Prevalence of Malnutrition in Pediatric Hospitals in Developed and In-Transition Countries: The Impact of Hospital Practices
Abstract
:1. Introduction
2. Methods
3. Malnutrition: A Broad Concept
4. Succinct Historical Reference
5. Nutrition Screening Tools
6. Prevalence of Acute Malnutrition
7. Hospital Practices
8. Hospital Food Services
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BMI | Body Mass Index |
| DRM | disease-related malnutrition |
| NRS | Nutritional Risk Score |
| PICU | Pediatric Intensive Care Unit |
| PNRS | Pediatric Nutritional Risk Score |
| PYMS | Pediatric Yorkhill Malnutrition Screening |
| SGNA | Subjective Global Nutrition Assessment |
| STAMP | Screening Tool for the Assessment of Malnutrition in Pediatrics |
| Strongkids | Screening Tool for Risk On Nutritional status and Growth |
| WFA | Weight for Age |
| WFH | weight-for-height |
| WHO | World Health Organization |
References
- Grover, Z.; Ee, L.C. Protein energy malnutrition. Pediatr. Clin. N. Am. 2009, 56, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.V.; Allen, S. Malnutrition in developing countries. Paediatr. Child Health 2015, 25, 422–427. [Google Scholar] [CrossRef]
- Perkins, J.M.; Kim, R.; Krishna, A.; McGovern, M.; Aguayo, V.M.; Subramanian, S.V. Understanding the association between stunting and child development in low- and middle-income countries: Next steps for research and intervention. Soc. Sci. Med. 2017, 193, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Balhara, K.S.; Silvestri, D.M.; Tyler Winders, W.; Selvam, A.; Kivlehan, S.M.; Becker, T.K.; Levine, A.C.; Global Emergency Medicine Literature Review Group (GEMLR). Impact of nutrition interventions on pediatric mortality and nutrition outcomes in humanitarian emergencies: A systematic review. Trop. Med. Int. Health 2017, 22, 1464–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysentruyt, K.; Alliet, P.; Raes, M.; Willekens, J.; De Schutter, I.; De Wachter, E.; Malfroot, A.; Devreker, T.; Goyens, P.; Vandenplas, Y.; et al. Nutritional status of children hospitalized for parapneumonic effusion. PLoS ONE 2014, 9, e94242. [Google Scholar] [CrossRef] [PubMed]
- Huysentruyt, K.; De Schepper, J.; Bontems, P.; Alliet, P.; Peeters, E.; Roelants, M.; Van Biervliet, S.; Hauser, B.; Vandenplas, Y.; Voedingscel van Vlaamse Vereniging voor Kindergeneeskunde, V.V.K. Proposal for An Algorithm for Screening for Undernutrition in Hospitalized Children. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e86–e91. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Pinho, C.; Wagner, J.A.; Brown, J.C.; Bertozzi-Villa, A.; Charlson, F.J.; Coffeng, L.E.; Dandona, L.; Erskine, H.E.; Ferrari, A.J.; et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016, 170, 267–287. [Google Scholar]
- Hecht, C.; Weber, M.; Grote, V.; Daskalou, E.; Dell’Era, L.; Flynn, D.; Gerasimidis, K.; Gottrand, F.; Hartman, C.; Hulst, J.; et al. Disease associated malnutrition correlates with length of hospital stay in children. Clin. Nutr. 2015, 34, 53–59. [Google Scholar] [CrossRef]
- Secker, D.J.; Jeejeebhoy, K.N. Subjective Global Nutritional Assessment for children. Am. J. Clin. Nutr. 2007, 85, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Rocha, G.A.; Rocha, E.J.; Martins, C.V. The effects of hospitalization on the nutritional status of children. J. Pediatr. 2006, 82, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Scrimshaw, N.S. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J. Nutr. 2003, 133, 316S–321S. [Google Scholar] [CrossRef] [PubMed]
- De Souza Menezes, F.; Leite, H.P.; Koch Nogueira, P.C. Malnutrition as an independent predictor of clinical outcome in critically ill children. Nutrition 2012, 28, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Radman, M.; Mack, R.; Barnoya, J.; Castaneda, A.; Rosales, M.; Azakie, A.; Mehta, N.; Keller, R.; Datar, S.; Oishi, P.; et al. The effect of preoperative nutritional status on postoperative outcomes in children undergoing surgery for congenital heart defects in San Francisco (UCSF) and Guatemala City (UNICAR). J. Thorac. Cardiovasc. Surg. 2014, 147, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysentruyt, K.; Alliet, P.; Muyshont, L.; Devreker, T.; Bontems, P.; Vandenplas, Y. Hospital-related undernutrition in children: Still an often unrecognized and undertreated problem. Acta Paediatr. 2013, 102, e460–e466. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, B.; Whitten, K.E.; Harrison, B.; Mitchell, M.; Kepreotes, H.; Sidler, M.; Lemberg, D.A.; Day, A.S. Prevalence of malnutrition and risk of under-nutrition in hospitalized children. Clin. Nutr. 2012, 31, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Joosten, K.F.; Zwart, H.; Hop, W.C.; Hulst, J.M. National malnutrition screening days in hospitalised children in The Netherlands. Arch. Dis. Child. 2010, 95, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J., Jr.; Hull, S.F.; Albert, N.; Waller, A.; Bringardener, S. Economic impact of malnutrition: A model system for hospitalized patients. JPEN. J. Parenter. Enter. Nutr. 1988, 12, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Freijer, K.; Tan, S.S.; Koopmanschap, M.A.; Meijers, J.M.; Halfens, R.J.; Nuijten, M.J. The economic costs of disease related malnutrition. Clin. Nutr. 2013, 32, 136–141. [Google Scholar] [CrossRef]
- Kac, G.; Camacho-Dias, P.; Silva-Coutinho, D.; Silveira-Lopes, R.; Marins, V.V.; Pinheiro, A.B. Length of stay is associated with incidence of in-hospital malnutrition in a group of low-income Brazilian children. Salud Publ. Mex. 2000, 42, 407–412. [Google Scholar] [CrossRef]
- De Moraes Silveira, C.R.; De Mello, E.D.; Antonacci Carvalho, P.R. Evolution of nutritional status of pediatric in patients of a tertiary care general hospital in Brazil. Nutr. Hosp. 2008, 23, 599–606. [Google Scholar] [PubMed]
- Brotherton, A.; Simmonds, N.; Stroud, M. Malnutrition Matters: Meeting Quality Standards in Nutritional Care; British Association for Parenteral and Enteral Nutrition (BAPEN): Redditch, UK, 2010. [Google Scholar]
- Freijer, K.; van Puffelen, E.; Joosten, K.F.; Hulst, J.M.; Koopmanschap, M.A. The costs of disease related malnutrition in hospitalized children. Clin. Nutr. ESPEN 2018, 23, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Wiskin, A.E.; Davies, J.H.; Wootton, S.A.; Beattie, R.M. Energy expenditure, nutrition and growth. Arch. Dis. Child. 2011, 96, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Han-Markey, T. Nutritional considerations in pediatric oncology. Semin. Oncol. Nurs. 2000, 16, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Alderman, H.; Behrman, J.R.; Glewwe, P.; Fernald, L.; Walker, S. Evidence of Impact of Interventions on Growth and Development during Early and Middle Childhood. In Child and Adolescent Health and Development; Bundy, D.A.P., Silva, N., Horton, S., Jamison, D.T., Patton, G.C., Eds.; The International Bank for Reconstruction and Development: Washington, DC, USA, 2017. [Google Scholar]
- Galler, J.R.; Koethe, J.R.; Yolken, R.H. Neurodevelopment: The Impact of Nutrition and Inflammation During Adolescence in Low-Resource Settings. Pediatrics 2017, 139, S72–S84. [Google Scholar] [CrossRef] [PubMed]
- Country Classification. Available online: http://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf (accessed on 6 January 2019).
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Chavez, R.; Frenk, S.; Galvan, R.R.; Munoz, J.C.; Vazquez, J. Mortality in second and third degree malnutrition. J. Trop. Pediatr. 1956, 2, 77–83. [Google Scholar] [CrossRef]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.N.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. JPEN. J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef]
- Waterlow, J.C. Classification and definition of protein-calorie malnutrition. Br. Med J. 1972, 3, 566–569. [Google Scholar] [CrossRef]
- World Health Organization. Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V.; Academy of Nutrition and Dietetics; American Society for Parenteral and Enteral Nutrition. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically ILL Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. Pediatr. Crit. Care Med. 2017, 18, 675–715. [Google Scholar] [CrossRef]
- Huysentruyt, K.; Vandenplas, Y.; De Schepper, J. Screening and assessment tools for pediatric malnutrition. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J. Nutritional Screening Tools among Hospitalized Children: From Past and to Present. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 79–85. [Google Scholar] [CrossRef]
- Rinninella, E.; Ruggiero, A.; Maurizi, P.; Triarico, S.; Cintoni, M.; Mele, M.C. Clinical tools to assess nutritional risk and malnutrition in hospitalized children and adolescents. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2690–2701. [Google Scholar]
- Reilly, H.M.; Martineau, J.K.; Moran, A.; Kennedy, H. Nutritional screening--evaluation and implementation of a simple Nutrition Risk Score. Clin. Nutr. 1995, 14, 269–273. [Google Scholar] [CrossRef]
- McCarthy, H.; Dixon, M.; Crabtree, I.; Eaton-Evans, M.J.; McNulty, H. The development and evaluation of the Screening Tool for the Assessment of Malnutrition in Paediatrics (STAMP©) for use by healthcare staff. J. Hum. Nutr. Diet. 2012, 25, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulst, J.M.; Zwart, H.; Hop, W.C.; Joosten, K.F. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin. Nutr. 2010, 29, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Poisson-Salomon, A.S.; Colomb, V.; Brusset, M.C.; Mosser, F.; Berrier, F.; Ricour, C. Simple pediatric nutritional risk score to identify children at risk of malnutrition. Am. J. Clin. Nutr. 2000, 72, 64–70. [Google Scholar] [CrossRef]
- White, M.; Lawson, K.; Ramsey, R.; Dennis, N.; Hutchinson, Z.; Soh, X.Y.; Matsuyama, M.; Doolan, A.; Todd, A.; Elliott, A.; et al. Simple Nutrition Screening Tool for Pediatric Inpatients. J. Parenter. Enter. Nutr. 2016, 40, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.C.; Marino, L.V.; Williams, S.A.; Beattie, R.M. Outcome of nutritional screening in the acute paediatric setting. Arch. Dis. Child. 2016, 101, 1119–1124. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Keane, O.; Macleod, I.; Flynn, D.M.; Wright, C.M. A four-stage evaluation of the Paediatric Yorkhill Malnutrition Score in a tertiary paediatric hospital and a district general hospital. Br. J. Nutr. 2010, 104, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagiozoglou-Lampoudi, T.; Daskalou, E.; Lampoudis, D.; Apostolou, A.; Agakidis, C. Computer-based malnutrition risk calculation may enhance the ability to identify pediatric patients at malnutrition-related risk for unfavorable outcome. J. Parenter. Enter. Nutr. 2015, 39, 418–425. [Google Scholar] [CrossRef]
- Baer, M.T.; Harris, A.B. Pediatric nutrition assessment: Identifying children at risk. J. Am. Diet. Assoc. 1997, 97, S107–S115. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Huysentruyt, K.; Devreker, T.; Dejonckheere, J.; De Schepper, J.; Vandenplas, Y.; Cools, F. Accuracy of Nutritional Screening Tools in Assessing the Risk of Undernutrition in Hospitalized Children. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 159–166. [Google Scholar] [CrossRef]
- Baker, J.P.; Detsky, A.S.; Wesson, D.E.; Wolman, S.L.; Stewart, S.; Whitewell, J.; Langer, B.; Jeejeebhoy, K.N. Nutritional assessment: A comparison of clinical judgement and objective measurements. N. Engl. J. Med. 1982, 306, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, K.M.; Duggan, C.; Gallagher, L.; Carlin, A.C.; Richardson, D.S.; Collier, S.B.; Simpson, W.; Lo, C. Malnutrition in hospitalized pediatric patients. Current prevalence. Arch. Pediatr. Adolesc. Med. 1995, 149, 1118–1122. [Google Scholar] [CrossRef]
- Pawellek, I.; Dokoupil, K.; Koletzko, B. Prevalence of malnutrition in paediatric hospital patients. Clin. Nutr. 2008, 27, 72–76. [Google Scholar] [CrossRef]
- Toole, B.J.; Toole, L.E.; Kyle, U.G.; Cabrera, A.G.; Orellana, R.A.; Coss-Bu, J.A. Perioperative nutritional support and malnutrition in infants and children with congenital heart disease. Congenit. Heart Dis. 2014, 9, 15–25. [Google Scholar] [CrossRef]
- Hendrikse, W.H.; Reilly, J.J.; Weaver, L.T. Malnutrition in a children’s hospital. Clin. Nutr. 1997, 16, 13–18. [Google Scholar] [CrossRef]
- Hankard, R.; Bloch, J.; Martin, P.; Randrianasolo, H.; Bannier, M.F.; Machinot, S.; Cezard, J.P. Nutritional status and risk in hospitalized children. Arch. Pediatr. 2001, 8, 1203–1208. [Google Scholar] [CrossRef]
- Groleau, V.; Thibault, M.; Doyon, M.; Brochu, E.E.; Roy, C.C.; Babakissa, C. Malnutrition in hospitalized children: Prevalence, impact, and management. Can. J. Diet. Pract. Res. 2014, 75, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.; Erkan, T.; Yalvac, S.; Altay, S.; Cokugras, F.C.; Aydin, A.; Kutlu, T. Nutritional status of patients hospitalized in pediatric clinic. Turk. J. Gastroenterol. 2005, 16, 212–216. [Google Scholar] [PubMed]
- Marteletti, O.; Caldari, D.; Guimber, D.; Mention, K.; Michaud, L.; Gottrand, F. Malnutrition screening in hospitalized children: Influence of the hospital unit on its management. Arch. Pediatr. 2005, 12, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Belanger, V.; McCarthy, A.; Marcil, V.; Marchand, V.; Boctor, D.L.; Rashid, M.; Noble, A.; Avinashi, V.; Davidson, B.; Groleau, V.; et al. Assessment of Malnutrition Risk in Canadian Pediatric Hospitals: A Multicenter Prospective Cohort Study. J. Pediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chourdakis, M.; Hecht, C.; Gerasimidis, K.; Joosten, K.F.; Karagiozoglou-Lampoudi, T.; Koetse, H.A.; Ksiazyk, J.; Lazea, C.; Shamir, R.; Szajewska, H.; et al. Malnutrition risk in hospitalized children: Use of 3 screening tools in a large European population. Am. J. Clin. Nutr. 2016, 103, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Ford-Chessel, C.; Berthiller, J.; Peretti, N.; Javouhey, E.; Valla, F.V. Nutritional status in pediatric intermediate care: Assessment at admission, progression during the stay and after discharge. Arch. Pediatr. 2016, 23, 333–339. [Google Scholar] [CrossRef]
- Lezo, A.; Diamanti, A.; Capriati, T.; Gandullia, P.; Fiore, P.; Lacitignola, L.; Gatti, S.; Spagnuolo, M.I.; Cecchi, N.; Verlato, G.; et al. Italian pediatric nutrition survey. Clin. Nutr. ESPEN 2017, 21, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Beser, O.F.; Cokugras, F.C.; Erkan, T.; Kutlu, T.; Yagci, R.V.; Group, T.S. Evaluation of malnutrition development risk in hospitalized children. Nutrition 2018, 48, 40–47. [Google Scholar] [CrossRef]
- Wyrick, S.; Hester, C.; Sparkman, A.; O’Neill, K.M.; Dupuis, G.; Anderson, M.; Cordell, J.; Bogie, A. What role does body mass index play in hospital admission rates from the pediatric emergency department? Pediatr. Emerg. Care 2013, 29, 974–978. [Google Scholar] [CrossRef]
- Carvalho-Salemi, J.; Salemi, J.L.; Wong-Vega, M.R.; Spooner, K.K.; Juarez, M.D.; Beer, S.S.; Canada, N.L. Malnutrition among Hospitalized Children in the United States: Changing Prevalence, Clinical Correlates, and Practice Patterns between 2002 and 2011. J. Acad. Nutr. Diet. 2018, 118, 40–51. [Google Scholar] [CrossRef]
- Cao, J.; Peng, L.; Li, R.; Chen, Y.; Li, X.; Mo, B.; Li, X. Nutritional risk screening and its clinical significance in hospitalized children. Clin. Nutr. 2014, 33, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Sissaoui, S.; De Luca, A.; Piloquet, H.; Guimber, D.; Colomb, V.; Peretti, N.; Bouniol, A.; Breton, A.; Chouraqui, J.-P.; Coste, M.-E.; et al. Large scale nutritional status assessment in pediatric hospitals. e-SPEN J. 2013, 8, e68–e72. [Google Scholar] [CrossRef]
- Baker, J.P.; Detsky, A.S.; Whitwell, J.; Langer, B.; Jeejeebhoy, K.N. A comparison of the predictive value of nutritional assessment techniques. Hum. Nutr. Clin. Nutr. 1982, 36, 233–241. [Google Scholar]
- Butterworth, C.E., Jr. The skeleton in the hospital closet. Nutrition 1994, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Corish, C.A.; Kennedy, N.P. Protein-energy undernutrition in hospital in-patients. Br. J. Nutr. 2000, 83, 575–591. [Google Scholar] [CrossRef]
- Kubrak, C.; Jensen, L. Malnutrition in acute care patients: A narrative review. Int. J. Nurs. Stud. 2007, 44, 1036–1054. [Google Scholar] [CrossRef] [PubMed]
- Huysentruyt, K.; Goyens, P.; Alliet, P.; Bontems, P.; Van Hautem, H.; Philippet, P.; Vandenplas, Y.; De Schepper, J. More training and awareness are needed to improve the recognition of undernutrition in hospitalised children. Acta Paediatr. 2015, 104, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Bunting, J.; Weaver, L.T. Anthropometry in a children’s hospital: A study of staff knowledge, use and quality of equipment. J. Hum. Nutr. Diet. 1997, 10, 17–23. [Google Scholar] [CrossRef]
- Chen, R.S.; Shiffman, R.N. Assessing growth patterns--routine but sometimes overlooked. Clin. Pediatr. 2000, 39, 97–102. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Youde, L.S.; Allen, J.R.; Hanson, R.M.; Baur, L.A. Outcomes of a nutrition audit in a tertiary paediatric hospital: Implications for service improvement. J. Paediatr. Child Health 2004, 40, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.A.; John, H.; Davis, H.S.; McTimoney, C.M. Documentation of growth parameters and body mass index in a paediatric hospital. Paediatr. Child Health 2005, 10, 391–394. [Google Scholar] [PubMed]
- Ramsden, L.; Day, A.S. Paediatric growth charts: How do we use them and can we use them better? J. Paediatr. Child Health 2012, 48, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Wright, C.; Purcell, O.; Macleod, I.; Gerasimidis, K. Acquisition and utilisation of anthropometric measurements on admission in a paediatric hospital before and after the introduction of a malnutrition screening tool. J. Hum. Nutr. Diet. 2013, 26, 294–297. [Google Scholar] [CrossRef]
- Restier, L.; Duclos, A.; Jarri, L.; Touzet, S.; Denis, A.; Occelli, P.; Kassai-Koupai, B.; Lachaux, A.; Loras-Duclaux, I.; Colin, C.; et al. Incorrect evaluation of the frequency of malnutrition and of its screening in hospitalized children by health care professionals. J. Eval. Clin. Pract. 2015, 21, 958–962. [Google Scholar] [CrossRef] [PubMed]
- De Longueville, C.; Robert, M.; Debande, M.; Podlubnai, S.; Defourny, S.; Namane, S.A.; Pace, A.; Brans, C.; Cayrol, E.; Goyens, P.; et al. Evaluation of nutritional care of hospitalized children in a tertiary pediatric hospital. Clin. Nutr. ESPEN 2018, 25, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.H.; Yang, H.R. Current status of nutritional support for hospitalized children: A nationwide hospital-based survey in South Korea. Nutr. Res. Pract. 2018, 12, 215–221. [Google Scholar] [CrossRef]
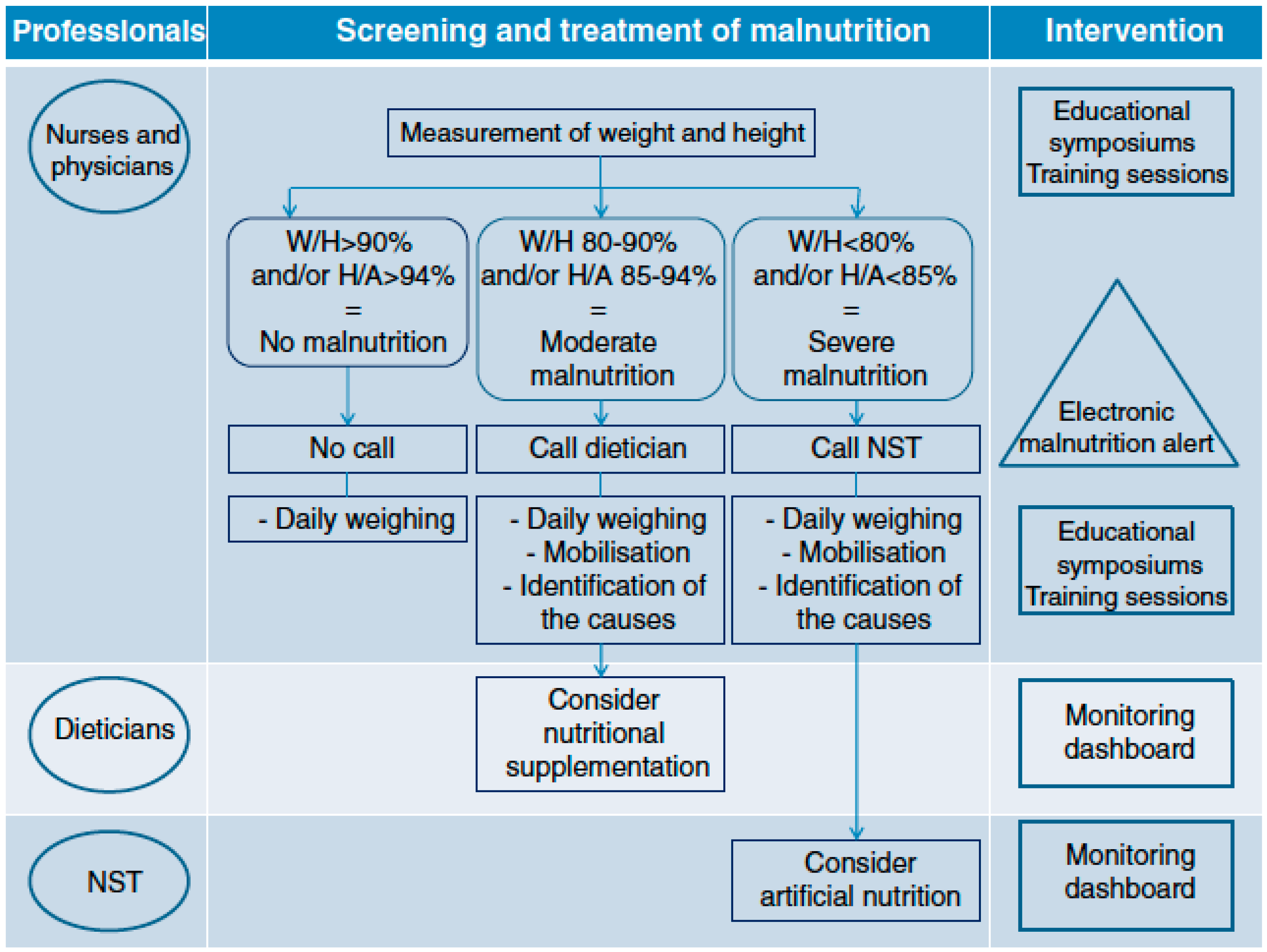
- Touzet, S.; Duclos, A.; Denis, A.; Restier-Miron, L.; Occelli, P.; Polazzi, S.; Betito, D.; Gamba, G.; Cour-Andlauer, F.; Colin, C.; et al. Multifaceted intervention to enhance the screening and care of hospitalised malnourished children: Study protocol for the PREDIRE cluster randomized controlled trial. BMC Health Serv. Res. 2013, 13, 107. [Google Scholar] [CrossRef]
- Duclos, A.; Touzet, S.; Restier, L.; Occelli, P.; Cour-Andlauer, F.; Denis, A.; Polazzi, S.; Colin, C.; Lachaux, A.; Peretti, N.; et al. Implementation of a computerized system in pediatric wards to improve nutritional care: A cluster randomized trial. Eur. J. Clin. Nutr. 2015, 69, 769–775. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Malnutrition at Hospital Admission-Contributors and Effect on Length of Stay: A Prospective Cohort Study from the Canadian Malnutrition Task Force. JPEN J. Parenter. Enter. Nutr. 2016, 40, 487–497. [Google Scholar] [CrossRef]
- Williams, R.; Virtue, K.; Adkins, A. Room service improves patient food intake and satisfaction with hospital food. J. Pediatr. Oncol. Nurs. 1998, 15, 183–189. [Google Scholar] [PubMed]
- McGlone, P.C.; Dickerson, J.W.; Davies, G.J. The feeding of patients in hospital: A review. J. R. Soc. Health 1995, 115, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Skolin, I.; Wahlin, Y.B.; Broman, D.A.; Koivisto Hursti, U.K.; Vikstrom Larsson, M.; Hernell, O. Altered food intake and taste perception in children with cancer after start of chemotherapy: Perspectives of children, parents and nurses. Support. Care Cancer 2006, 14, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Allard, J.; Vesnaver, E.; Laporte, M.; Gramlich, L.; Bernier, P.; Davidson, B.; Duerksen, D.; Jeejeebhoy, K.; Payette, H. Barriers to food intake in acute care hospitals: A report of the Canadian Malnutrition Task Force. J. Hum. Nutr. Diet. 2015, 28, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Kuperberg, K.; Caruso, A.; Dello, S.; Mager, D. How will a room service delivery system affect dietary intake, food costs, food waste and patient satisfaction in a paediatric hospital? A pilot study. J. Foodserv. 2008, 19, 255–261. [Google Scholar] [CrossRef]
- Wadden, K.; Wolf, B.; Mayhew, A. Traditional versus room service menu styles for pediatric patients. Can. J. Diet. Pract. Res. 2006, 67, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J. Can food intake in hospitals be improved? Clin. Nutr. 2001, 20, 153–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
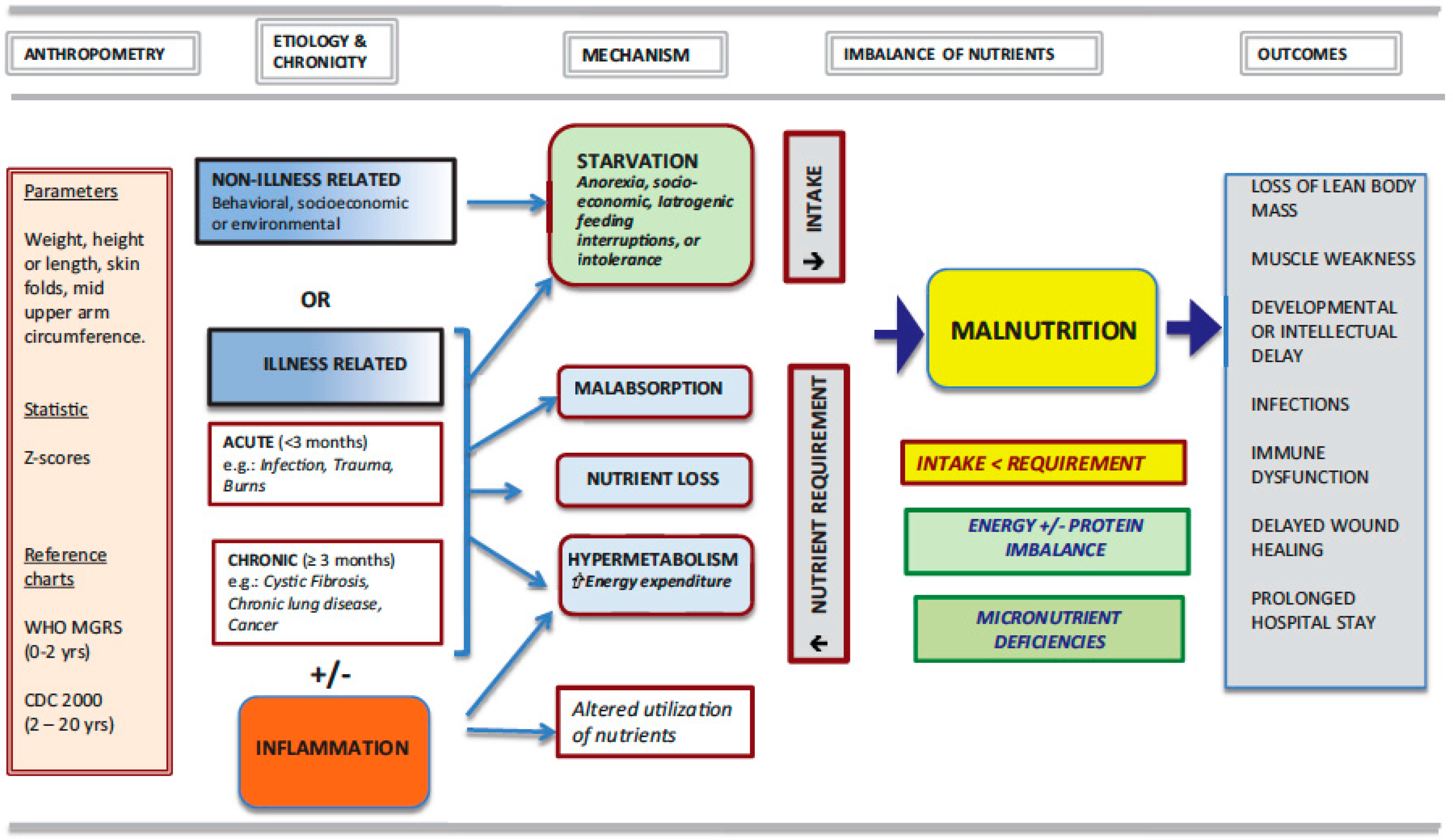
| Tools | Anthropometric Evaluation | Nutritional Intake | Medical Condition | Others | Score | Ref |
|---|---|---|---|---|---|---|
| STAMP | Weight & height measurement | Nutritional intake | Pathology Medical condition | Score ≥ 4 = HNR | [40] | |
| STRONGkids | Reported weight loss or no gain | Nutritional and Impaired intake | Pathology/high risk disease | Subjective clinical assessment (diminished fat &/or muscle mass &/or hollow face) | Score ≥ 3 = HNR | [41] |
| PNRS | Weight loss | Food intake <50% | Feeding interference Medical condition | Pain | Score ≥ 3 = HNR | [42] |
| PNST | Reported recent weight loss | Reported feeding in the last few weeks | Not Fully validated | Score ≥ 4 = HNR | [43] | |
| PMST | Weight & height, BMI | Food intake | Pathology Medical condition | Score ≥ 4 = HNR | [44] | |
| PYMS | Body Mass Index (BMI) <2% pertentile. (<−2 SD) on UK 1990 growth chart Weight loss | Changes in nutritional intake | Pathology Medical condition | Score ≥ 2 = HNR | [45] | |
| PeDiSMART | WFA (z Score) | Nutritional intake | Disease impact | Computer/Not fully validated | Score ≥ 18 = HNR | [46] |
| Geographical Location | Population Studied | Clinical Setting | Screening Tools | Anthropometric Parameters | Prevalence | Ref |
|---|---|---|---|---|---|---|
| Belgium | 0.8–17 y N = 379 | Tertiary & secondary care facilities | No specific | WFH < −2 SD BMI < −2 SD %WFH < 80% MUAC < −2 SD Any one variable | 9.0% 9.8% 2.4% 3.8% 13.5% | [14] |
| Canada | Birth–18 y N = 173 | General pediatric unit | PNRS | Scoring | 20.2% | [56] |
| Canada | 1 m–18 y N = 307 | Tertiary Pediatric Care Facilities | STRONGkids | Scoring WFA < −2 SD HFA < −2 SD WFH or BMI < −2 SD Any one variable | 26.6% 10.4% 14.0% 9.1% 19.5% | [59] |
| Europe | 1 m–18 y N = 1258 | 14 Hospital Centres General pediatric wards & pediatric surgery | PYMS STAMP STRONGkids | Scoring | 22% 22% 10% | [60] |
| France | 1–≥72 m N = 296 | Tertiary care facility | PNRS | PIBW < 85% | 26% | [42] |
| France | >6 m N = 52 | Tertiary care facility | NRS | BMI < −2 SD Scoring | 12% 26% | [55] |
| France | 2 m–16 y N = 280 | Tertiary care facility | No specific | WFH < −2 SD | 11% | [58] |
| France | 1 d–16 y N = 923 | Primary & Tertiary Care Facilities | No specific | WFH < −2 SD | 11.9% | [61] |
| Germany | 7.9 ± 5 y N = 475 | Tertiary care facility | Waterlow classification | Median WFH < 80% TST < 10% Perc. | 6.1% 17.2% | [52] |
| Italy | 1 m–20 y N = 1994 | Tertiary care & General pediatric wards | No specific | BMI or WFH<−2 SD | 13.2% | [62] |
| Turkey | 1 m–23 y N = 528 | General pediatric unit | No specific | WFA < −2 SDWFH < −2 SDBMI < −2 SD | 36.6% 27.7% 7.4% | [57] |
| Turkey | 1 m–18 y N = 1513 | Nationwide hospitals | PYMS N = 919 STRONGkids | Scoring BMI < −2 SD WFA < −2 SD HFA < −2 SD Scoring | 39.7% 9.5% 14.8% 16.2% 3.6% | [63] |
| UK | 0.6–16 y N = 226 | Tertiary care facility | No specific | WFA < −2 SD or <5% Perc. HFA < −2 SD or <5% Perc. WFH: <80% of STD | 8% 11% 16% | [54] |
| US | <2–18 y N = 268 | Tertiary care facilities | Waterlow classification | Median WFH < 80% | 7.1% | [51] |
| US | <24 m N = 121 | Cardiac intensive tertiary care facility | Waterlow classification | Median WFH < 80% | 17.4% | [53] |
| US | 2–18 y N = 1747 | Tertiary care facility | No specific | BMI ≤ 5% Perc. | 24.5% | [64] |
| US | 1 m–17 y N = 2.14 × 106 | Nationwide hospitals | No specific | % discharges | 2.6% | [65] |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, A.; Delvin, E.; Marcil, V.; Belanger, V.; Marchand, V.; Boctor, D.; Rashid, M.; Noble, A.; Davidson, B.; Groleau, V.; et al. Prevalence of Malnutrition in Pediatric Hospitals in Developed and In-Transition Countries: The Impact of Hospital Practices. Nutrients 2019, 11, 236. https://doi.org/10.3390/nu11020236
McCarthy A, Delvin E, Marcil V, Belanger V, Marchand V, Boctor D, Rashid M, Noble A, Davidson B, Groleau V, et al. Prevalence of Malnutrition in Pediatric Hospitals in Developed and In-Transition Countries: The Impact of Hospital Practices. Nutrients. 2019; 11(2):236. https://doi.org/10.3390/nu11020236
Chicago/Turabian StyleMcCarthy, Andrea, Edgard Delvin, Valerie Marcil, Veronique Belanger, Valerie Marchand, Dana Boctor, Mohsin Rashid, Angela Noble, Bridget Davidson, Veronique Groleau, and et al. 2019. "Prevalence of Malnutrition in Pediatric Hospitals in Developed and In-Transition Countries: The Impact of Hospital Practices" Nutrients 11, no. 2: 236. https://doi.org/10.3390/nu11020236






