Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature
Abstract
:1. The Effects of Dieting on Health
2. Anatomy and Physiology of the Skin
3. Epidemiology of Skin-Related Disorders in the Middle East and North Africa (MENA) Region
4. Effects of Fasting on Skin Homeostasis
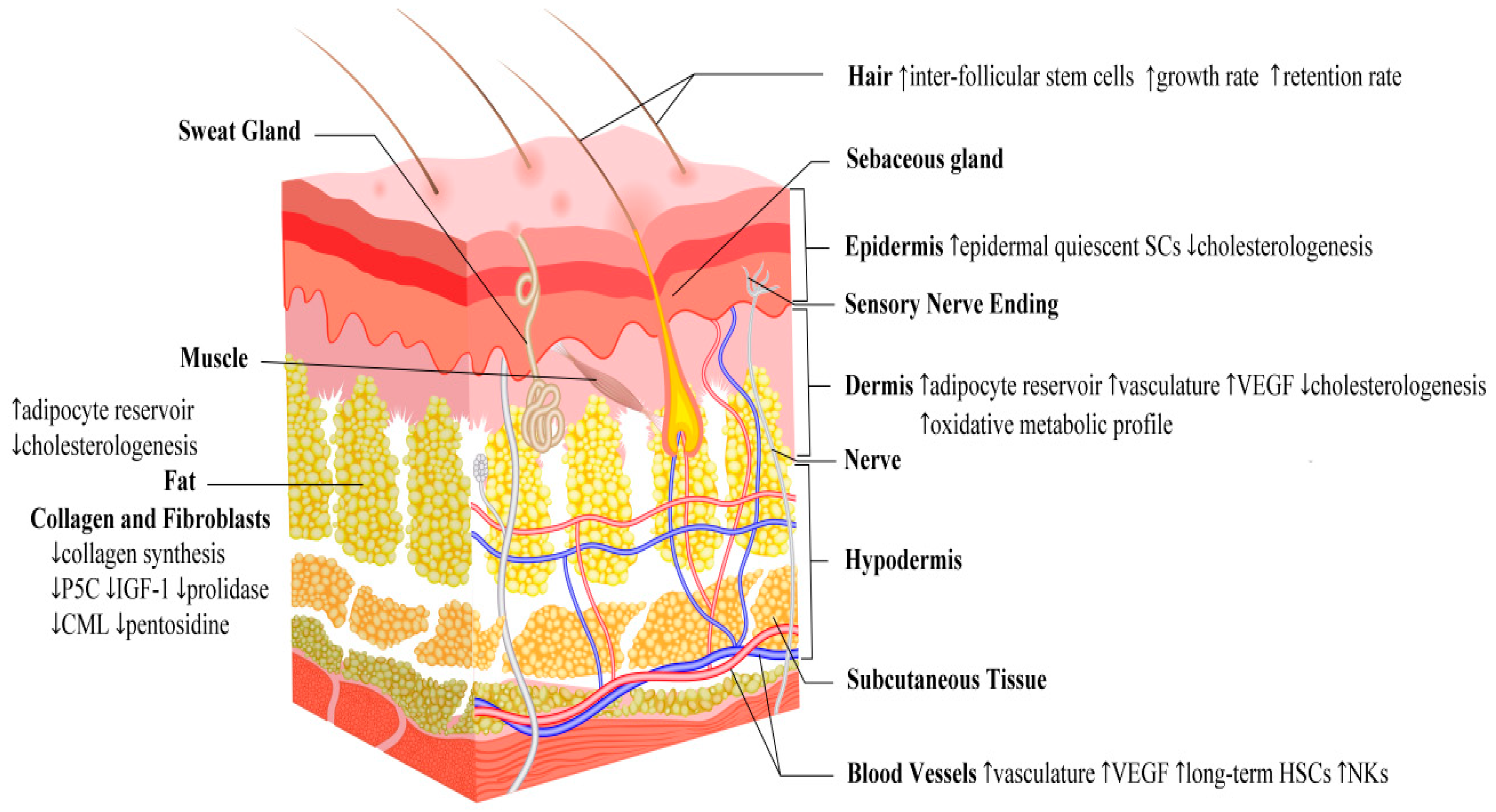
4.1. Fasting and Skin Structural and Functional Adaptation
4.2. Fasting and Wound Healing
4.3. Fasting and the Immune System
4.4. Fasting and Skin Growth Regulation
4.5. Fasting and Skin Aging
4.6. Fasting and Skin Effects: A Summary
5. Fasting and Autoimmune/Inflammatory Dermatoses
6. Fasting and Skin Cancer
7. Ramadan, Chronotherapy and Skin
8. Future Prospects
9. Conclusions
Funding
Conflicts of Interest
References
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Hollestein, L.M.; Nijsten, T. An insight into the global burden of skin diseases. J. Investig. Dermatol. 2014, 134, 1499–1501. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global Burden of Skin Disease: Inequities and Innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef]
- Lewis, V.; Finlay, A.Y. 10 years experience of the Dermatology Life Quality Index (DLQI). J. Investig. Dermatol. Symp. Proc. 2004, 9, 169–180. [Google Scholar] [CrossRef]
- Cecchi, L.; D’Amato, G.; Annesi-Maesano, I. External exposome and allergic respiratory and skin diseases. J. Allergy Clin. Immunol. 2018, 141, 846–857. [Google Scholar] [CrossRef]
- Katta, R.; Desai, S.P. Diet and dermatology: The role of dietary intervention in skin disease. J. Clin. Aesthet. Dermatol. 2014, 7, 46–51. [Google Scholar]
- Barrea, L.; Nappi, F.; Di Somma, C.; Savanelli, M.C.; Falco, A.; Balato, A.; Balato, N.; Savastano, S. Environmental Risk Factors in Psoriasis: The Point of View of the Nutritionist. Int. J. Environ. Res. Public Health 2016, 13, 743. [Google Scholar] [CrossRef]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Dréno, B. Acne and nutrition: Hypotheses, myths and facts. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1631–1637. [Google Scholar] [CrossRef]
- Saha, K.; Hornyak, T.J.; Eckert, R.L. Epigenetic cancer prevention mechanisms in skin cancer. AAPS J. 2013, 15, 1064–1071. [Google Scholar] [CrossRef]
- Alhamdan, B.A.; Garcia-Alvarez, A.; Alzahrnai, A.H.; Karanxha, J.; Stretchberry, D.R.; Contrera, K.J.; Utria, A.F.; Cheskin, L.J. Alternate-day versus daily energy restriction diets: Which is more effective for weight loss? A systematic review and meta-analysis. Obes. Sci. Pract. 2016, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes. (Lond.) 2015, 39, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell MeTable 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.S.; He, X.C.; Li, L. Stem Cells Matter in Response to Fasting. Cell Rep. 2015, 13, 2325–2326. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Qoran, Surat 2 “Al-Baqarah”, ayyat (verse) 183. Available online: https://quran.com/2/183 (accessed on 1 December 2018).
- Berbari, A.E.; Daouk, N.A.; Mallat, S.G.; Jurjus, A.R. Ramadan Fasting in Health and Disease; Berbari, A.E., Mancia, G., Eds.; Special Issues in Hypertension; Springer: Milan, Italy, 2012. [Google Scholar]
- Günaydin, G.P.; Dogan, N.O.; Çevik, Y.; Korkmaz, H.; Savrun, A.; Çıkrıkçı, G. Evaluation of Patients with Renal Colic that Present to an Emergency Department During the Month of Ramadan. J. Acad. Emerg. Med. 2013, 12, 24–26. [Google Scholar]
- Qoran, Surat 2 “Al-Baqarah”, ayyat (verses) 185–186. Available online: https://quran.com/2/185-186 (accessed on 1 December 2018).
- Al Wakeel, J.; Mitwalli, A.H.; Alsuwaida, A.; Al Ghonaim, M.; Usama, S.; Hayat, A.; Shah, I.H. Recommendations for fasting in Ramadan for patients on peritoneal dialysis. Perit. Dial. Int. 2013, 33, 86–91. [Google Scholar] [CrossRef]
- Emami-Naini, A.; Roomizadeh, P.; Baradaran, A.; Abedini, A.; Abtahi, M. Ramadan fasting and patients with renal diseases: A mini review of the literature. J. Res. Med. Sci. 2013, 18, 711–716. [Google Scholar]
- Bragazzi, N.L. Ramadan fasting and chronic kidney disease: Does estimated glomerular filtration rate change after and before Ramadan? Insights from a mini meta-analysis. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 53–57. [Google Scholar] [CrossRef]
- Bragazzi, N.L. Ramadan fasting and chronic kidney disease: A systematic review. J. Res. Med. Sci. 2014, 19, 665–676. [Google Scholar]
- Oláh, A.; Szöllősi, A.G.; Bíró, T. The channel physiology of the skin. Rev. Physiol. Biochem. Pharmacol. 2012, 163, 65–131. [Google Scholar]
- Nie, J.; Fu, X.; Han, W. Microenvironment-dependent homeostasis and differentiation of epidermal basal undifferentiated keratinocytes and their clinical applications in skin repair. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Tareen, S.H.K.; Kutmon, M.; Adriaens, M.E.; Mariman, E.C.M.; de Kok, T.M.; Arts, I.C.W.; Evelo, C.T. Exploring the cellular network of metabolic flexibility in the adipose tissue. Genes Nutr. 2018, 13, 17. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Sil, P.; Wong, S.W.; Martinez, J. More Than Skin Deep: Autophagy Is Vital for Skin Barrier Function. Front. Immunol. 2018, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Żmijewski, M.A.; Piotrowska, A.; Nedoszytko, B.; Lange, M.; Tuckey, R.C.; Slominski, A.T. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol. Cell. Endocrinol. 2016, 437, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Al-Zoman, A.Y.; Al-Asmari, A.K. Pattern of skin diseases at Riyadh Military Hospital. Egyp. Dermatol. Online J. 2008, 4, 4. [Google Scholar]
- El-Essawi, D.; Musial, J.L.; Hammad, A.; Lim, H.W. A survey of skin disease and skin-related issues in Arab Americans. J. Am. Acad. Dermatol. 2007, 56, 933–938. [Google Scholar] [CrossRef]
- Varady, K.A.; Roohk, D.J.; McEvoy-Hein, B.K.; Gaylinn, B.D.; Thorner, M.O.; Hellerstein, M.K. Modified alternate-day fasting regimens reduce cell proliferation rates to a similar extent as daily calorie restriction in mice. FASEB J. 2008, 22, 2090–2096. [Google Scholar] [CrossRef] [Green Version]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell MeTable 2015, 22, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Nieto, R.; Palmer, R.M.; Fernández-Fígares, I.; Pérez, L.; Prieto, C. Effect of dietary protein quality, feed restriction and short-term fasting on protein synthesis and turnover in tissues of the growing chicken. Br. J. Nutr. 1994, 72, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, A.J. Beyond interventions: Caloric restriction as a scientific model. Psychosom. Med. 2012, 74, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Alverez, L.C.; Peters, D.J.; Murad, H.; Wright, E.T.; McGhee, G.; Drenick, E.J. Changes in the epidermis during prolonged fasting. Am. J. Clin. Nutr. 1975, 28, 866–871. [Google Scholar] [CrossRef] [Green Version]
- Forni, M.F.; Peloggia, J.; Braga, T.T.; Chinchilla, J.E.O.; Shinohara, J.; Navas, C.A.; Camara, N.O.S.; Kowaltowski, A.J. Caloric Restriction Promotes Structural and Metabolic Changes in the Skin. Cell Rep. 2017, 20, 2678–2692. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Rabbiosi, G. Aspects of carbohydrate metabolism in rat skin in various experimental conditions. II. Changes induced by fasting. Boll. Soc. Ital. Biol. Sper. 1967, 43, 337–338. [Google Scholar] [PubMed]
- MacDonald, I. Dietary carbohydrates and skin lipids. Br. J. Dermatol. 1967, 79, 119–121. [Google Scholar]
- Wu-Pong, S.; Elias, P.M.; Feingold, K.R. Influence of altered serum cholesterol levels and fasting on cutaneous cholesterol synthesis. J. Investig. Dermatol. 1994, 102, 799–802. [Google Scholar] [CrossRef]
- Varani, J.; Bhagavathula, N.; Aslam, M.N.; Fay, K.; Warner, R.L.; Hanosh, A.; Barron, A.G.; Miller, R.A. Inhibition of retinoic acid-induced skin irritation in calorie-restricted mice. Arch. Dermatol. Res. 2008, 300, 27–35. [Google Scholar] [CrossRef]
- Hayati, F.; Maleki, M.; Pourmohammad, M.; Sardari, K.; Mohri, M.; Afkhami, A. Influence of Short-term, Repeated Fasting on the Skin Wound Healing of Female Mice. Wounds 2011, 23, 38–43. [Google Scholar]
- Hunt, N.D.; Li, G.D.; Zhu, M.; Miller, M.; Levette, A.; Chachich, M.E.; Spangler, E.L.; Allard, J.S.; Hyun, D.H.; Ingram, D.K.; et al. Effect of calorie restriction and refeeding on skin wound healing in the rat. Age (Dordr.) 2012, 34, 1453–1458. [Google Scholar] [CrossRef]
- Miltyk, W.; Palka, J.A. Potential role of pyrroline 5-carboxylate in regulation of collagen biosynthesis in cultured human skin fibroblasts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 265–271. [Google Scholar] [CrossRef]
- Cechowska-Pasko, M.; Pałka, J. Expression of IGF-binding protein-1 phosphoisoforms in fasted rat skin and its role in regulation of collagen biosynthesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 703–711. [Google Scholar] [CrossRef]
- Cechowska-Pasko, M.; Palka, J.; Bańkowski, E. Fasting-induced inhibition of collagen biosynthesis in rat skin. A possible role for phosphoenolpyruvate in this process. Mol. Cell. Biochem. 2004, 265, 203–208. [Google Scholar] [CrossRef]
- Cheng, C.W.; Adams, G.B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.S.; Da Sacco, S.; Mirisola, M.; Quinn, D.I.; Dorff, T.B.; et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014, 14, 810–823. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Briki, W.; Khabbache, H.; Rammouz, I.; Chamari, K.; Demaj, T.; Re, T.S.; Zouhir, M. Ramadan Fasting and Patients with Cancer: State-of-the-Art and Future Prospects. Front. Oncol. 2016, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Pelling, J.C.; White, L.T.; Dimitroff, K.; Barnett, T. Influence of diet and calorie restriction on the initiation and promotion of skin carcinogenesis in the SENCAR mouse model. Cancer Res. 1991, 51, 1851–1854. [Google Scholar]
- Lu, J.; Xie, L.; Sylvester, J.; Wang, J.; Bai, J.; Baybutt, R.; Wang, W. Different gene expression of skin tissues between mice with weight controlled by either calorie restriction or physical exercise. Exp. Biol. Med. (Maywood) 2007, 232, 473–480. [Google Scholar]
- Xie, L.; Jiang, Y.; Ouyang, P.; Chen, J.; Doan, H.; Herndon, B.; Sylvester, J.E.; Zhang, K.; Molteni, A.; Reichle, M.; et al. Effects of dietary calorie restriction or exercise on the PI3K and Ras signaling pathways in the skin of mice. J. Biol. Chem. 2007, 282, 28025–28035. [Google Scholar] [CrossRef]
- Bhattacharyya, T.K.; Merz, M.; Thomas, J.R. Modulation of cutaneous aging with calorie restriction in Fischer 344 rats: A histological study. Arch. Facial Plast. Surg. 2005, 7, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Bell-Farrow, A.D.; Wang, Z.Q.; Sonntag, W.E.; Fu, M.X.; Baynes, J.W.; Thorpe, S.R. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, B337–B341. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L., Jr.; Mattison, J.A.; Desmond, R.A.; Gardner, J.P.; Kimura, M.; Roth, G.S.; Ingram, D.K.; Allison, D.B.; Aviv, A. Telomere dynamics in rhesus monkeys: No apparent effect of caloric restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1163–1168. [Google Scholar] [CrossRef]
- Peterka, E.S.; Fusaro, R.M. Cutaneous carbohydrate studies. IV. The skin glucose content of fasting diabetics with and without infection. J. Investig. Dermatol. 1966, 46, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Peterka, E.S.; Fusaro, R.M. Cutaneous carbohydrate studies. III. Comparison of the fasting glucose content of the skin of the back, arm, abdomen and thigh. J. Investig. Dermatol. 1966, 47, 410–411. [Google Scholar] [CrossRef]
- Longo, V.D.; Fontana, L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010, 31, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bronsnick, T.; Murzaku, E.C.; Rao, B.K. Diet in dermatology: Part I. Atopic dermatitis, acne, and nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2014, 71, 1039.e1–1039.e12. [Google Scholar] [CrossRef] [PubMed]
- Lithell, H.; Bruce, A.; Gustafsson, I.B.; Höglund, N.J.; Karlström, B.; Ljunghall, K.; Sjölin, K.; Venge, P.; Werner, I.; Vessby, B. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm.Venereol. 1983, 63, 397–403. [Google Scholar]
- Francis, N.; Wong, S.H.; Hampson, P.; Wang, K.; Young, S.P.; Deigner, H.P.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim. Biophys. Acta (BBA)-Mol.Cell Res. 2011, 1813, 1822–1826. [Google Scholar] [CrossRef] [Green Version]
- Wolters, M. Diet and psoriasis: Experimental data and clinical evidence. Br. J. Dermatol. 2005, 153, 706–714. [Google Scholar] [CrossRef]
- Smith, R.N.; Braue, A.; Varigos, G.A.; Mann, N.J. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J. Dermatol. Sci. 2008, 50, 41–52. [Google Scholar] [CrossRef]
- Downing, D.T.; Strauss, J.S.; Pochi, P.E. Changes in skin surface lipid composition induced by severe caloric restriction in man. Am. J. Clin. Nutr. 1972, 25, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Pochi, P.E.; Downing, D.T.; Strauss, J.S. Sebaceous gland response in man to prolonged total caloric deprivation. J. Investig. Dermatol. 1970, 55, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, M.; Kehdy, J.; Kibbi, A.G.; Ghosn, S. Prurigo pigmentosa: A clinicopathologic study of 4 cases from the middle East. Am. J. Dermatopathol. 2014, 36, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Corazzari, M.; Pereira, G.; Fimia, G.M.; Piacentini, M.; Smaili, S. Fasting boosts sensitivity of human skin melanoma to cisplatin-induced cell death. Biochem. Biophys. Res. Commun. 2017, 485, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Beltran, L.; Carbajal, S.; Hursting, S.D.; DiGiovanni, J. Energy balance modulates mouse skin tumor promotion through altered IGF-1R and EGFR crosstalk. Cancer Prev. Res. 2012, 5, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.K.; FitzGerald, G.A. Circadian Clocks and Metabolism: Implications for Microbiome and Aging. Trends Genet. 2017, 33, 760–769. [Google Scholar] [CrossRef]
- Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological Rhythms in the Skin. Int. J. Mol. Sci. 2016, 17, 801. [Google Scholar] [CrossRef]
- Le Fur, I.; Reinberg, A.; Lopez, S.; Morizot, F.; Mechkouri, M.; Tschachler, E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J. Investig. Dermatol. 2001, 117, 718–724. [Google Scholar] [CrossRef]
- Adawi, M.; Watad, A.; Brown, S.; Aazza, K.; Aazza, H.; Zouhir, M.; Sharif, K.; Ghanayem, K.; Farah, R.; Mahagna, H.; et al. Ramadan fasting exerts immunomodulatory effects: insights from a systematic review. Front. Immunol. 2017, 8, 1144. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Sackett-Lundeen, L.; Goon, A.; Yiong Huak, C.; Leok Goh, C.; Haus, E. Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non-irritated and irritated skin-effect of topical corticosteroids. J. Investig. Dermatol. 2004, 122, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Nohroudi, K.; Born, J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 2007, 30, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Chiang, B.L. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int. J. Mol. Sci. 2016, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, L.A.; Wenger, C.B.; O’Donovan, B.H.; Nadel, E.R. Circadian rhythm in sweating and cutaneous blood flow. Am. J. Physiol. 1984, 246, R321–R324. [Google Scholar] [CrossRef]
- Haeck, I.M.; Timmer-de Mik, L.; Lentjes, E.G.; Buskens, E.; Hijnen, D.J.; Guikers, C.; Bruijnzeel-Koomen, C.A.; de Bruin-Weller, M.S. Low basal serum cortisol in patients with severe atopic dermatitis: Potent topical corticosteroids wrongfully accused. Br. J. Dermatol. 2007, 156, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Katsunuma, T.; Iikura, Y.; Kato, H.; Saito, H.; Akasawa, A. Adrenocortical function in patients with severe atopic dermatitis. Ann. Allerg. Asthma Immunol. 2000, 85, 35–39. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yamatodani, A.; Okakura, K.; Horii, A.; Inagaki, N.; Wada, H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol. Behav. 1992, 51, 391–394. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ishimaru, K.; Shibata, S.; Nakao, A. Regulation of plasma histamine levels by the mast cell clock and its modulation by stress. Sci. Rep. 2017, 7, 39934. [Google Scholar] [CrossRef] [Green Version]
- Janich, P.; Toufighi, K.; Solanas, G.; Luis, N.M.; Minkwitz, S.; Serrano, L.; Lehner, B.; Benitah, S.A. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem. Cell. 2013, 13, 745–753. [Google Scholar] [CrossRef]
- Dridi, I.; Grissa, I.; Ezzi, L.; Chakroun, S.; Ben-Cherif, W.; Haouas, Z.; Aouam, K.; Ben-Attia, M.; Reinberg, A.; Boughattas, N.A. Circadian variation of cytotoxicity and genotoxicity induced by an immunosuppressive agent “Mycophenolate Mofetil” in rats. Chronobiol. Int. 2016, 33, 1208–1221. [Google Scholar] [CrossRef]
- BaHammam, A.; Alrajeh, M.; Albabtain, M.; Bahammam, S.; Sharif, M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite 2010, 54, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Magdum, A.; Ghura, V. Does fasting during Ramadan affect the use of topical dermatological treatment by Muslim patients in the UK? Clin. Exp. Dermatol. 2012, 37, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [PubMed]



| Animal Model | Fasting Regimen | Results | Explanation | References |
|---|---|---|---|---|
| Swiss Mice | Long term caloric restriction to 60% for 6 months | Maintained/preserved thermal homeostasis | Maintenance of fur due to increase of HFSC pool Expansion of dermal vasculature due to increased levels of VEGF | Forni et al., 2017 [38] |
| Hairless Mice | Caloric restriction | Compromised stratum corneum permeability barrier | Decreased synthesis of epidermal cholesterol | Wu-Pong et al., 1994 [41] |
| UM-HET3 Mice | Caloric restriction to 70% for 3–18 months | Decreased retinoid-induced skin irritation without interfering with treatment efficacy | Increased local antioxidant levels Inhibitory effect on transcription of MMP genes involved in tissue destruction | Varani et al., 2008 [42] |
| Suri Mice | Short-term fast for 4 day/2weeks for 2 months | Enhancement of wound healing | Increased MQ production and release of TNF-α and VEGF | Hayati et al., 2011 [43] |
| Fisher-344 Rats | 40% calorie restricted diet begun 48 hours before wounding and continued during healing | Delayed wound healing | Decreased collagen synthesis | Hunt et al., 2012 [44] |
| Rats | One time 72-hour fast with access to water only | Delayed wound healing | Decreased level of the proline precursor, P5C, resulting in suppression of IGF-1 dependent stimulation of collagen synthesis | Miltyk and Palka, 2000 [45] |
| Rats | One time 72-hour fast with access to water only | Delayed wound healing | Decreased availability of IGF-1 due to up-regulation of IGFBP-1 that has high affinity to IGF-1 | Cechowska-Pasko et al., 2003 [46] |
| Rats | One time 72-hour fast with access to water only | Delayed wound healing | Decreased prolidase activity leading to decreased proline salvage and reduction of collagen synthesis | Cechowska-Pasko et al., 2004 [47] |
| Mice | 2-day water-only fast before chemotherapy | Protective effect against toxic effects of chemotherapy on HSCs | Decreased levels of IGF-1 and PKA, resulting in modulation of HSC, promoting self-renewal, lineage regeneration and proliferation | Cheng et al., 2014 [48] |
| SENCAR Mice | 20% calorie restricted diet for 5 days/week for 10 weeks | Anti-carcinogenic role | Decrease in mitogenesis downstream signaling cascade of IGF-1 (PI3K-AKT and Ras-MAPK) | Xie et al., 2007 [52] |
| Rodent | Long term 60% caloric restriction | Decreased aging | Decreased concentration of glycoxidation products (CML and pentosidine) in cutaneous collagen | Cefalu et al., 1995 [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.; Kimak, M.; Pigatto, P.D.M.; Damiani, G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients 2019, 11, 249. https://doi.org/10.3390/nu11020249
Bragazzi NL, Sellami M, Salem I, Conic R, Kimak M, Pigatto PDM, Damiani G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients. 2019; 11(2):249. https://doi.org/10.3390/nu11020249
Chicago/Turabian StyleBragazzi, Nicola Luigi, Maha Sellami, Iman Salem, Rosalynn Conic, Mark Kimak, Paolo Daniele Maria Pigatto, and Giovanni Damiani. 2019. "Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature" Nutrients 11, no. 2: 249. https://doi.org/10.3390/nu11020249
APA StyleBragazzi, N. L., Sellami, M., Salem, I., Conic, R., Kimak, M., Pigatto, P. D. M., & Damiani, G. (2019). Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients, 11(2), 249. https://doi.org/10.3390/nu11020249







