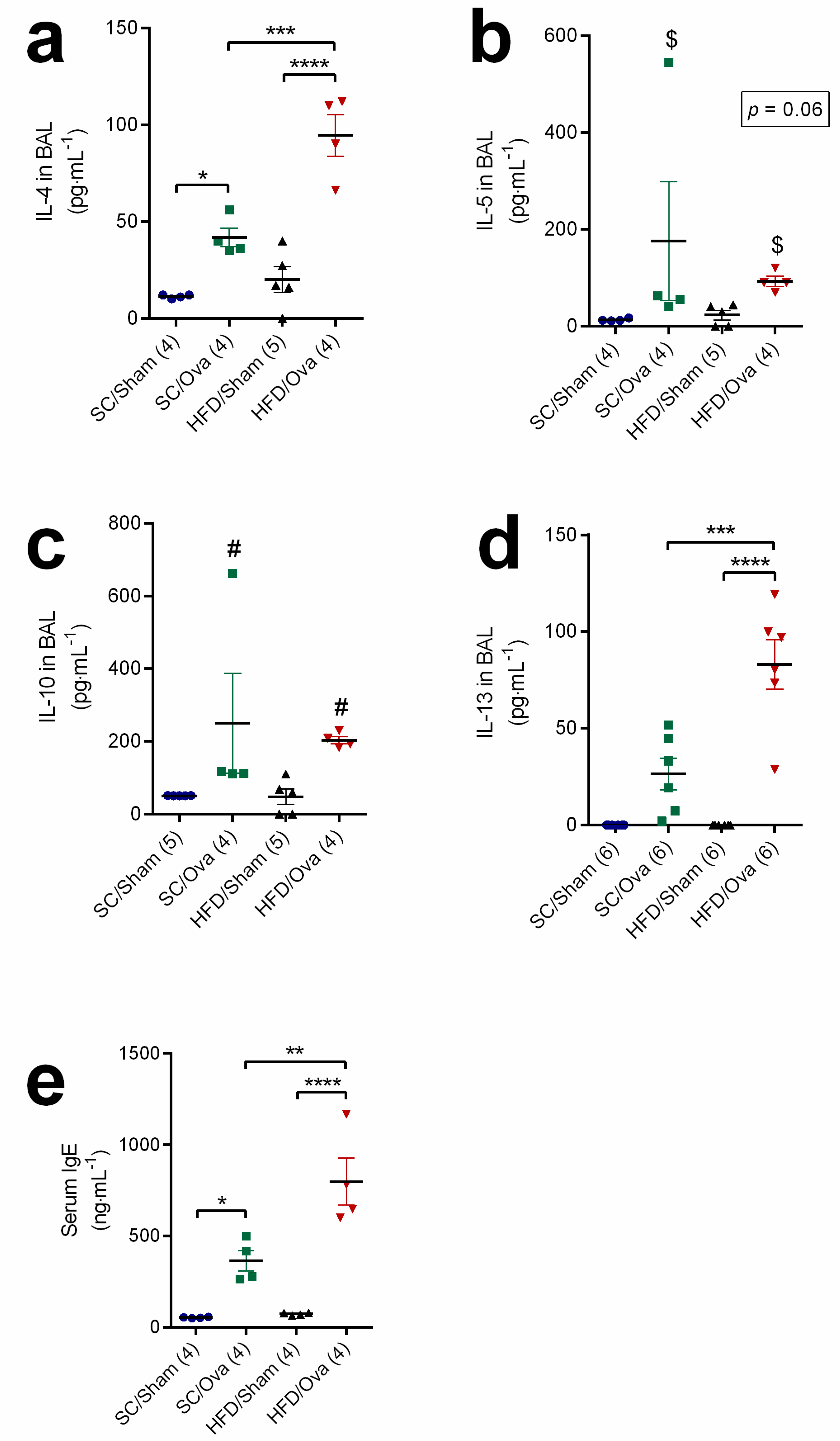
3.3. Increased BAL Levels of Interleukins of the TH2 Response and Serum Level of IgE in OVA-Challenged Mice Born to HFD-Fed Mice
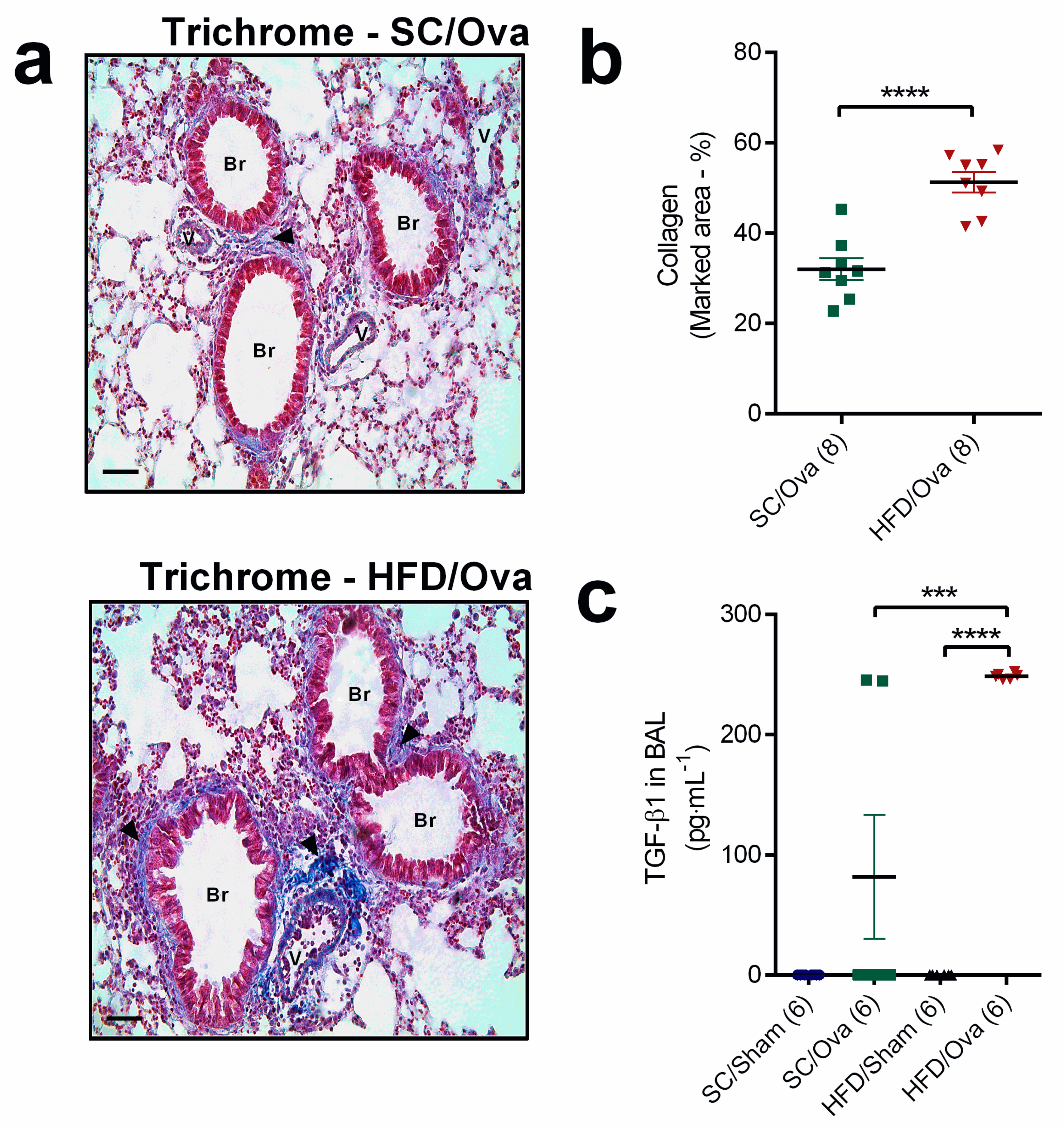
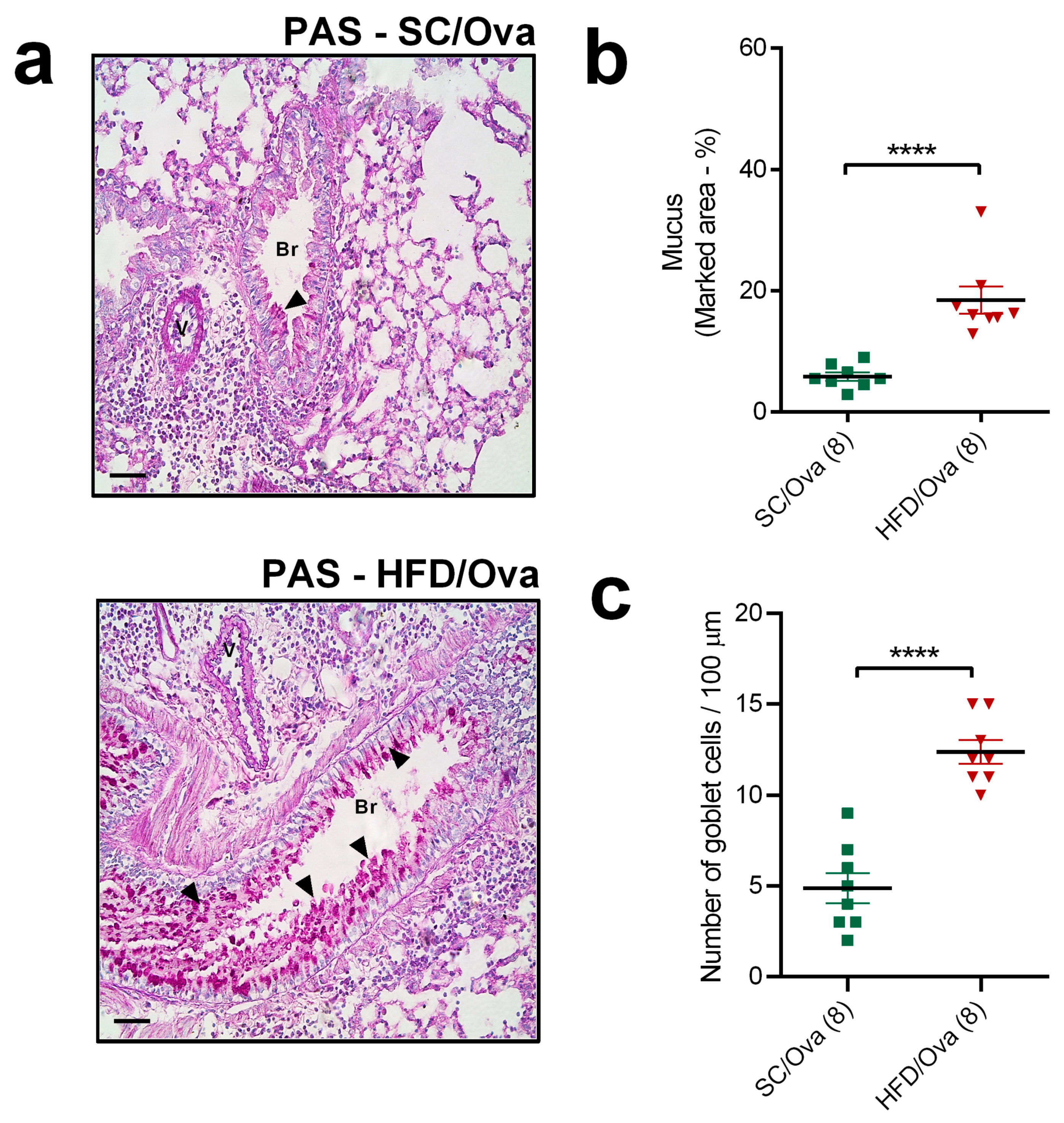
3.4. Intensification of Lung Remodeling in OVA-Challenged Mice Born to HFD-Fed Mice
3.5. Mice Born to HFD-Fed Mothers Have Exacerbated TNF-α level and Increased TNF-α Signaling after Being Challenged with OVA
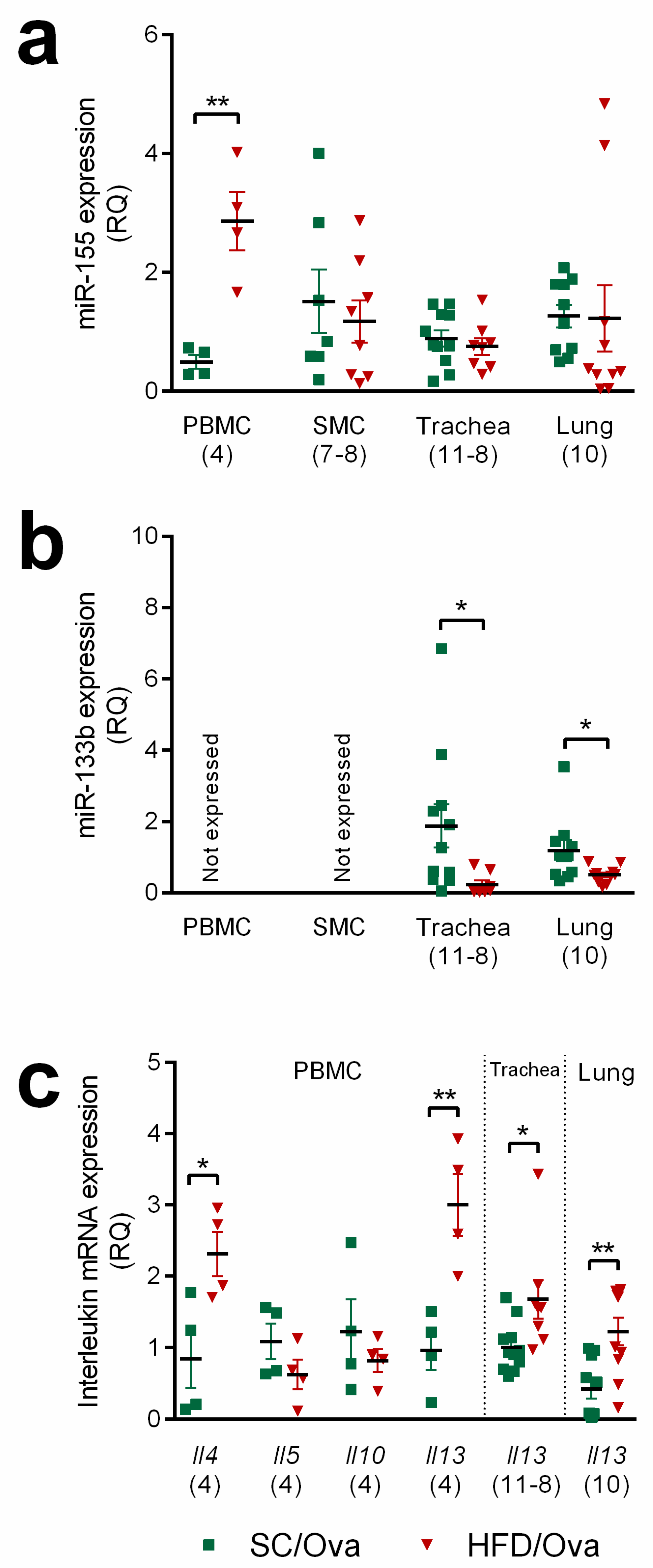
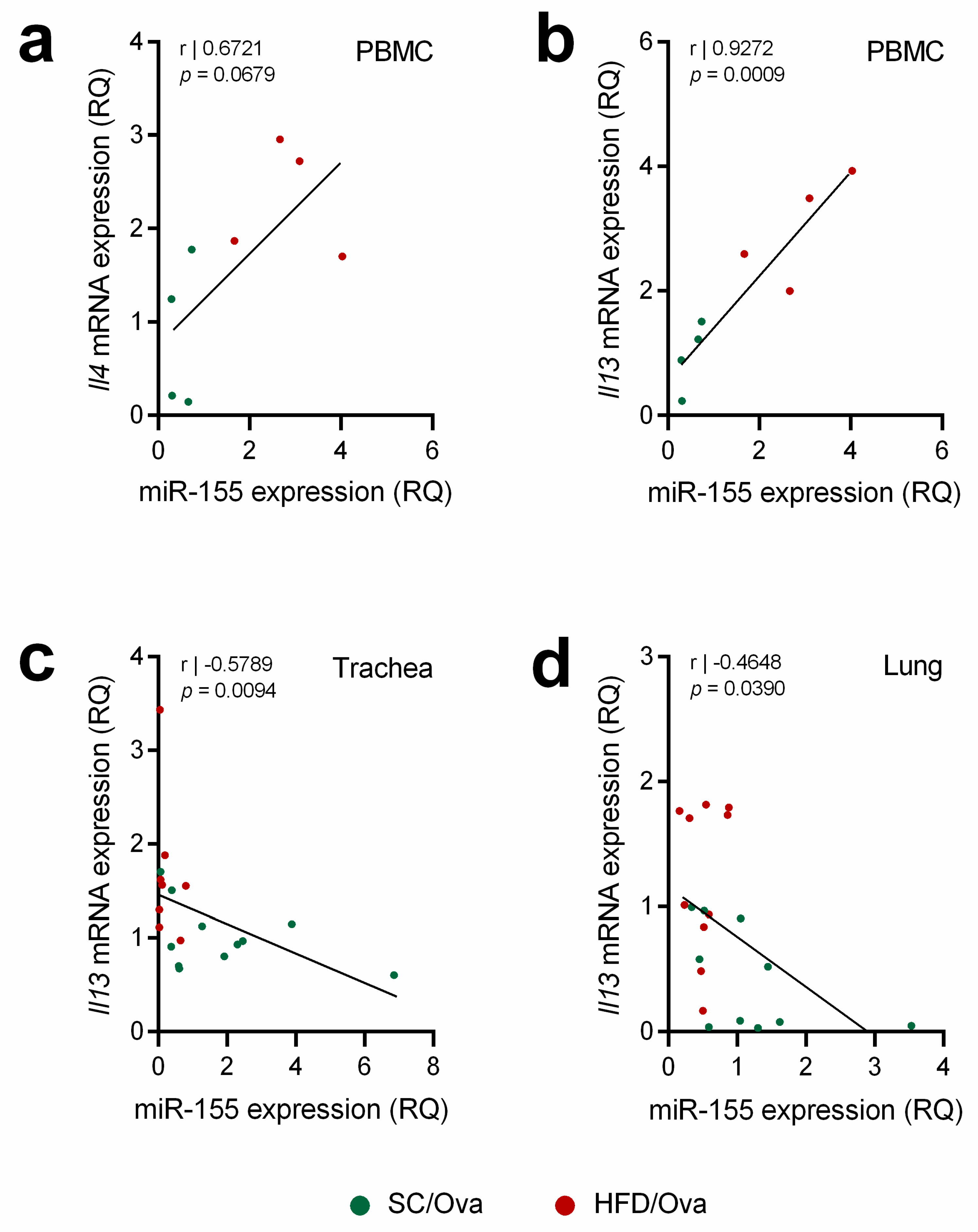
3.6. Mice Born to HFD-Fed Mice Have Increased miRNAs That Are Associated with the Exacerbation of the TH2 Response and IL-13 Signaling
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Backman, H.; Räisänen, P.; Hedman, L.; Stridsman, C.; Andersson, M.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016—results from three population surveys. Clin. Exp. Allergy 2017, 47, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.S.; Maltby, S.; Rosenberg, H.F.; Tay, H.L.; Hogan, S.P.; Collison, A.M.; Yang, M.; Kaiko, G.E.; Hansbro, P.M.; Kumar, R.K.; et al. Modeling T H 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol. Rev. 2017, 278, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Grünig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Herbert, C.; Siegle, J.S.; Vuppusetty, C.; Hansbro, N.; Thomas, P.S.; Foster, P.S.; Barnes, P.J.; Kumar, R.K. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am. J. Respir. Cell Mol. Biol. 2008, 39, 543–550. [Google Scholar] [CrossRef]
- Herbert, C.; Scott, M.M.; Scruton, K.H.; Keogh, R.P.; Yuan, K.C.; Hsu, K.; Siegle, J.S.; Tedla, N.; Foster, P.S.; Kumar, R.K. Alveolar macrophages stimulate enhanced cytokine production by pulmonary CD4+ T-lymphocytes in an exacerbation of murine chronic asthma. Am. J. Pathol. 2010, 177, 1657–1664. [Google Scholar] [CrossRef]
- Romieu, I.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am. J. Epidemiol. 2003, 158, 165–174. [Google Scholar] [CrossRef]
- Camargo, C.A.; Weiss, S.T.; Zhang, S.; Willett, W.C.; Speizer, F.E. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch. Intern. Med. 1999, 159, 2582–2588. [Google Scholar] [CrossRef]
- Baltieri, L.; Cazzo, E.; de Souza, A.L.; Alegre, S.M.; de Paula Vieira, R.; Antunes, E.; de Mello, G.C.; Claudio Martins, L.; Chaim, E.A. Influence of weight loss on pulmonary function and levels of adipokines among asthmatic individuals with obesity: One-year follow-up. Respir. Med. 2018, 145, 48–56. [Google Scholar] [CrossRef]
- Grotta, M.B.; Squebola-Cola, D.M.; Toro, A.A.D.C.; Ribeiro, M.A.G.O.; Mazon, S.B.; Ribeiro, J.D.; Antunes, E. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Calixto, M.C.; Lintomen, L.; Schenka, A.; Saad, M.J.; Zanesco, A.; Antunes, E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br. J. Pharmacol. 2010, 159, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Dietze, J.; Böcking, C.; Heverhagen, J.T.; Voelker, M.N.; Renz, H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Everaere, L.; Ait-Yahia, S.; Molendi-Coste, O.; Vorng, H.; Quemener, S.; LeVu, P.; Fleury, S.; Bouchaert, E.; Fan, Y.; Duez, C.; et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J. Allergy Clin. Immunol. 2016, 138, 1309–1318.e11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, D.; Wu, X.; Zhang, X.; Zhou, Q.; Luo, Y.; Yang, X.; Chock, C.J.; Liu, M.; Yang, X.O. Leptin Promotes Allergic Airway Inflammation through Targeting the Unfolded Protein Response Pathway. Sci. Rep. 2018, 8, 8905. [Google Scholar] [CrossRef]
- Forno, E.; Young, O.M.; Kumar, R.; Simhan, H.; Celedón, J.C. Maternal Obesity in Pregnancy, Gestational Weight Gain, and Risk of Childhood Asthma. Pediatrics 2014, 134, e535–e546. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- MacDonald, K.D.; Moran, A.R.; Scherman, A.J.; McEvoy, C.T.; Platteau, A.S. Maternal high-fat diet in mice leads to innate airway hyperresponsiveness in the adult offspring. Physiol. Rep. 2017, 5, e13082. [Google Scholar] [CrossRef]
- Griffiths, P.S.; Walton, C.; Samsell, L.; Perez, M.K.; Piedimonte, G. Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr. Res. 2016, 79, 278–286. [Google Scholar] [CrossRef]
- Dinger, K.; Kasper, P.; Hucklenbruch-Rother, E.; Vohlen, C.; Jobst, E.; Janoschek, R.; Bae-Gartz, I.; Van Koningsbruggen-Rietschel, S.; Plank, C.; Dötsch, J.; et al. Early-onset obesity dysregulates pulmonary adipocytokine/insulin signaling and induces asthma-like disease in mice. Sci. Rep. 2016, 6, 24168. [Google Scholar] [CrossRef]
- Pantaleão, L.C.; Murata, G.; Teixeira, C.J.; Payolla, T.B.; Santos-Silva, J.C.; Duque-Guimaraes, D.E.; Sodré, F.S.; Lellis-Santos, C.; Vieira, J.C.; De Souza, D.N.; et al. Prolonged fasting elicits increased hepatic triglyceride accumulation in rats born to dexamethasone-Treated mothers. Sci. Rep. 2017, 7, 10367. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.P.; Wang, Y.; Howrylak, J.; Chinchilli, V.M.; Craig, T.J.; August, A.; Ishmael, F.T. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J. Allergy Clin. Immunol. 2016, 137, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Jiang, L.; Hu, Q.; Li, Y. MicroRNA-133b Ameliorates Allergic Inflammation and Symptom in Murine Model of Allergic Rhinitis by Targeting Nlrp3. Cell. Physiol. Biochem. 2017, 42, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, W.; Liu, F.; Lin, F.; He, J.; Liu, C.; Lin, Y.; Dong, S. MicroRNA-133b regulates the growth and migration of vascular smooth muscle cells by targeting matrix metallopeptidase 9. Pathol. Res. Pract. 2019, 215, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Naves-Díaz, M.; Carrillo-López, N.; Martínez-Arias, L.; Fernández-Martín, J.L.; Ruiz-Torres, M.P.; Cannata-Andía, J.B.; Rodríguez, I. MicroRNAs 29b, 133b, and 211 regulate vascular smooth muscle calcification mediated by high phosphorus. J. Am. Soc. Nephrol. 2016, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.J.; Qi, J.; Kong, X.J.; Huang, T.; Qian, X.Q.; Xu, D.; Liang, J.H.; Kang, J. MiR-133 modulates TGF-β1-induced bladder smooth muscle cell hypertrophic and fibrotic response: Implication for a role of microRNA in bladder wall remodeling caused by bladder outlet obstruction. Cell. Signal. 2015, 27, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Malmhäll, C.; Alawieh, S.; Lu, Y.; Sjöstrand, M.; Bossios, A.; Eldh, M.; Rådinger, M. MicroRNA-155 is essential for TH2-mediated allergen-induced eosinophilic inflammation in the lung. J. Allergy Clin. Immunol. 2014, 133. [Google Scholar] [CrossRef]
- Zech, A.; Ayata, C.K.; Pankratz, F.; Meyer, A.; Baudiß, K.; Cicko, S.; Yegutkin, G.G.; Grundmann, S.; Idzko, M. MicroRNA-155 modulates P2R signaling and Th2 priming of dendritic cells during allergic airway inflammation in mice. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1121–1129. [Google Scholar] [CrossRef]
- Johansson, K.; Malmhäll, C.; Ramos-Ramírez, P.; Rådinger, M. MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation. J. Allergy Clin. Immunol. 2017, 139, 1007–1016.e9. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, E.; Li, X.; Zhang, M.; Tang, Z.; He, L.; Lv, K. miR-155 contributes to Df1-induced asthma by increasing the proliferative response of Th cells via CTLA-4 downregulation. Cell. Immunol. 2017, 314, 1–9. [Google Scholar] [CrossRef]
- Okoye, I.S.; Czieso, S.; Ktistaki, E.; Roderick, K.; Coomes, S.M.; Pelly, V.S.; Kannan, Y.; Perez-Lloret, J.; Zhao, J.L.; Baltimore, D.; et al. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc. Natl. Acad. Sci. USA 2014, 111, E3081–E3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, E.; Roff, A.; Hsu, M.-H.; Panganiban, R.; Lambert, K.; Ishmael, F. Effects of allergic stimulation and glucocorticoids on miR-155 in CD4+ T-cells. Am. J. Clin. Exp. Immunol. 2018, 7, 57–66. [Google Scholar] [PubMed]
- Shi, Y.; Fu, X.; Cao, Q.; Mao, Z.; Chen, Y.; Sun, Y.; Liu, Z.; Zhang, Q. Overexpression of miR-155-5p inhibits the proliferation and migration of IL-13-induced human bronchial smooth muscle cells by suppressing TGF-ß-activated kinase 1/MAP3K7-binding protein 2. Allergy Asthma Immunol. Res. 2018, 10, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polinski, K.J.; Liu, J.; Boghossian, N.S.; McLain, A.C. Maternal Obesity, Gestational Weight Gain, and Asthma in Offspring. Prev. Chronic Dis. 2017, 14, 170196. [Google Scholar] [CrossRef]
- Bergstedt-Lindqvist, S.; Moon, H.-B.; Persson, U.; Möller, G.; Heusser, C.; Severinson, E. Interleukin 4 instructs uncommitted B lymphocytes to switch to IgGl and IgE. Eur. J. Immunol. 1988, 18, 1073–1077. [Google Scholar] [CrossRef]
- Hogan, S.P.; Mould, A.; Kikutani, H.; Ramsay, A.J.; Foster, P.S. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J. Clin. Investig. 1997, 99, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Jutel, M.; Akdis, M.; Budak, F.; Aebischer-Casaulta, C.; Wrzyszcz, M.; Blaser, K.; Akdis, C.A. IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003, 33, 1205–1214. [Google Scholar] [CrossRef]
- Böhm, L.; Maxeiner, J.; Meyer-Martin, H.; Reuter, S.; Finotto, S.; Klein, M.; Schild, H.; Schmitt, E.; Bopp, T.; Taube, C. IL-10 and Regulatory T Cells Cooperate in Allergen-Specific Immunotherapy To Ameliorate Allergic Asthma. J. Immunol. 2015, 194, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Shahabuddin, S.; Ponath, P.; Schleimer, R.P. Migration of Eosinophils Across Endothelial Cell Monolayers: Interactions Among IL-5, Endothelial-Activating Cytokines, and C-C Chemokines. J. Immunol. 2014, 164, 3847–3854. [Google Scholar] [CrossRef]
- Januskevicius, A.; Gosens, R.; Sakalauskas, R.; Vaitkiene, S.; Janulaityte, I.; Halayko, A.J.; Hoppenot, D.; Malakauskas, K. Suppression of eosinophil integrins prevents remodeling of airway smooth muscle in asthma. Front. Physiol. 2017, 7, 680. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Hogan, S.P.; Henry, P.J.; Matthaei, K.I.; McKenzie, A.N.J.; Young, I.G.; Rothenberg, M.E.; Foster, P.S. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am. J. Respir. Cell Mol. Biol. 2001, 25, 522–530. [Google Scholar] [CrossRef]
- Alimam, M.Z.; Piazza, F.M.; Selby, D.M.; Letwin, N.; Huang, L.; Rose, M.C. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am. J. Respir. Cell Mol. Biol. 2000, 22, 253–260. [Google Scholar] [CrossRef]
- Shim, J.J.; Dabbagh, K.; Takeyama, K.; Burgel, P.R.; Dao-Pick, T.P.; Ueki, I.F.; Nadel, J.A. Suplatast tosilate inhibits goblet-cell metaplasia of airway epithelium in sensitized mice. J. Allergy Clin. Immunol. 2000, 105, 739–745. [Google Scholar] [CrossRef]
- Dabbagh, K.; Takeyama, K.; Lee, H.M.; Ueki, I.F.; Lausier, J.A.; Nadel, J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999, 162, 6233–6237. [Google Scholar]
- Narala, V.R.; Ranga, R.; Smith, M.R.; Berlin, A.A.; Standiford, T.J.; Lukacs, N.W.; Reddy, R.C. Pioglitazone is as effective as dexamethasone in a cockroach allergen-induced murine model of asthma. Respir. Res. 2007, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Rankin, J.A.; Picarella, D.E.; Geba, G.P.; Temann, U.A.; Prasad, B.; DiCosmo, B.; Tarallo, A.; Stripp, B.; Whitsett, J.; Flavell, R.A. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: Lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc. Natl. Acad. Sci. USA 2002, 93, 7821–7825. [Google Scholar] [CrossRef] [Green Version]
- Coutts, A.; Chen, G.; Stephens, N.; Hirst, S.; Douglas, D.; Eichholtz, T.; Khalil, N. Release of biologically active TGF-β from airway smooth muscle cells induces autocrine synthesis of collagen. Am. J. Physiol. Cell. Mol. Physiol. 2017, 280, L999–L1008. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, W.; Liu, C.; Li, Y.; Xia, Y.; Yang, X.; Sun, W.; Bai, H.; Li, Q.; Peng, Z. Immunization against TGF-β1 reduces collagen deposition but increases sustained inflammation in a murine asthma model. Hum. Vaccines Immunother. 2016, 12, 1876–1885. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.-I.; Yoon, H.-Y.; Lee, Y.-R.; Won, M.; Chung, M.J.; Park, J.-W.; Hur, G.M.; Lee, H.-K.; Park, B.-H. A20 Attenuates Allergic Airway Inflammation in Mice. J. Immunol. 2009, 183, 1488–1495. [Google Scholar] [CrossRef] [Green Version]
- Sasse, S.K.; Altonsy, M.O.; Kadiyala, V.; Cao, G.; Panettieri, R.A.; Gerber, A.N. Glucocorticoid and TNF signaling converge at A20 (TNFAIP3) to repress airway smooth muscle cytokine expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L421–L432. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Czermak, B.J.; Bless, N.M.; Ward, P.A. NF-κB activation during IgG immune complex-induced lung injury: Requirements for TNF-α and IL-1β but not complement. Am. J. Pathol. 1998, 152, 1327. [Google Scholar] [PubMed]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijod, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-α from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5360–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firszt, R.; Francisco, D.; Church, T.D.; Thomas, J.M.; Ingram, J.L.; Kraft, M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase- 2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur. Respir. J. 2014, 43, 464–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blease, K.; Jakubzick, C.; Westwick, J.; Lukacs, N.; Kunkel, S.L.; Hogaboam, C.M. Therapeutic Effect of IL-13 Immunoneutralization During Chronic Experimental Fungal Asthma. J. Immunol. 2001, 166, 5219–5224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohir, W.; Ratcliffe, E.M.; Sloboda, D.M. Of the bugs that shape us: Maternal obesity, the gut microbiome, and long-term disease risk. Pediatr. Res. 2015, 77, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [Green Version]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; MacIa, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]



| SC Dams | HFD Dams | P | |
|---|---|---|---|
| N | 12 | 12 | - |
| Body mass (g) | 21.68 ± 1.24 | 27.03 ± 3.89 | *** |
| Triglycerides (mg/dL) | 114.3 ± 27.4 | 158.1 ± 36.6 | ** |
| Glucose (mg/dL) | 128.8 ± 14.9 | 170.5 ± 15.54 | **** |
| Cholesterol (mg/dL) | 94.15 ± 7.9 | 141.2 ± 32.9 | *** |
| Leptin (ng/mL) | 1.555 ± 0.297 | 11.070 ± 2.130 | *** |
| Number of pups per litter | 6.1 ± 1.6 | 5 ± 1.9 | nd |
| SC Offspring | HFD Offspring | P | |
|---|---|---|---|
| N | 8 | 8 | - |
| Triglycerides (mg/dL) | 82.02 ± 29.5 | 67.16 ± 24.87 | nd |
| Glucose (mg/dL) | 150.4 ± 24.29 | 142.4 ± 18.28 | nd |
| Cholesterol (mg/dL) | 78.36 ± 12.24 | 67.07 ± 12.08 | nd |
| Leptin (ng/mL) | 0.588 ± 0.162 | 0.587 ± 0.064 | nd |
| Body mass at the 3rd day of life (g) | 3.17 ± 0.14 | 2.52 ± 0.14 | ** |
| Body mass at the 21st day of life (g) | 9.36 ± 0.61 | 7.56 ± 0.19 | * |
| Body mass at the 8th week of life (g) | 26.13 ± 2.34 | 24.38 ± 0.73 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
e-Lacerda, R.R.; Teixeira, C.J.; Bordin, S.; Antunes, E.; Anhê, G.F. Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring. Nutrients 2019, 11, 2902. https://doi.org/10.3390/nu11122902
e-Lacerda RR, Teixeira CJ, Bordin S, Antunes E, Anhê GF. Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring. Nutrients. 2019; 11(12):2902. https://doi.org/10.3390/nu11122902
Chicago/Turabian Stylee-Lacerda, Rodrigo Rodrigues, Caio Jordão Teixeira, Silvana Bordin, Edson Antunes, and Gabriel Forato Anhê. 2019. "Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring" Nutrients 11, no. 12: 2902. https://doi.org/10.3390/nu11122902
APA Stylee-Lacerda, R. R., Teixeira, C. J., Bordin, S., Antunes, E., & Anhê, G. F. (2019). Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring. Nutrients, 11(12), 2902. https://doi.org/10.3390/nu11122902






