Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotyping
2.2. Vitamin D Quantification
2.3. Statistical Analysis
3. Results
3.1. Association of VDR Polymorphism and Breast Cancer Risk
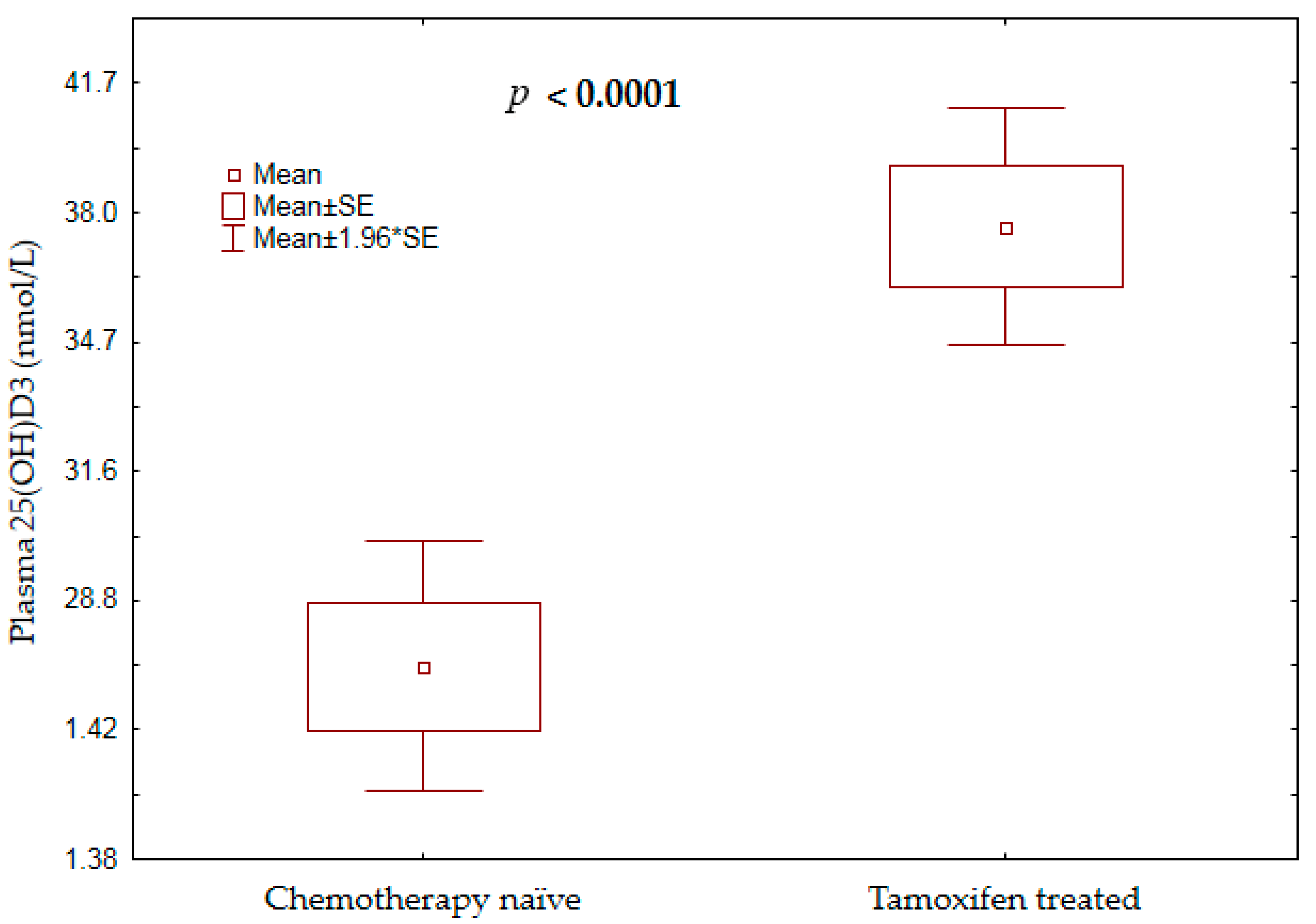
3.2. Plasma 25(OH)D3 Concentration and Vitamin D Status among Treatment-Naïve Versus Tamoxifen-Treated Patients
3.3. Vitamin D Status and Tumor Characteristics
3.4. VDR Genotype with Plasma 25(OH)D3 Concentration and Vitamin D Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suda, T.; Masuyama, R.; Bouillon, R.; Carmeliet, G. Physiological functions of vitamin D: What we have learned from global and conditional VDR knockout mouse studies. Curr. Opin. Pharmacol. 2015, 22, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. The physiology of vitamin D—far more than calcium and bone. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and Breast Cancer. The Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev. Med. 1990, 19, 614–622. [Google Scholar] [CrossRef]
- Gorham, E.D.; Garland, F.C.; Garland, C.F. Sunlight and breast cancer incidence in the USSR. Int. J. Epidemiol. 1990, 19, 820–824. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; LaPorta, E.; Simmons, K.M.; Beaudin, S.; Welsh, J. The impact of vitamin D in breast cancer: Genomics, pathways, metabolism. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. Fundamentals of vitamin D hormone-regulated gene expression. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. ActaDerm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, L.M.; Langman, C.B.; Ariza, A.J.; Ali, F.N.; Dilley, K.; Price, H.; Liu, X.; Zhang, S.; Hong, X.; Wang, B.; et al. Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent twins. J. Clin. Endocrinol. Metab. 2009, 94, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 2010, 121, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; van der Veer, E.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013, 52, 1115–1125. [Google Scholar] [CrossRef]
- Laird, E.; Thurston, S.W.; van Wijngaarden, E.; Shamlaye, C.F.; Myers, G.J.; Davidson, P.W.; Watson, G.E.; McSorley, E.M.; Mulhern, M.S.; Yeates, A.J.; et al. Maternal Vitamin D Status and the Relationship with Neonatal Anthropometric and Childhood Neurodevelopmental Outcomes: Results from the Seychelles Child Development Nutrition Study. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Green, R.J.; Samy, G.; Miqdady, M.S.; El-Hodhod, M.; Akinyinka, O.O.; Saleh, G.; Haddad, J.; Alsaedi, S.A.; Mersal, A.Y.; Edris, A.; et al. Vitamin D deficiency and insufficiency in Africa and the Middle East, despite year-round sunny days. South Afr. Med. J. Suid-Afr. Tydskr. VirGeneeskd. 2015, 105, 603–605. [Google Scholar] [CrossRef]
- Cusick, S.E.; Opoka, R.O.; Lund, T.C.; John, C.C.; Polgreen, L.E. Vitamin D Insufficiency Is Common in Ugandan Children and Is Associated with Severe Malaria. PLoS ONE 2014, 9, e113185. [Google Scholar] [CrossRef] [PubMed]
- Nansera, D.; Graziano, F.M.; Friedman, D.J.; Bobbs, M.K.; Jones, A.N.; Hansen, K.E. Vitamin D and calcium levels in Ugandan adults with human immunodeficiency virus and tuberculosis. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2011, 15, 1522–1527. [Google Scholar] [CrossRef]
- Kibirige, D.; Mutebi, E.; Ssekitoleko, R.; Worodria, W.; Mayanja-Kizza, H. Vitamin D deficiency among adult patients with tuberculosis: A cross sectional study from a national referral hospital in Uganda. BMC Res. Notes 2013, 6, 293. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Stoecker, B.J. Vitamin D insufficiency in a sunshine-sufficient area: Southern Ethiopia. Food Nutr. Bull. 2013, 34, 429–433. [Google Scholar] [CrossRef]
- Wakayo, T.; Belachew, T.; Vatanparast, H.; Whiting, S.J. Vitamin D Deficiency and Its Predictors in a Country with Thirteen Months of Sunshine: The Case of School Children in Central Ethiopia. PLoS ONE 2015, 10, e0120963. [Google Scholar] [CrossRef]
- Nylén, H.; Habtewold, A.; Makonnen, E.; Yimer, G.; Bertilsson, L.; Burhenne, J.; Diczfalusy, U.; Aklillu, E. Prevalence and risk factors for efavirenz-based antiretroviral treatment-associated severe vitamin D deficiency: A prospective cohort study. Medicine (Baltimore) 2016, 95, e4631. [Google Scholar] [CrossRef]
- Bilinski, K.; Boyages, J. Association between 25-hydroxyvitamin D concentration and breast cancer risk in an Australian population: An observational case-control study. Breast Cancer Res. Treat. 2013, 137, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Clayton, S.; Chlebowski, R.; Vadgama, J.V. Association of Vitamin D3 Level with Breast Cancer Risk and Prognosis in African-American and Hispanic Women. Cancers 2017, 9. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs. <20 ng/ml (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef] [PubMed]
- Köstner, K.; Denzer, N.; Müller, C.S.L.; Klein, R.; Tilgen, W.; Reichrath, J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: A review of the literature. Anticancer Res. 2009, 29, 3511–3536. [Google Scholar] [PubMed]
- Tang, C.; Chen, N.; Wu, M.; Yuan, H.; Du, Y. Fok1 polymorphism of vitamin D receptor gene contributes to breast cancer susceptibility: A meta-analysis. Breast Cancer Res. Treat. 2009, 117, 391–399. [Google Scholar] [CrossRef]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin D receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2014, 810, 69–105. [Google Scholar] [PubMed]
- Vaughan-Shaw, P.G.; O’Sullivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br. J. Cancer 2017, 116, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, L. Association between vitamin D receptor gene polymorphisms and breast cancer risk: A meta-analysis of 39 studies. PLoS ONE 2014, 9, e96125. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, P.; Hirvonen, A.; Kataja, V.; Eskelinen, M.; Kosma, V.-M.; Uusitupa, M.; Vainio, H.; Mitrunen, K. Vitamin D receptor gene polymorphism as an important modifier of positive family history related breast cancer risk. Pharmacogenetics 2004, 14, 239–245. [Google Scholar] [CrossRef]
- Shahbazi, S.; Alavi, S.; Majidzadeh-A, K.; Ghaffarpour, M.; Soleimani, A.; Mahdian, R. BsmI but not FokI polymorphism of VDR gene is contributed in breast cancer. Med. Oncol. Northwood Lond. Engl. 2013, 30, 393. [Google Scholar] [CrossRef] [PubMed]
- Elzehery, R.R.; Baiomy, A.A.; Hegazy, M.A.-F.; Fares, R.; El-Gilany, A.-H.; Hegazi, R. Vitamin D status, receptor gene BsmI (A/G) polymorphism and breast cancer in a group of Egyptian females. Egypt. J. Med. Hum. Genet. 2017, 18, 269–273. [Google Scholar] [CrossRef]
- Sinotte, M.; Rousseau, F.; Ayotte, P.; Dewailly, E.; Diorio, C.; Giguère, Y.; Bérubé, S.; Brisson, J. Vitamin D receptor polymorphisms (FokI, BsmI) and breast cancer risk: Association replication in two case–control studies within French Canadian population. Endocr. Relat. Cancer 2008, 15, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Mena, J.M.; Schöttker, B.; Saum, K.U.; Holleczek, B.; Burwinkel, B.; Wang, T.J.; Brenner, H. No Association of Vitamin D Pathway Genetic Variants with Cancer Risks in a Population-Based Cohort of German Older Adults. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 1459–1461. [Google Scholar] [CrossRef]
- Abd-Elsalam, E.A.-E.; Ismaeil, N.A.; Abd-Alsalam, H.S. Vitamin D receptor gene polymorphisms and breast cancer risk among postmenopausal Egyptian women. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 6425–6431. [Google Scholar] [CrossRef]
- Dean, A.; Sullivan, K.; Soe, M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. 2013. Available online: http://www.openepi.com/OE2.3/Menu/OpenEpiMenu.htm (accessed on 4 June 2018).
- Hatta, F.H.M.; Aklillu, E. P450 (Cytochrome) Oxidoreductase Gene (POR) Common Variant (POR*28) Significantly Alters CYP2C9 Activity in Swedish, But Not in Korean Healthy Subjects. Omics J. Integr. Biol. 2015, 19, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Ng, K.; Wolpin, B.M.; Meyerhardt, J.A.; Wu, K.; Chan, A.T.; Hollis, B.W.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br. J. Cancer 2009, 101, 916–923. [Google Scholar] [CrossRef]
- Zgaga, L.; Theodoratou, E.; Farrington, S.M.; Din, F.V.N.; Ooi, L.Y.; Glodzik, D.; Johnston, S.; Tenesa, A.; Campbell, H.; Dunlop, M.G. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2430–2439. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Fakih, M.G.; Trump, D.L.; Johnson, C.S.; Tian, L.; Muindi, J.; Sunga, A.Y. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int. J. Colorectal Dis. 2009, 24, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Schoenmakers, I.; Jones, K.S.; Jarjou, L.M.A.; Goldberg, G.R. Vitamin D Deficiency and Its Health Consequences in Africa. Clin. Rev. Bone Miner. Metab. 2009, 7, 94–106. [Google Scholar] [CrossRef] [PubMed]
- FMHACA—Standards Directives Guidelines. Available online: http://www.fmhaca.gov.et/standardsdirectivesguidelines.html (accessed on 19 January 2019).
- Tessema, B.; Moges, F.; Habte, D.; Hiruy, N.; Yismaw, S.; Melkieneh, K.; Kassie, Y.; Girma, B.; Melese, M.; Suarez, P.G. Vitamin D deficiency among smear positive pulmonary tuberculosis patients and their tuberculosis negative household contacts in Northwest Ethiopia: A case-control study. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, S.; Mazurek, J.; Rehn, A.; Lemma, B.; Aderaye, G.; Bekele, A.; Assefa, G.; Chanyalew, M.; Aseffa, A.; Andersson, J.; et al. Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Galluzzo, S.; Vincenzi, B.; Zoccoli, A.; Ferraro, E.; Lippi, C.; Altomare, V.; Tonini, G.; Bertoldo, F. Longitudinal evaluation of vitamin D plasma levels during anthracycline- and docetaxel-based adjuvant chemotherapy in early-stage breast cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Shane, E.; Cremers, S.; McMahon, D.J.; Irani, D.; Hershman, D.L. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Pascussi, J.M.; Robert, A.; Nguyen, M.; Walrant-Debray, O.; Garabedian, M.; Martin, P.; Pineau, T.; Saric, J.; Navarro, F.; Maurel, P.; et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J. Clin. Invest. 2005, 115, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Maalmi, H.; Walter, V.; Jansen, L.; Boakye, D.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Association between Blood 25-Hydroxyvitamin D Levels and Survival in Colorectal Cancer Patients: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Koh, B.S.; Yu, J.H.; Lee, J.W.; Son, B.H.; Kim, S.B.; Ahn, S.H. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur. J. Cancer Oxf. Engl. 1990 2014, 50, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Caniggia, A.; Lorè, F.; di Cairano, G.; Nuti, R. Main endocrine modulators of vitamin D hydroxylases in human pathophysiology. J. Steroid Biochem. 1987, 27, 815–824. [Google Scholar] [PubMed]
- Lu, D.; Jing, L.; Zhang, S. Vitamin D Receptor Polymorphism and Breast Cancer Risk: A Meta-Analysis. Medicine (Baltimore) 2016, 95, e3535. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.; Lowe, L.C.; Bretherton-Watt, D.; Mansi, J.L.; Peckitt, C.; Bliss, J.; Wilson, R.G.; Thomas, V.; Colston, K.W. Vitamin D receptor gene polymorphisms and breast cancer risk. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 5472–5481. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Wu, Y.; Sarkissyan, M.; Sarkissyan, S.; Chen, Z.; Shang, X.; Ong, M.; Heber, D.; Koeffler, H.P.; Vadgama, J.V. Vitamin D Receptor Gene Polymorphisms and Prognosis of Breast Cancer among African-American and Hispanic Women. PLoS ONE 2013, 8, e57967. [Google Scholar] [CrossRef]
- McCullough, M.L.; Stevens, V.L.; Diver, W.R.; Feigelson, H.S.; Rodriguez, C.; Bostick, R.M.; Thun, M.J.; Calle, E.E. Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: A nested case-control study. Breast Cancer Res. 2007, 9, R9. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Peng, X.; Murillo, G.; Mehta, R.G. Functional Significance of Vitamin D Receptor FokI Polymorphism in Human Breast Cancer Cells. PLoS ONE 2011, 6, e16024. [Google Scholar] [CrossRef]
- Batina, N.G.; Trentham-Dietz, A.; Gangnon, R.E.; Sprague, B.L.; Rosenberg, M.A.; Stout, N.K.; Fryback, D.G.; Alagoz, O. Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res. Treat. 2013, 138, 519–528. [Google Scholar] [CrossRef]
- Amend, K.; Hicks, D.; Ambrosone, C.B. Breast cancer in African-American women: Differences in tumor biology from European-American women. Cancer Res. 2006, 66, 8327–8330. [Google Scholar] [CrossRef]
- Yao, S.; Zirpoli, G.; Bovbjerg, D.H.; Jandorf, L.; Hong, C.C.; Zhao, H.; Sucheston, L.E.; Tang, L.; Roberts, M.; Ciupak, G.; et al. Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: A case-control study. Breast Cancer Res. 2012, 14, R58. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value | |
|---|---|---|
| Socio-demographics | ||
| Age (years, mean ± SD)♣ | 40.77 ± 10.79 | |
| BSA (m2, mean ± SD) | 1.61 ± 0.19 | |
| BMI (Kg/m2, mean ± SD) | 23.91 ± 4.61 | |
| Baseline laboratory results | ||
| WBC (103/mm3; median, IQR) | 6.67 (2.74) | |
| ANC (103/mm3; median, IQR) Hgb (gm/dL; median, IQR) | 3.59 (2.12) 13.9 (1.8) | |
| HCT (%; median, IQR) | 41.35 (4.48) | |
| PLT (103/mm3; median, IQR) | 295.5 (105) | |
| ALT (U/L; median, IQR) | 18 (14) | |
| AST (U/L; median, IQR) | 24 (11) | |
| ALP (U/L; median, IQR) | 214 (141) | |
| SCr (mean ± SD) | 0.91 ± 0.18 | |
| BUN (median; IQR) | 18 + 10 | |
| Tumor characteristics | N, % | |
| Site of tumor | Left | 200 (51.7) |
| Right | 177 (45.7) | |
| Bilateral | 10 (2.6) | |
| Histologic type of tumor | Ductal | 332 (84.7) |
| Lobular | 17 (4.3) | |
| Mixed | 4 (1) | |
| Other | 39 (10) | |
| Degree of differentiation | Well differentiated | 33 (13.9) |
| Moderately differentiated | 116 (48.9) | |
| Poorly differentiated | 88 (37.1) | |
| Lymph node involvement | Negative | 52 (16.7) |
| Positive | 259 (83.3) | |
| Distant metastatic site | No known distant metastasis | 63 (19.1) |
| Bone, skin, or lung only | 189 (57.3) | |
| Liver, CNS, lung + other organs | 78 (23.6) |
| SNP | Genotype | Genotype Frequency by Group, N (%) | Allele Frequency by Group, N (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | p-Value | Allele | Cases | Controls | p-Value | ||
| rs7975232 (ApaI, A > C) | AA | 145 (37.5) | 84 (43.7) | 0.34 | A | 474 (61.2) | 249 (64.8) | 0.23 |
| AC | 184 (47.5) | 81 (42.2) | C | 300 (38.8) | 135 (35.2) | |||
| CC | 58 (15) | 27 (14.1) | ||||||
| rs2228570 (FokI, T > C) | AA | 23 (5.9) | 12 (6.4) | 0.12 | A | 168 (21.5) | 98 (26.2) | 0.078 |
| AG | 122 (31.3) | 74 (39.6) | G | 612 (78.5) | 276 (73.8) | |||
| GG | 245 (62.8) | 101 (54) | ||||||
| rs731236 (TaqI, T > C) | AA | 149 (38.3) | 74 (38.3) | 0.33 | A | 481 (61.8) | 230 (59.6) | 0.46 |
| AG | 183 (47) | 82 (42.5) | G | 297 (38.2) | 156 (40.4) | |||
| GG | 57 (14.7) | 37 (19.2) | ||||||
| Chi-square test | Gene | Genotype | Presence of Cancer | p-Value | |
| Yes | No | ||||
| rs7975232 (ApaI, A > C) | AA + AC | 329 (66.6) | 165 (33.4) | 0.77 | |
| CC | 58 (68.2) | 27 (31.8) | |||
| rs2228570 (FokI, T > C) | AA + AG | 145 (62.8) | 86 (37.2) | 0.04 | |
| GG | 245 (70.8) | 101 (29.2) | |||
| rs731236 (TaqI, T > C) | AA + AG | 332 (68) | 156 (32) | 0.16 | |
| GG | 57 (60.6) | 37 (39.4) | |||
| Logistic regression | Univariate Analysis | Multivariate Analysis | |||
| ‡ OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| rs2228570 (FokI, T > C) | 0.04 | ||||
| AG + AA | 1 | 1 | 1 | ||
| GG | 1.44 (1.01–2.05) | 0.044 | 1.44 (1.01–2.06) | ||
| rs731236 (TaqI, T > C) | - | ||||
| AG + AA | 1 | - | |||
| GG | 0.72 (0.469–1.14) | 0.164 | - | ||
| Vitamin D Status ♣ | Chemotherapy Naïve | Tamoxifen Group | p-Value |
|---|---|---|---|
| SVDD (<25 nmol/L) | 46 (41.1%) | 10 (11.2%) | <0.001 |
| VDD (25–50 nmol/L) | 56 (50%) | 60 (67.4%) | |
| Insufficient (51–72.5 nmol/L) | 9 (8%) | 12 (14.6%) | |
| Normal (72.5–250 nmol/L) | 1 (0.9%) | 6 (6.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, J.H.; Makonnen, E.; Fotoohi, A.; Yimer, G.; Seifu, D.; Assefa, M.; Tigeneh, W.; Aseffa, A.; Howe, R.; Aklillu, E. Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia. Nutrients 2019, 11, 289. https://doi.org/10.3390/nu11020289
Ahmed JH, Makonnen E, Fotoohi A, Yimer G, Seifu D, Assefa M, Tigeneh W, Aseffa A, Howe R, Aklillu E. Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia. Nutrients. 2019; 11(2):289. https://doi.org/10.3390/nu11020289
Chicago/Turabian StyleAhmed, Jemal Hussien, Eyasu Makonnen, Alan Fotoohi, Getnet Yimer, Daniel Seifu, Mathewos Assefa, Wondmagegnehu Tigeneh, Abraham Aseffa, Rawleigh Howe, and Eleni Aklillu. 2019. "Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia" Nutrients 11, no. 2: 289. https://doi.org/10.3390/nu11020289
APA StyleAhmed, J. H., Makonnen, E., Fotoohi, A., Yimer, G., Seifu, D., Assefa, M., Tigeneh, W., Aseffa, A., Howe, R., & Aklillu, E. (2019). Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia. Nutrients, 11(2), 289. https://doi.org/10.3390/nu11020289





