The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Tissue Collection
2.3. Liver Triglycerides and Liver Gene Expression
2.4. Quantification of Hop Prenylflavonoids in Hop Extract and Serum
2.5. Bile Acid Determination
2.6. Short Chain Fatty Acid Determination
2.7. Intestinal Permeability and Inflammation
2.8. DNA Extraction and 16S Sequencing
2.9. Statistical Analysis
3. Results
3.1. Weight Gain, Adiposity and Liver Triglycerides
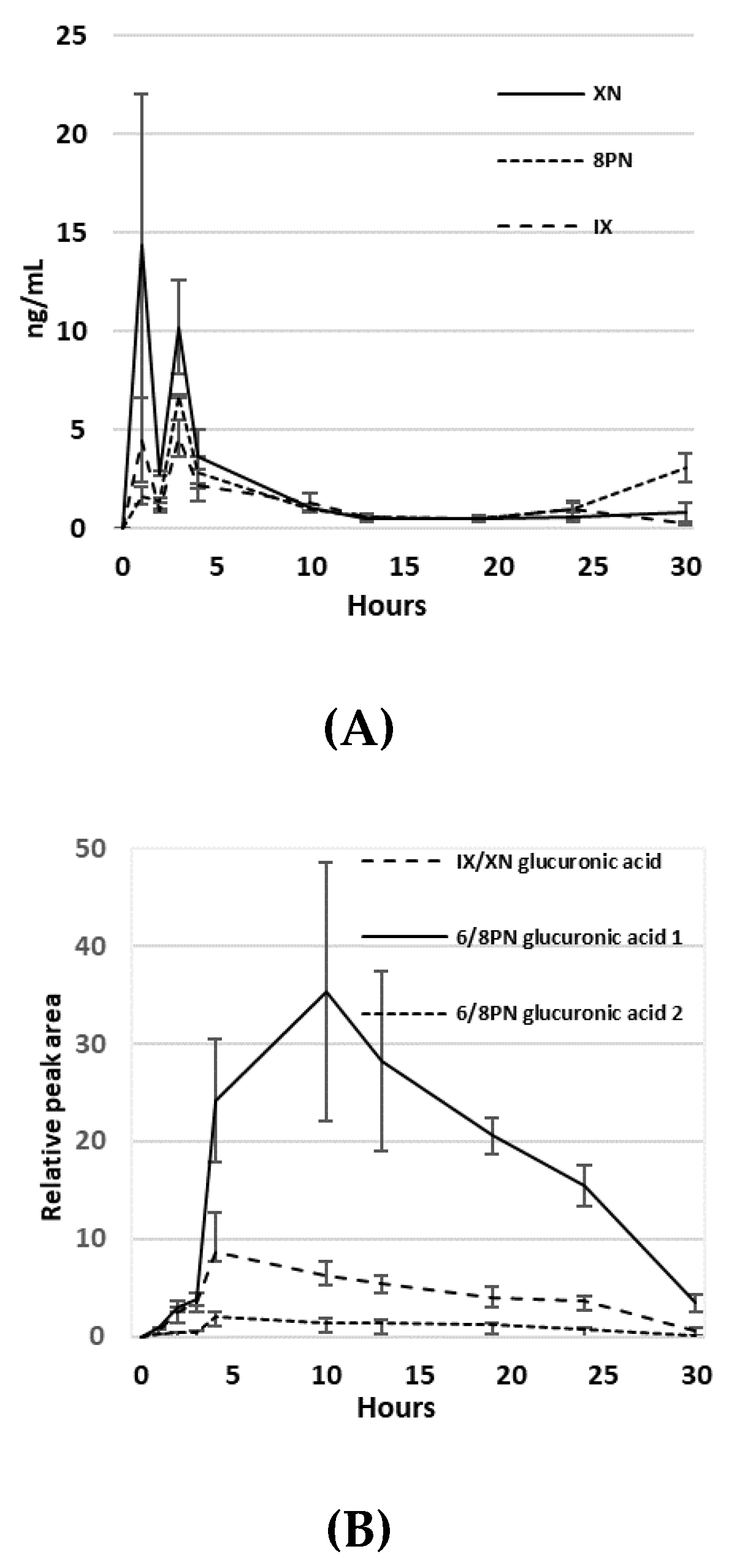
3.2. Pharmacokinetics of Hop Prenylvlavonoids in Serum
3.3. Bile Acid and Short Chain Fatty Acid Quantification
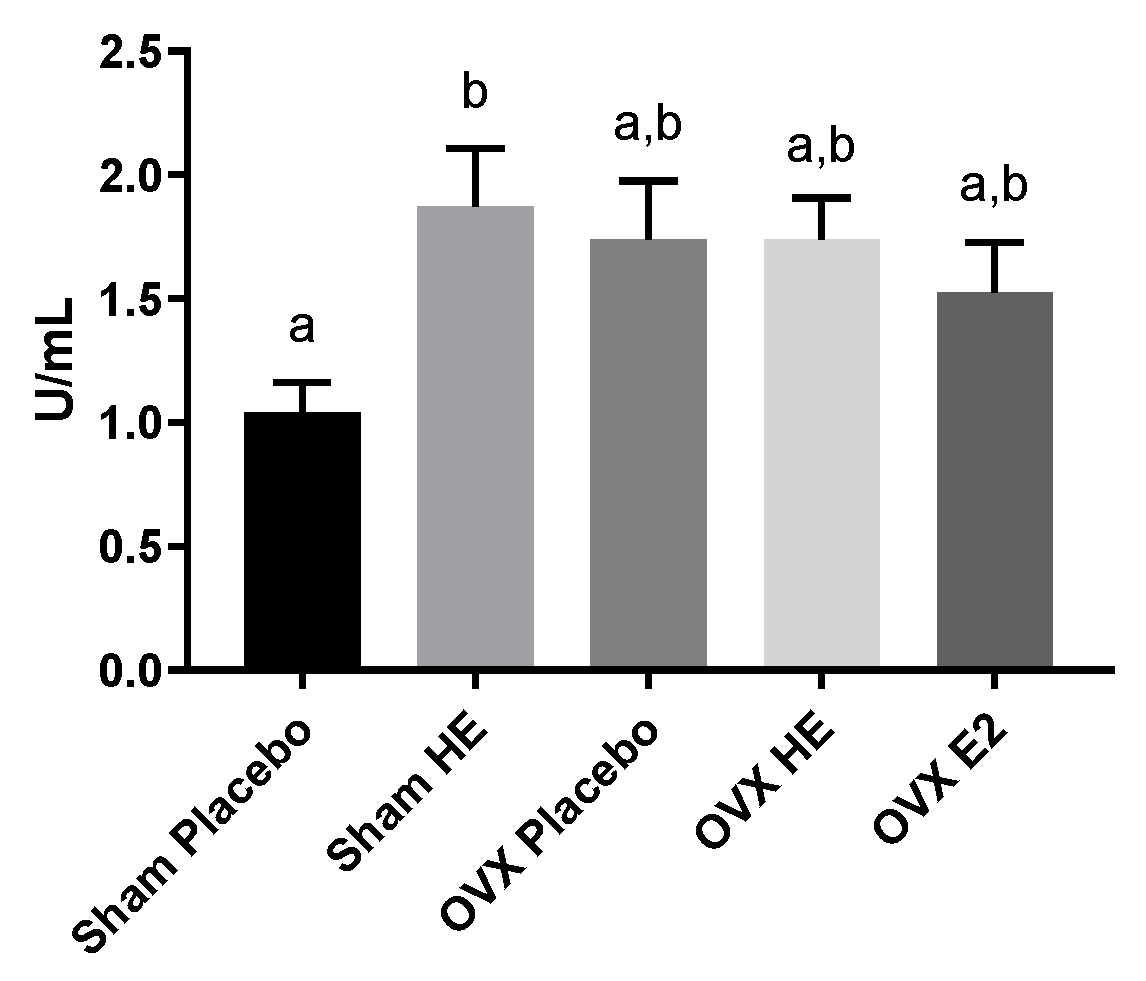
3.4. Intestinal Function
3.5. Microbiota Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.C.; McNamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease: The Framingham study. Ann. Intern. Med. 1976, 85, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taha, M.; Rius, C.; Hermenegildo, C.; Noguera, I.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Jose, P.J.; Cortijo, J.; Morcillo, E.J.; Sanz, M.-J. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J. Immunol. 2009, 183, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Estrogens, breast cancer, and intestinal flora. Rev. Infect. Dis. 1984, 6, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Watson, S.E.; Thomas, L.N.; Allred, C.D.; Dabney, A.; Azcarate-Peril, M.A.; Sturino, J.M. Diet complexity and estrogen receptor β-status affect the composition of the murine intestinal microbiota. Appl. Environ. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cox-York, K.A.; Sheflin, A.M.; Foster, M.T.; Gentile, C.L.; Kahl, A.; Koch, L.G.; Britton, S.L.; Weir, T.L. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep. 2015, 3, e12488. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Janes, M.; Robert, J.; Martin, R.J.; Raggio, A.M.; McCutcheon, K.L.; Pelkman, C.; Tulley, R.; Goita, M.F.; Durham, H.A. Resistant starch from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity 2013, 21, 981–984. [Google Scholar] [CrossRef]
- Liu, T.-W.; Park, Y.-M.; Holscher, H.D.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K.S. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Sánchez-Garrido, M.Á.; Martín-Núñez, G.M.; Pérez-Jiménez, F.; Tena-Sempere, M.; Tinahones, F.J.; Queipo-Ortuño, M.I. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology 2016, 157, 4888–4898. [Google Scholar] [CrossRef]
- Melvin, R. The Effects of Exercise and Estrogen on Gut Microbiota in Female Mice. Master’s Thesis, Rutgers University-Graduate School-New Brunswick, New Brunswick, NJ, USA, 2016. [Google Scholar]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Gass, M.; Lane, D.S.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Stefanick, M.L.; Ockene, J.; Sarto, G.E. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010, 304, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Santen, R.J.; Allred, D.C.; Ardoin, S.P.; Archer, D.F.; Boyd, N.; Braunstein, G.D.; Burger, H.G.; Colditz, G.A.; Davis, S.R.; Gambacciani, M. Postmenopausal hormone therapy: An Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010, 95, s1–s66. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Roberts, H.; Hickey, M. Managing the menopause: An update. Maturitas 2016, 86, 53–58. [Google Scholar] [CrossRef]
- Jungbauer, A.; Medjakovic, S. Phytoestrogens and the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2014, 139, 277–289. [Google Scholar] [CrossRef]
- Milligan, S.; Kalita, J.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249. [Google Scholar] [CrossRef]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 2002, 123, 235–242. [Google Scholar] [CrossRef]
- Bowe, J.; Li, X.F.; Kinsey-Jones, J.; Heyerick, A.; Brain, S.; Milligan, S.; O’Byrne, K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol. 2006, 191, 399–405. [Google Scholar] [CrossRef]
- Erkkola, R.; Vervarcke, S.; Vansteelandt, S.; Rompotti, P.; De Keukeleire, D.; Heyerick, A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine 2010, 17, 389–396. [Google Scholar] [CrossRef]
- Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur. J. Pharmacol. 2009, 620, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Rabot, S.; Espín, J.C.; Bruneau, A.; Philippe, C.; González-Sarrías, A.; Heyerick, A.; Tomás-Barberán, F.A.; De Keukeleire, D.; Verstraete, W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr. 2008, 138, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, I.L.; Plagmann, L.S.; Yang, L.; Zielke, R.; Gombart, A.F.; Maier, C.S.; Sikora, A.E.; Blakemore, P.R.; Stevens, J.F. Reductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the intestinal bacterium Eubacterium ramulus. Mol. Nutr. Food Res. 2019, 63, e1800923. [Google Scholar] [CrossRef]
- Ingberg, E.; Theodorsson, A.; Theodorsson, E.; Strom, J. Methods for long-term 17β-estradiol administration to mice. Gen. Comp. Endocrinol. 2012, 175, 188–193. [Google Scholar] [CrossRef]
- Overk, C.R.; Yao, P.; Chadwick, L.R.; Nikolic, D.; Sun, Y.; Cuendet, M.A.; Deng, Y.; Hedayat, A.; Pauli, G.F.; Farnsworth, N.R. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem. 2005, 53, 6246–6253. [Google Scholar] [CrossRef][Green Version]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- Manter, D.K.; Korsa, M.; Tebbe, C.; Delgado, J.A. myPhyloDB: A local web server for the storage and analysis of metagenomic data. Database J. Biol. Databases Curation 2016. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.; Morris, J.S.; Davies, C.R.; Weber-Hall, S.J.; Duffy, M.-A.; Heath, V.J.; Bell, A.K.; Ferrier, R.K.; Sandilands, G.P.; Gusterson, B.A. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2003, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garrido, M.A.; Ruiz-Pino, F.; Manfredi-Lozano, M.; Leon, S.; Heras, V.; Castellano, J.M.; Castaño, J.P.; Luque, R.M.; Vázquez, M.J.; Roa, J. Metabolic and gonadotropic impact of sequential obesogenic insults in the female: Influence of the loss of ovarian secretion. Endocrinology 2015, 156, 2984–2998. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Alekel, L.; Hollis, B.W.; Amin, D. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J. Nutr. 1996, 126, 161. [Google Scholar] [CrossRef]
- Davis, S.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.; Nappi, R.; Shah, D.; Villaseca, P. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef]
- Madak-Erdogan, Z.; Gong, P.; Zhao, Y.C.; Xu, L.; Wrobel, K.U.; Hartman, J.A.; Wang, M.; Cam, A.; Iwaniec, U.T.; Turner, R.T. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol. Nutr. Food Res. 2016, 60, 369–380. [Google Scholar] [CrossRef]
- Pouliot, M.-C.; Després, J.-P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Frank, A.; Brown, L.M.; Clegg, D.J. The role of hypothalamic estrogen receptors in metabolic regulation. Front. Neuroendocrinol. 2014, 35, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nikolic, D.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159. [Google Scholar] [CrossRef]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; van Breemen, R.B. In vitro studies of intestinal permeability and hepatic and intestinal metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus L.). Pharm. Res. 2006, 23, 864–872. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Legette, L.L.; Luna, A.Y.M.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Hop (Humulus lupulus L.) extract inhibits obesity in mice fed a high-fat diet over the long term. Br. J. Nutr. 2013, 109, 162–172. [Google Scholar] [CrossRef]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; France, M.; Boester, A.; Sawai, M.; Scholl, J.; Witkin, S.S. Influence of pregnancy history on the vaginal microbiome of pregnant women in their first trimester. Sci. Rep. 2017, 7, 10201. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Rosenthal, M.D.; Miller, K.R.; Martindale, R.G. The gut in trauma. Curr. Opin. Crit. Care 2016, 22, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.A.; Liu, X.; Zhao, Y.C.; Hieronymi, K.; Rossi, G.; Auvil, L.S.; Welge, M.; Bushell, C.; Smith, R.L.; Carlson, K.E. Long-Term Administration of conjugated estrogen and Bazedoxifene decreased murine fecal β-glucuronidase activity without impacting overall microbiome community. Sci. Rep. 2018, 8, 8166. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2013, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Karlsson, C.L.; Önnerfält, J.; Xu, J.; Molin, G.; Ahrné, S.; Thorngren-Jerneck, K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.; Larsen, L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes 2015, 5, e159. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Ahn, J.; Schatzkin, A.; Lacey, J.V.; Albanes, D.; Ballard-Barbash, R.; Adams, K.F.; Kipnis, V.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch. Intern. Med. 2007, 167, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Tankó, L.B.; Bagger, Y.Z.; Qin, G.; Alexandersen, P.; Larsen, P.J.; Christiansen, C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation 2005, 111, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.; Katz, M.; Dunn, J. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int. J. Obes. 1991, 15, 471–478. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 1987, 316, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.K.; Weir, T.L. Editorial on “Cancer and the microbiota” published in Science. Ann. Transl. Med. 2015, 3, 175. [Google Scholar]
- Takahashi, K.; Osada, K. Effect of dietary purified Xanthohumol from hop (Humulus lupulus L.) pomace on adipose tissue mass, fasting blood glucose level, and lipid metabolism in KK-Ay mice. J. Oleo Sci. 2017, 66, 531–541. [Google Scholar] [CrossRef]
- Mendes, V.; Monteiro, R.; Pestana, D.; Teixeira, D.; Calhau, C.; Azevedo, I. Xanthohumol influences preadipocyte differentiation: Implication of antiproliferative and apoptotic effects. J. Agric. Food Chem. 2008, 56, 11631–11637. [Google Scholar] [CrossRef]
- Costa, R.; Rodrigues, I.; Guardão, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhães, J.; Ferreira-de-Almeida, M.; Negrão, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef]
- Lupinacci, E.; Meijerink, J.; Vincken, J.-P.; Gabriele, B.; Gruppen, H.; Witkamp, R.F. Xanthohumol from hop (Humulus lupulus L.) is an efficient inhibitor of monocyte chemoattractant protein-1 and tumor necrosis factor-α release in LPS-stimulated RAW 264.7 mouse macrophages and U937 human monocytes. J. Agric. Food Chem. 2009, 57, 7274–7281. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Kraus, B.; Motyl, M.; Weiss, T.S.; Gehrig, M.; Schölmerich, J.; Heilmann, J.; Hellerbrand, C. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol. Nutr. Food Res. 2010, 54, S205–S213. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-C.; Kim, H.J.; Kim, Y.-J.; Lee, K.Y.; Choi, H.J.; Lee, I.-S.; Kang, B.Y. Differential anti-inflammatory pathway by xanthohumol in IFN-γ and LPS-activated macrophages. Int. Immunopharmacol. 2008, 8, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Heyerick, A.; Vervarcke, S.; Depypere, H.; Bracke, M.; De Keukeleire, D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas 2006, 54, 164–175. [Google Scholar] [CrossRef]



| Daily Intake (g) | Total Calories | % Calories Fat | % Calories Protein | % Calories Carbohydrate | |
|---|---|---|---|---|---|
| Diet | 2.99 | 10.75 | 10% | 14% | 76% |
| Cookie | 0.20 | 1.04 | 49% | 5% | 48% |
| Oil | 0.03 | 0.27 | 100% | 0% | 0% |
| Total: | 3.22 | 12.06 | 15.6% | 12.7% | 71.9% |
| Target Gene | Sequence |
|---|---|
| ABCG5 | For: 5′-CAGGGACCGAATTGTGATTG-3′ |
| Rev: 5′-GAACACCAACTCTCCGTAAG-3′ | |
| ABCG8 | For: 5′-CTGGAATCCTGAGAGGATAG-3′ |
| Rev: 5′-TAGGTCGCCCTTTGTATTGG-3′ | |
| FASn | For: 5′- AGACTACAGACGACAGCAACC-3′ |
| Rev: 5’-CTCTCAGACAGGCACTCAGC-3’ | |
| SREBP1c | For: 5′-TGGTGGGCACTGAAGCAAAG-3′ |
| Rev: 5′-CACTTCGTAGGGTCAGGTTCTC-3′ | |
| AcCoA carboxylase | For: 5′-CTTCGCCATAACCAAGTAGAG-3′ |
| Rev: 5′-GTTTCCGAGAGGATGAGTTTC-3′ | |
| Hormone sensitive lipase | For: 5′-CAGTCAATGGAGACACTTGG-3′ |
| Rev: 5′-GGGTCTCACTTCATCTTTGG-3′ |
| Molecule | Parent m/z | Fragment m/z | CV | CE |
|---|---|---|---|---|
| Xanthohumol | 353.3 | 119 | 50 | 26 |
| Xanthohumol | 353.3 | 233.2 | 50 | 18 |
| 8-prenylnaringenin | 339.3 | 219.2 | 60 | 22 |
| 8-prenylnaringenin | 339.3 | 119 | 60 | 30 |
| Naringenin | 271.2 | 151.1 | 50 | 18 |
| Naringenin | 271.2 | 119 | 50 | 26 |
| 2,4-dihydroxychalcone | 239.2 | 135.1 | 60 | 22 |
| 2,4-dihydroxychalcone | 239.2 | 197.1 | 60 | 20 |
| Xanthohumol-glucuronic acid | 529.3 | 353.3 | 50 | 25 |
| 8-prenylnaringenin-glucuronic acid | 515.3 | 339.3 | 50 | 25 |
| Sham Placebo | Sham HE | p-Value | OVX Placebo | p-Value | OVX HE | p-Value | OVX E2 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| BW | 31.0 | 30.1 | 0.96 | 34.3 | 0.09 | 33.9 | 0.16 | 31.0 | 0.99 |
| Cecum | 296.7 | 317.6 | 0.99 | 243.9 | 0.91 | 274.7 | 0.99 | 297.7 | 0.99 |
| Uterus | 107.7 | 89.5 | 0.32 | 28.0 | <0.001 * | 31.2 | <0.001 * | 78.9 | 0.023 * |
| SAT | 1.24 | 1.17 | 0.99 | 1.67 | 0.21 | 1.54 | 0.57 | 1.16 | 0.99 |
| BAT | 0.11 | 0.12 | 0.99 | 0.14 | 0.78 | 0.15 | 0.55 | 0.13 | 0.93 |
| VAT | 2.259 | 2.288 | 0.99 | 3.68 | <0.001 * | 3.27 | 0.063 | 2.02 | 0.97 |
| VAT/Total AT | 0.627 | 0.638 | 0.98 | 0.671 | 0.09 | 0.655 | 0.48 | 0.608 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamm, A.K.; Manter, D.K.; Kirkwood, J.S.; Wolfe, L.M.; Cox-York, K.; Weir, T.L. The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice. Nutrients 2019, 11, 3004. https://doi.org/10.3390/nu11123004
Hamm AK, Manter DK, Kirkwood JS, Wolfe LM, Cox-York K, Weir TL. The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice. Nutrients. 2019; 11(12):3004. https://doi.org/10.3390/nu11123004
Chicago/Turabian StyleHamm, Alison K., Daniel K. Manter, Jay S. Kirkwood, Lisa M. Wolfe, Kimberly Cox-York, and Tiffany L. Weir. 2019. "The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice" Nutrients 11, no. 12: 3004. https://doi.org/10.3390/nu11123004
APA StyleHamm, A. K., Manter, D. K., Kirkwood, J. S., Wolfe, L. M., Cox-York, K., & Weir, T. L. (2019). The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice. Nutrients, 11(12), 3004. https://doi.org/10.3390/nu11123004





