Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models
Abstract
:1. Introduction
2. Materials and Methods
3. Results
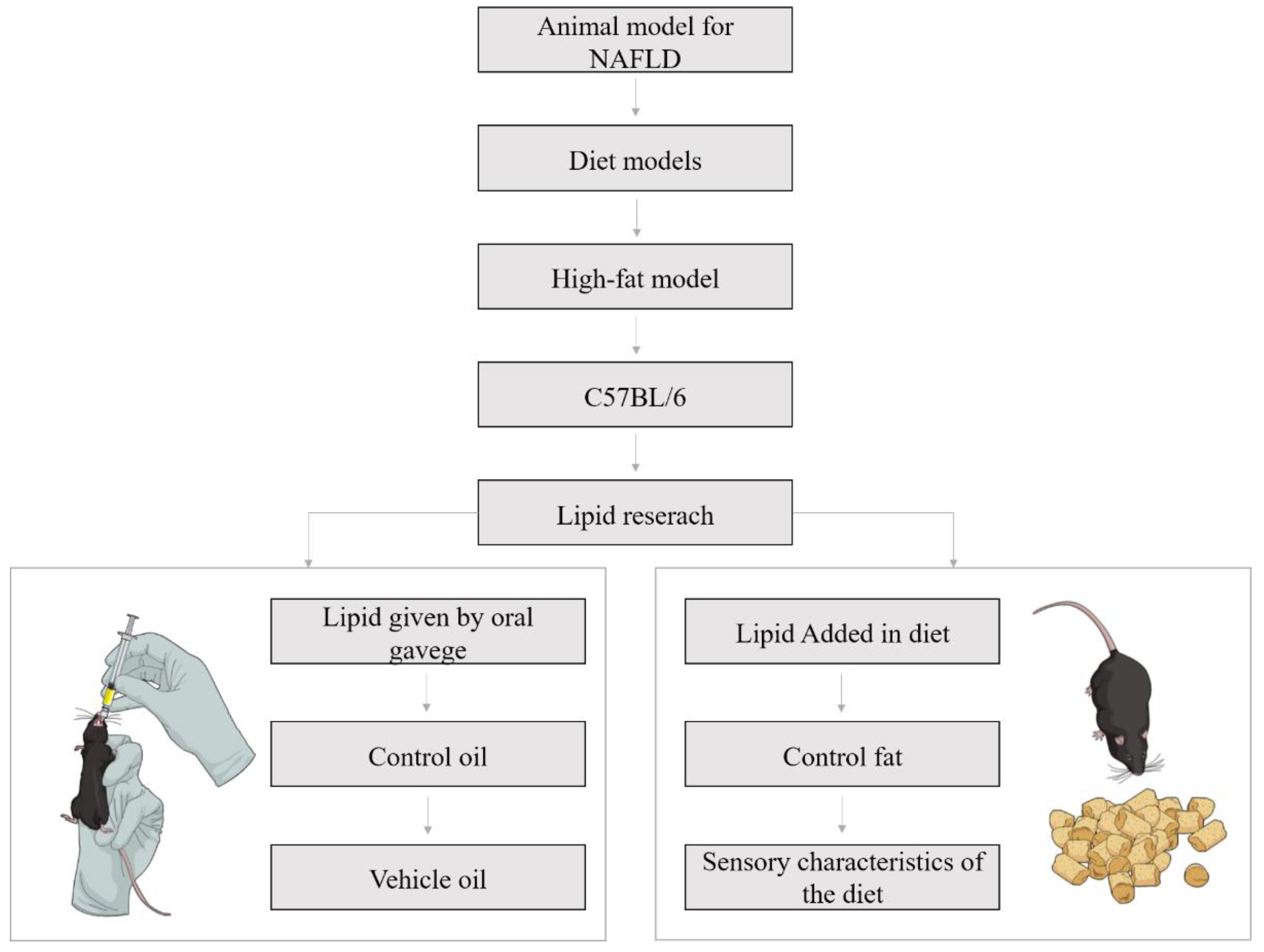
3.1. Experimental Design: General Aspects
3.2. Oil Administration by Oral Gavage
3.2.1. Diet
3.2.2. Dose
3.2.3. Vehicle
3.2.4. Control Substance
3.3. Oil Administration Included in the Diet
3.3.1. Diet
3.3.2. Dose and Control Substance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medic-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L. Nonalcoholic fatty liver disease in obese adolescents: The role of genetic polymorphisms. Hepatobiliary Surg. Nutr. 2019, 8, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Abenavoli, L.; Di Renzo, L.; Boccuto, L.; Alwardat, N.; Gratteri, S.; De Lorenzo, A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 873–881. [Google Scholar]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Machado, M.; Marques-Vidal, P.; Cortez-pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006, 45, 600–606. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Health Observatory (GHO) Data. Available online: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/ (accessed on 15 February 2019).
- FAO; OPAS. Panorama de la Seguridad Alimentaria y Nutricional; FAO: Santiago, Chile, 2017. [Google Scholar]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Kanunfre, C.C. PPAR - Receptor ativado por proliferadores de peroxissoma: um receptor nuclear para ácidos graxos. In Entendendo a Gordura: Os Ácidos Graxos; Curi, R., Pompéia, C., Miyasaka, C.K., Procopio, J., Eds.; Manole: Barueri, Brazil, 2002; pp. 227–248. ISBN 85-204-1355-2. [Google Scholar]
- White, P.A.S.; Cercato, L.M.; Araújo, J.M.D.; Souza, L.A.; Soares, A.F.; Barbosa, A.P.O.; Neto, J.M.D.R.; Marçal, A.C.; Machado, U.F.; Camargo, E.A.; et al. Modelo de obesidade induzida por dieta hiperlipídica e associada à resistência à ação da insulina e intolerância à glicose. Arq. Bras. Endocrinol. Metabol. 2013, 57, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jump, D.B.; Depner, C.M.; Tripathy, S.; Lytle, K.A. Impact of dietary fat on the development of non-alcoholic fatty liver disease in Ldlr-/- mice. Proc. Nutr. Soc. 2016, 75, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Riordan, J.D.; Nadeau, J.H. Modeling progressive non-alcoholic fatty liver disease in the laboratory mouse. Mamm. Genome 2014, 25, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Soejima, Y.; Fukusato, T. Animal models of nonalcoholic fatty liver disease / nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2300–2308. [Google Scholar] [CrossRef]
- Jacobs, A.; Warda, A.; Verbeek, J.; Cassiman, D.; Spincemaille, P. An Overview of Mouse Models of Nonalcoholic Steatohepatitis: From Past to Present. Curr. Protoc. Mouse Biol. 2016, 6, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Ka, J.; Lau, C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2017, 241, 36–44. [Google Scholar]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, A.P.; Marker, P.; Cervenka, T. Time course of insulin resistance associated with feeding dogs a high- fat diet. Am. J. Physiol.Endocrinol. Metab. 1997, 272, E147–E154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Smith, D.L., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2015, 22, 2147–2155. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Lai, Y.S.; Lee, W.C.; Lin, Y.E.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Panyod, S.; Chu, Y.L.; Sheen, L.Y. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Sun, J.; Ran, L.; Li, Y.; Wang, N.; Yu, T.; Gao, W.; Jia, W.; Jiang, R.; et al. Microalgal Oil from Schizochytrium sp. Prevents HFD-Induced Abdominal Fat Accumulation in Mice. J. Am. Coll. Nutr. 2017, 36, 347–356. [Google Scholar] [CrossRef]
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.Y.; Sheen, L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Jo, H.K.; Chung, S.H. Ginseng seed oil ameliorates hepatic lipid accumulation in vitro and in vivo. J. Ginseng Res. 2018, 42, 419–428. [Google Scholar] [CrossRef] [PubMed]
- González-Mañán, D.; D’Espessailles, A.; Dossi, C.G.; Martín, M.S.; Rodrigo; Mancilla, A.R.; Gladys, S.T. Rosa Mosqueta Oil Prevents Oxidative Stress and Inflammation through the Upregulation of PPAR-a and NRF2 in C57BL/6J Mice Fed a High-Fat Diet. J. Nutr. 2017, 147, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Rodas, M.C.; Valenzuela, R.; Echeverría, F.; Rincón-Cervera, M.Á.; Espinosa, A.; Illesca, P.; Muñoz, P.; Corbari, A.; Romero, N.; Gonzalez-Mañan, D.; et al. Supplementation with Docosahexaenoic Acid and Extra Virgin Olive Oil Prevents Liver Steatosis Induced by a High-Fat Diet in Mice through PPAR-α and Nrf2 Upregulation with Concomitant SREBP-1c and NF-kB Downregulation. Mol. Nutr. Food Res. 2017, 61, 1–6. [Google Scholar] [CrossRef]
- Bashir, S.; Ali, S.; Khan, F. Partial reversal of obesity-induced insulin resistance owing to anti-inflammatory immunomodulatory potential of flaxseed oil. Immunol. Investig. 2015, 44, 451–469. [Google Scholar] [CrossRef]
- Jurado-Ruiz, E.; Varela, L.M.; Luque, A.; Berná, G. An extra virgin olive oil-rich diet intervention ameliorates the non-alcoholic steatohepatitis induced by a high-fat “Western type” diet in mice. Mol. Nutr. Food Res. 2016, 61, 1600549. [Google Scholar] [CrossRef]
- Park, S.; Shin, S.; Lim, Y.; Shin, J.H.; Seong, J.K.; Han, S.N. Korean pine nut oil attenuated hepatic triacylglycerol accumulation in high-fat diet-induced obese mice. Nutrients 2016, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Bargut, T.C.L.; Frantz, E.D.C.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef]
- De Oliveira Chamma, C.M.; Bargut, T.C.L.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. A rich medium-chain triacylglycerol diet benefits adiposity but has adverse effects on the markers of hepatic lipogenesis and beta-oxidation. Food Funct. 2017, 8, 778–787. [Google Scholar] [CrossRef]
- Beppu, F.; Li, H.; Yoshinaga, K.; Nagai, T.; Yoshinda, A.; Kubo, A.; Kanda, J.; Gotoh, N. Dietary Starfish Oil Prevents Hepatic Steatosis and Hyperlipidemia in C57BL / 6N Mice Fed High-fat Diet. J Oleo Sci. 2017, 769, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Soni, N.K.; Nookaew, I.; Sandberg, A.S.; Gabrielsson, B.G. Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.H.; Inoue, S.; Taniguchi, Y.; Miyahara, H.; Iwasaki, Y.; Takeo, J.; Sakaue, H.; Nakaya, Y. Long-term dietary supplementation with saury oil attenuates metabolic abnormalities in mice fed a high-fat diet: Combined beneficial effect of omega-3 fatty acids and long-chain monounsaturated fatty acids. Lipids Health Dis. 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazic, M.; Inzaugarat, M.E.; Povero, D.; Zhao, I.C.; Chen, M.; Nalbandian, M.; Miller, Y.I.; Cherñavsky, A.C.; Feldstein, A.E.; Sears, D.D. Reduced dietary omega-6 to omega-3 fatty acid ratio and 12/15-lipoxygenase deficiency are protective against chronic high fat diet-induced steatohepatitis. PLoS ONE 2014, 9, e107658. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57B1/6J mice. Int. J. Obes. 2000, 24, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patten, A.R.; Fontaine, C.J.; Christie, B.R. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front. Pediatr. 2014, 2, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.P.; Boyd, K.L.; Wallace, J.M. Evaluation of Mice Undergoing Serial Oral Gavage While Awake or Anesthetized. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 805–810. [Google Scholar] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Della Vedova, M.C.; Muñoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germanó, M.J.; Rinaldi Tosi, M.E.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Gomez Mejiba, S.E.; et al. A mouse model of diet-induced obesity resembling most features of human metabolic syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Tetri, L.H.; Basaranoglu, M.; Brunt, E.M.; Yerian, L.M.; Neuschwander-Tetri, B.A. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 987–995. [Google Scholar] [CrossRef]
- Cahova, M.; Dankova, H.; Palenickova, E.; Papackova, Z.; Kazdova, L. The opposite effects of high-sucrose and high-fat diet on fatty acid oxidation and very low density lipoprotein secretion in rat model of metabolic syndrome. J. Nutr. Metab. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alcala, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Ramos, M.P.; Viana, M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS ONE 2017, 12, e0186579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gavage | |||||
|---|---|---|---|---|---|
| Ref. | Gender | Number of Animals Per Group | Age of Animals | Weeks of Adaptation | Experiment Duration after Adaptation (Weeks) |
| [22] | Male | 8 | 6-weeks-old | 2 | 12 |
| [23] | Male | 12 | 3–4-weeks-old | 1 | 16 |
| [24] | Male | 10 | - | 2 | 12 |
| [25] | − | 7 or 8 | 5-weeks-old | 1 | 12 |
| [26] | Male | 5 in control groups | 4-weeks-old | 0 | 12 |
| 9 in high-fat groups | |||||
| [27] | Male | 10 or 11 | − | − | 12 |
| [28] | − | 5 | − | − | 4 |
| Lipids Included in Diet | |||||
| [29] | Male | 6 | 5-weeks-old | − | 12 weeks to promote NAFLD + 24 weeks of treatment |
| [30] | Male | – | 5-weeks-old | 3 days | 12 weeks |
| [31] | Male | 10 | 3 months of age | − | 8 weeks |
| [32] | Male | 10 | 3 months of age | − | 12 weeks |
| [33] | Male | − | 4-weeks-old | 7 days | 8 weeks |
| [34] | Male | 12 | 9-10-weeks-old | − | 12 weeks |
| [35] | Male | 10 | 5-weeks-old | 2 weeks | 18 weeks |
| [36] | Male | − | 14-15-weeks-old | − | 15 weeks |
| Control Groups | Treatment Groups | |||
|---|---|---|---|---|
| Ref. | Lean Control | Obese Control | Lean Treatment | Obese Treatment |
| [22] | 13.5% kcal from fat | 60% kcal fat content | − | 60% kcal fat content |
| [23] | 10% kcal from fat | 60% kcal fat content | − | 60% kcal fat content |
| [24] | 13.5% kcal from fat | 60% kcal fat content | − | 60% kcal fat content |
| [25] | 10% kcal from fat | 60% kcal fat content | − | 60% kcal fat content |
| [26] | 10% kcal from fat | 60% kcal fat content | 10% kcal from fat | 60% kcal fat content |
| [27] | 10% kcal from fat | 60% kcal fat content | 10% kcal from fat | 60% kcal fat content |
| [28] | standard mice diet | 60% kcal fat content | − | 60% kcal fat content |
| Ref. | Dosage of Oil Given by Oral Gavage | Gavage Vehicle | Substance Administered to Control Groups |
|---|---|---|---|
| [22] | 12.5, 62.5 or 125 mg/kg/day | − | Olive oil |
| [23] | 100 mg/kg/day of Omega-3 PUFAS from fish oil, MO low DHA purity or MO high DHA purity | Corn oil | Corn oil |
| [24] | 25, 50 or 100 mg/kg/day | Olive oil | Olive oil |
| [25] | 250 or 500 mg/kg/week | − | Vehicle |
| [26] | 1.94 mg ALA/g animal body weight/day | − | Saline solution |
| [27] | 50 mg/kg/day | − | Saline solution |
| [28] | 4, 8 or 16 mg/kg/day | − | − |
| Control Group | Treatment Group | |||
|---|---|---|---|---|
| Ref. | Lean Control | Obese Control | Lean Treatment | Obese Treatment |
| [29] | 13% kcal from fat | 49% kcal fat content | Not applicable | 49% kcal fat content |
| [30] | 10% kcal from fat | 35% kcal fat content | 10% kcal from fat | 35% kcal fat content |
| [31] | 10% kcal from fat | 50% kcal fat content | 10% kcal from fat | 50% kcal fat content |
| [32] | 10% kcal from fat | 50% kcal fat content | Not applicable | 50% kcal fat content |
| [33] | − | 46% kcal fat content | Not applicable | 46% kcal fat content |
| [34] | 12% kcal from fat | 32% kcal fat content | Not applicable | 32% kcal fat content |
| [35] | − | 60% kcal fat content | Not applicable | 60% kcal fat content |
| [36] | − | 46,3% kcal fat content | Not applicable | 46,3% kcal fat content |
| Ref. | Diet Composition |
|---|---|
| [29] | HFD Control: Lard-based diet 49% total kcal of diet from fat |
| HFD Treatment: 41.7% total kcal of diet from treatment oils | |
| [30] | Lean Control: 45 g/1045.0 g (10% of kcal) from soybean oil and 350 g/1045.0 g of sucrose |
| Lean Treatment: 45 g/1045.0 g (10% of kcal) from pine nut oil and 350 g/1045.0 g of sucrose | |
| HFD Control: 157.5 g/848.1 g of lard (35% of kcal), 45 g/848.1 g (10% of kcal) from soybean oil and 172.8 g/848.1 g of sucrose | |
| HFD Treatment: 157.5 g/848.1 g of lard (35% of kcal), 45 g/848.1 g (10% of kcal) from pine nut oil and 172.8 g/848.1 g of sucrose | |
| [31] | Lean Control: 40 g/kg of soybean oil, 100 g/kg of sucrose and 140 g/kg of casein |
| Lean Treatment: 36 g/kg of fish oil, 4 g/kg of soybean oil, 100 g/kg of sucrose and 140 g/kg of casein | |
| HFD Control: 238 g/kg of lard, 40 g/kg of soybean oil, 100 g/kg of sucrose and 175 g/kg of casein | |
| HFD Treatment: 238 g/kg of fish oil, 40 g/kg of soybean oil, 100 g/kg of sucrose and 175 g/kg of casein | |
| [32] | Lean Control: 42 g/kg of soybean oil, 622.69 g/kg of cornstarch, 100 g/kg of sucrose, 42 g/kg of soybean oil and 136 g/kg of casein |
| HFD Control: 236 g/kg of lard, 42 g/kg of soybean oil, 352.192 g/kg of cornstarch, 100 g/kg of sucrose, 42 g/kg of soybean oil and 170 g/kg of casein | |
| HFD + 25% MCT: 177 g/kg of lard, 42 g/kg of soybean oil, 61.5 g/kg of medium-chain triacylglycerol, 350.192 g/kg of cornstarch, 100 g/kg of sucrose, and 170 g/kg of casein | |
| HFD + 75% MCT: 59 g/kg of lard, 42 g/kg of soybean oil, 184,5 g/kg of medium-chain triacylglycerol, 345.192 g/kg of cornstarch, 100 g/kg of sucrose, and 170 g/kg of casein | |
| HFD + 100% MCT: 42 g/kg of soybean oil, 246 g/kg of medium-chain triacylglycerol, 342.692 g/kg of cornstarch, 100 g/kg of sucrose, and 170 g/kg of casein | |
| [33] | HFD Control: 200 g/kg of lard, 200 g/kg of casein, 250 g/kg of corn starch, 100 g/kg of fructose, 100 g/kg of sucrose, and 50 g/kg of soybean oil |
| HFD + 2% of Starfish Oil: 180 g/kg of lard, 200 g/kg of casein, 250 g/kg of corn starch, 100 g/kg of fructose, 100 g/kg of sucrose, 20 g/kg of starfish oil and 50 g/kg of soybean oil | |
| HFD + 5% of Starfish Oil: 150 g/kg of lard, 200 g/kg of casein, 250 g/kg of corn starch, 100 g/kg of fructose, 100 g/kg of sucrose, 50 g/kg of starfish and 50 g/kg of soybean oil | |
| [34] | Lean Control: 222 g/kg of casein, 50 g/kg of sucrose, 560 g/kg of corn starch, 50 g/kg of cellulose, 25 g/kg of corn oil and 25 g/kg of coconut oil |
| HFD Corn Oil Control: 256 g/kg of casein, 100 g/kg of sucrose, 348 g/kg of corn starch, 58 g/kg of cellulose, 100 g/kg of coconut oil and 50 g/kg of corn oil | |
| HFD Treatments: 256 g/kg of casein, 100 g/kg of sucrose, 348 g/kg of corn starch, 58 g/kg of cellulose, 100 g/kg of coconut oil, 30 g/kg of corn oil + 20 g/100 g of EPA and DHA-enriched oils | |
| [35] | HFD Control: 258 g/kg of casein, 162 g/kg of maltodextrin, 89 g/kg of sucrose, 65 g/kg of cellulose, 320 g/kg of lard, and 32 g/kg of soybean oil |
| HFD Treatment: 258 g/kg of casein, 162 g/kg of maltodextrin, 89 g/kg of sucrose, 65 g/kg of cellulose, 220 g/kg of lard, 32 g/kg of soybean oil and 100 g/kg of saury oil | |
| [36] | HFD Control: 40.8% SFA, 42.0% MUFA and 17.3% PUFA (15.9% Omega-6 and 1.4% Omega-3) (Lard and soybean-based diet) |
| HFD Treatment: 41.5% SFA, 41.4% MUFA and 17.1% PUFA (12.5% Omega-6 and 4.6% Omega-3) (Lard, soybean oil and Menhaden fish oil-based diet) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recena Aydos, L.; Aparecida do Amaral, L.; Serafim de Souza, R.; Jacobowski, A.C.; Freitas dos Santos, E.; Rodrigues Macedo, M.L. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. https://doi.org/10.3390/nu11123067
Recena Aydos L, Aparecida do Amaral L, Serafim de Souza R, Jacobowski AC, Freitas dos Santos E, Rodrigues Macedo ML. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients. 2019; 11(12):3067. https://doi.org/10.3390/nu11123067
Chicago/Turabian StyleRecena Aydos, Leonardo, Luane Aparecida do Amaral, Roberta Serafim de Souza, Ana Cristina Jacobowski, Elisvânia Freitas dos Santos, and Maria Lígia Rodrigues Macedo. 2019. "Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models" Nutrients 11, no. 12: 3067. https://doi.org/10.3390/nu11123067
APA StyleRecena Aydos, L., Aparecida do Amaral, L., Serafim de Souza, R., Jacobowski, A. C., Freitas dos Santos, E., & Rodrigues Macedo, M. L. (2019). Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients, 11(12), 3067. https://doi.org/10.3390/nu11123067





