Reduced Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by Prophylactic Diet Supplementation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. TBI Injury
2.3. Cathepsin B Activity Assay
2.4. Cathepsin B and Bax Western Blot Analyses
2.5. ELISA Analysis
2.6. Rotarod Assay
2.7. Wire Hanging Test
2.8. Grid Walking and Foot-Fault Test
2.9. Cylinder Test and the Morris Water Maze Test
2.10. Brain Lesion Volume Analysis
2.11. Neuronal Cell Density Determination
2.12. Statistical Analysis
3. Results
3.1. Food Intake and Weight Changes
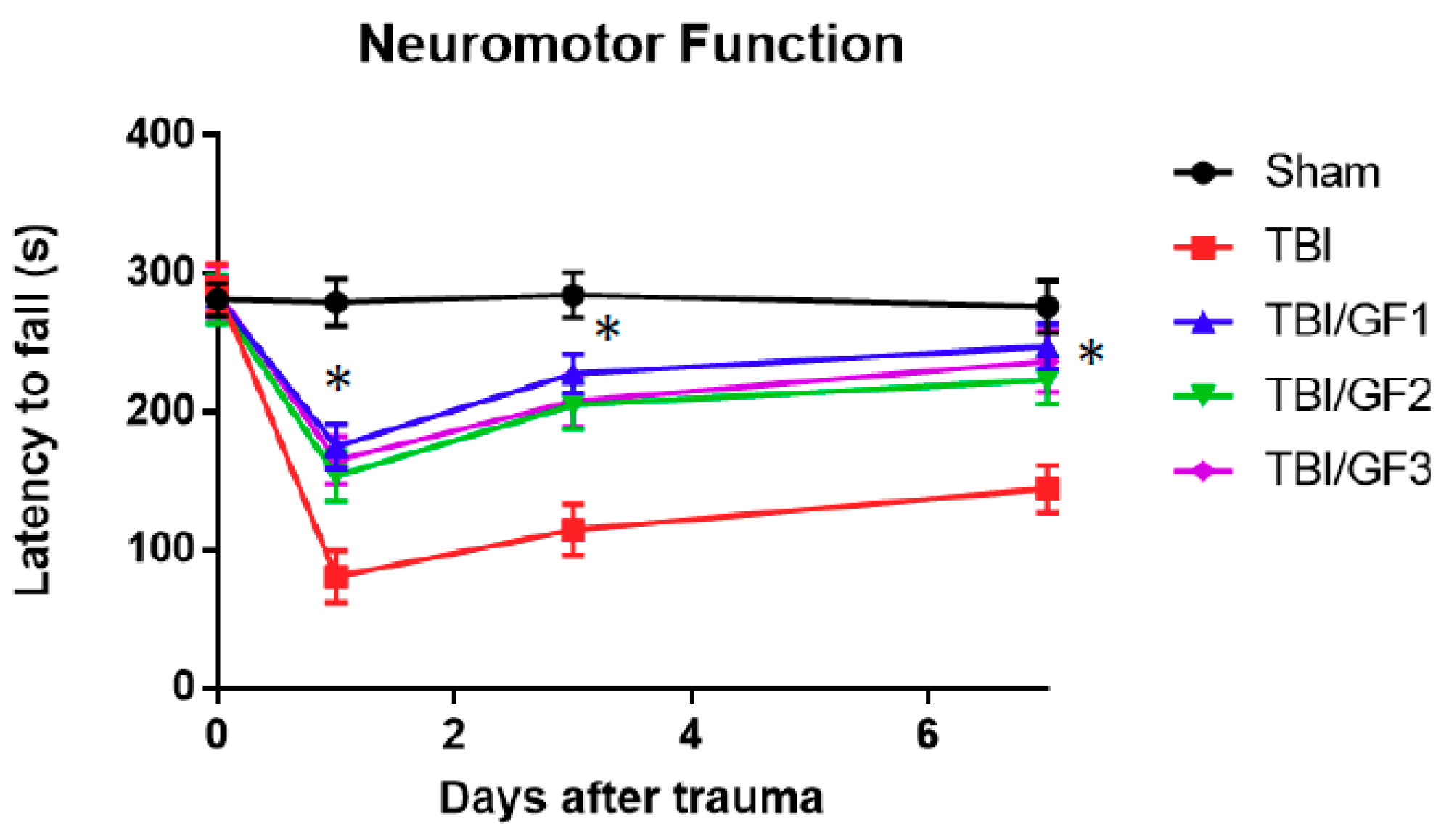
3.2. Impact of Diet on Neuromotor Activity Following TBI
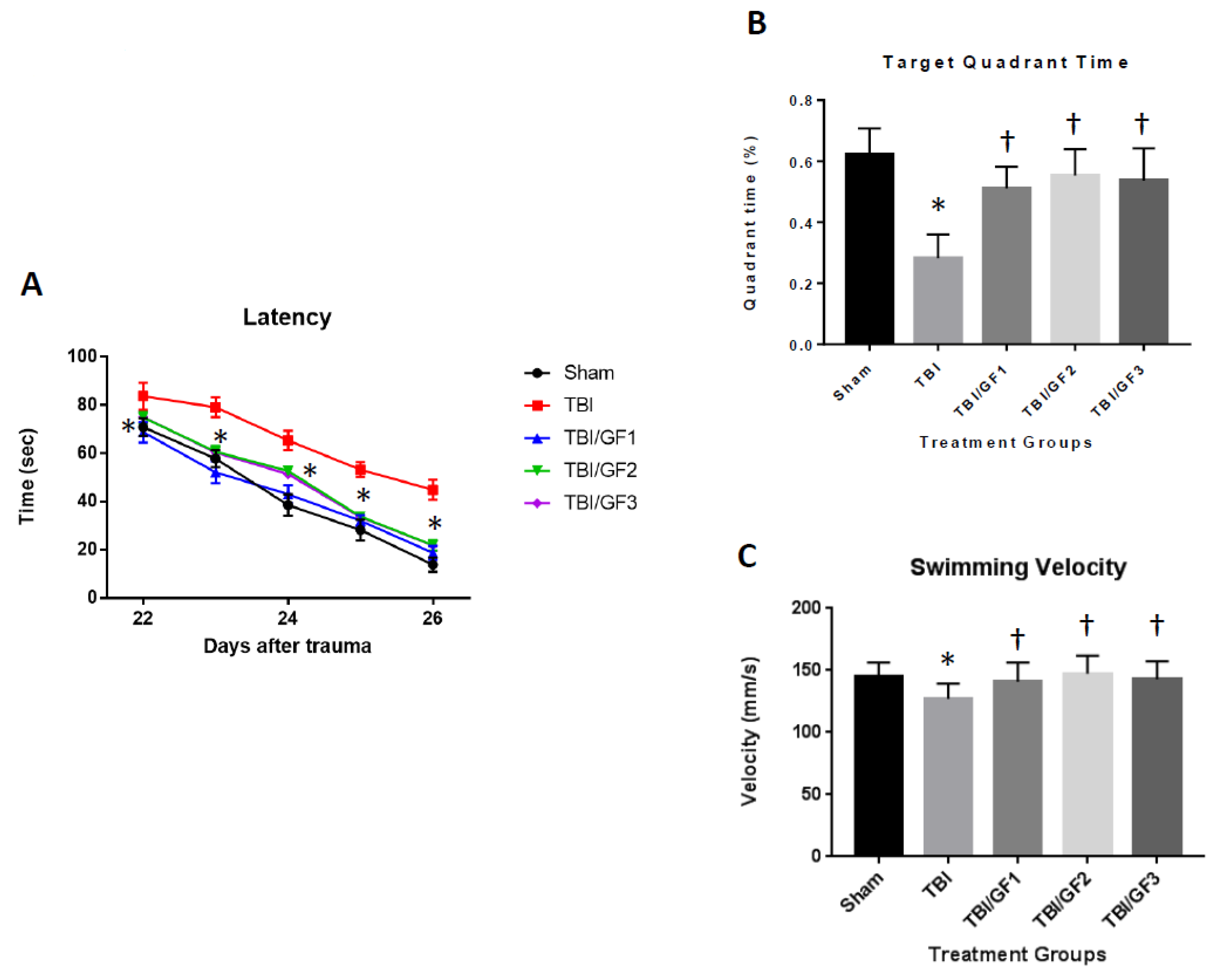
3.3. Improved Cognitive Deficits with GF Diets
3.4. Improvement in Sensorimotor Deficits Following TBI
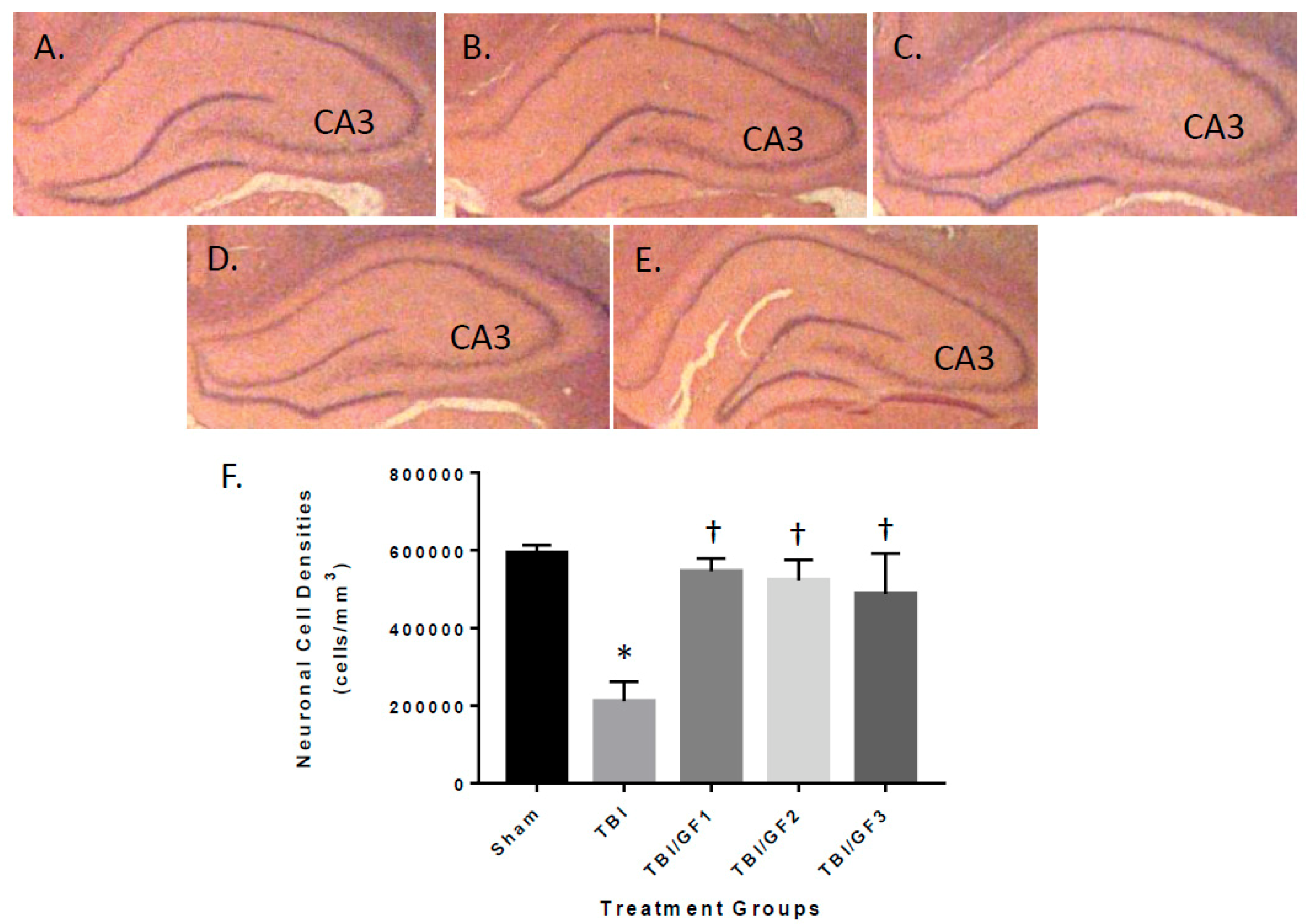
3.5. Protection of the Brain with GF Diets Following TBI
3.6. Diet-Induced Reduction in Neuroinflammation Following TBI
3.7. Altered Cathepsin B Activity in Diet Treated TBI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Xu, L.X.; Wald, M.; Coronado, V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2010. [Google Scholar]
- Cruz-Haces, M.; Tang, J.; Acosta, G.; Fernandez, J.; Shi, R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl. Neurodegener. 2017, 6, 20–29. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.A.; Nelson, L.D.; Guskiewicz, K. Diagnosis and Management of Acute Concussion. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Ercole, A. Critical care management of traumatic brain injury. Handb. Clin. Neurol. 2017, 140, 239–274. [Google Scholar]
- McAllister, T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 287–300. [Google Scholar] [PubMed]
- Beauchamp, K.; Mutlak, H.; Smith, W.R.; Shohami, E.; Stahel, P.F. Pharmacology of traumatic brain injury: Where is the “golden bullet”? Mol. Med. 2008, 14, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.K.; Michel, M.E.; Ansell, B.; Baethmann, A.; Biegon, A.; Bracken, M.B.; Bullock, M.R.; Choi, S.C.; Clifton, G.L.; Contant, C.F.; et al. Clinical trials in head injury. J. Neurotrauma 2002, 19, 503–557. [Google Scholar] [CrossRef]
- Ashbaugh, A.; McGrew, C. The Role of Nutritional Supplements in Sports Concussion Treatment. Curr. Sports Med. Rep. 2016, 15, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Curtis, L.; Epstein, P. Nutritional treatment for acute and chronic traumatic brain injury patients. J. Neurosurg. Sci. 2014, 58, 151–160. [Google Scholar]
- Guseva, M.V.; Kamenskii, A.A.; Gusev, V.B. Optimization of choline administration regimen for correction of cognitive functions in rats after brain injury. Bull. Exp. Biol. Med. 2013, 155, 197–199. [Google Scholar] [CrossRef]
- Barrett, E.C.; McBurney, M.I.; Ciappio, E.D. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv. Nutr. 2014, 5, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Härtl, R.; Gerber, L.M.; Ni, Q.; Ghajar, J. Effect of early nutrition on deaths due to severe traumatic brain injury. J. Neurosurg. 2008, 109, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, H.; Gattoni-Celli, S.; Taheri, S.; Kindy, M.S. Dietary supplementation of GrandFusion(®) mitigates cerebral ischemia-induced neuronal damage and attenuates inflammation. Nutr. Neurosci. 2016, 19, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, H.; Perry, S.; Taheri, S.; Kindy, M.S. Daily supplementation with GrandFusion® improves memory and learning in aged rats. Aging 2017, 9, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, H.; Perry, S.; Taheri, S.; Kindy, M.S. The effect of diet on improved endurance in male C57BL/6 mice. Nutrients 2018, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Hook, G.R.; Yu, J.; Sipes, N.; Pierschbacher, M.D.; Hook, V.; Kindy, M.S. The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury. J. Neurotrauma 2014, 31, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Hook, V.Y.; Kindy, M.; Reinheckel, T.; Peters, C.; Hook, G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 2009, 386, 284–288. [Google Scholar] [CrossRef]
- Elvington, A.; Atkinson, C.; Zhu, H.; Yu, J.; Takahashi, K.; Stahl, G.L.; Kindy, M.S.; Tomlinson, S. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J. Immunol. 2012, 189, 4640–4647. [Google Scholar] [CrossRef]
- Hamm, R.J.; Pike, B.R.; O’Dell, D.M.; Lyeth, B.G.; Jenkins, L.W. The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 1994, 11, 187–196. [Google Scholar] [CrossRef]
- Kawashita, E.; Kanno, Y.; Ikeda, K.; Kuretake, H.; Matsuo, O.; Matsuno, H. Altered behavior in mice with deletion of the alpha2-antiplasmin gene. PLoS ONE 2014, 9, e97947. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav. Brain Res. 2015, 281, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Carriere, C.H.; Kang, N.H.; Niles, L.P. Bilateral upregulation of α-synuclein expression in the mouse substantia nigra by intracranial rotenone treatment. Exp. Toxicol. Pathol. 2017, 69, 109–114. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Ostergaard, K.; Andreassen, O.A.; Finsen, B. Estimation of the number of somatostatin neurons in the striatum: An in situ hybridization study using the optical fractionator method. J. Comp. Neurol. 1996, 370, 11–22. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.; Serra-Grabulosa, J.M.; Junque, C.; Ramirez, B.; Mataro, M.; Poca, A.; Bargallo, N.; Sahuquillo, J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 2006, 44, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.R.; Smith, D.H.; Lowenstein, D.H.; Saint Marie, R.; McIntosh, T.K. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma 1993, 10, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.; Bullock, M.R. Past, Present, and Future of Traumatic Brain Injury Research. Neurosurg. Clin. N. Am. 2016, 27, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M.; Borovnik Lesjak, V.; Vujanović, V.; Križmarić, M. Predictors of mortality in patients with isolated severe traumatic brain injury. Wien. Klin. Wochenschr. 2017, 129, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Hardy, J.; Zetterberg, H. Neurological consequences of traumatic brain injuries in sports. Mol. Cell. Neurosci. 2015, 66, 114–122. [Google Scholar] [CrossRef]
- McKee, C.A.; Lukens, J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016, 7, 556. [Google Scholar] [CrossRef]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 2016, 272, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.W.; Vagni, V.M.; Kochanek, P.M.; Clark, R.S. Combined Neurotrauma Models: Experimental Models Combining Traumatic Brain Injury and Secondary Insults. Methods Mol. Biol. 2016, 1462, 393–411. [Google Scholar] [PubMed]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Steenerson, K.; Starling, A.J. Pathophysiology of Sports-Related Concussion. Neurol. Clin. 2017, 35, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Prasad, N.; Kuwar, R.; Haldar, D.; Abdul-Muneer, P.M. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 2017, 64, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.; Baker, J.G.; Haider, M.N.; Hinds, A.; Willer, B. A Physiological Approach to Prolonged Recovery From Sport-Related Concussion. J. Athl. Train. 2017, 52, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.; Schwartzbauer, G.; Jia, X. Brain Monitoring in Critically Neurologically Impaired Patients. Int. J. Mol. Sci. 2016, 18, 43. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Lewis, M.D. Concussions, Traumatic Brain Injury, and the Innovative Use of Omega-3s. J. Am. Coll. Nutr. 2016, 35, 469–475. [Google Scholar] [CrossRef]
- Costello, L.A.; Lithander, F.E.; Gruen, R.L.; Williams, L.T. Nutrition therapy in the optimisation of health outcomes in adult patients with moderate to severe traumatic brain injury: Findings from a scoping review. Injury 2014, 45, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Redmond, C.; Lipp, J. Traumatic brain injury in the pediatric population. Nutr. Clin. Pract. 2006, 21, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Daradkeh, G.; Essa, M.M.; Al-Adawi, S.S.; Subash, S.; Mahmood, L.; Kumar, P.R. Nutritional status, assessment, requirements and adequacy of traumatic brain injury patients. Pak. J. Biol. Sci. 2014, 17, 1089–1097. [Google Scholar] [PubMed]
- Guseva, M.V.; Hopkins, D.M.; Scheff, S.W.; Pauly, J.R. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J. Neurotrauma 2008, 25, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Freire Royes, L.F.; Cassol, G. The Effects of Creatine Supplementation and Physical Exercise on Traumatic Brain Injury. Mini Rev. Med. Chem. 2016, 16, 29–39. [Google Scholar] [CrossRef]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Bao, Z.; Hou, L.; Liu, Y.; Wang, X.; You, Y.; et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017, 290, 115–122. [Google Scholar] [CrossRef]
- Cope, E.C.; Morris, D.R.; Gower-Winter, S.D.; Brownstein, N.C.; Levenson, C.W. Effect of zinc supplementation on neuronal precursor proliferation in the rat hippocampus after traumatic brain injury. Exp. Neurol. 2016, 279, 96–103. [Google Scholar] [CrossRef]
- Vonder Haar, C.; Peterson, T.C.; Martens, K.M.; Hoane, M.R. Vitamins and nutrients as primary treatments in experimental brain injury: Clinical implications for nutraceutical therapies. Brain Res. 2016, 1640, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Hatton, J.; Kryscio, R.; Ryan, M.; Ott, L.; Young, B. Systemic metabolic effects of combined insulin-like growth factor-I and growth hormone therapy in patients who have sustained acute traumatic brain injury. J. Neurosurg. 2006, 105, 843–852. [Google Scholar] [CrossRef]
- Taha, A.A.; Badr, L.; Westlake, C.; Dee, V.; Mudit, M.; Tiras, K.L. Effect of early nutritional support on intensive care unit length of stay and neurological status at discharge in children with severe traumatic brain injury. J. Neurosci. Nurs. 2011, 43, 291–297. [Google Scholar] [CrossRef]
- Fan, M.-C.; Wang, Q.-L.; Fang, W.; Jiang, Y.-X.; Li, L.-D.; Sun, P.; Wang, Z.-H. Early Enteral Combined with Parenteral Nutrition Treatment for Severe Traumatic Brain Injury: Effects on Immune Function, Nutritional Status and Outcomes. Chin. Med. Sci. J. 2016, 31, 213–220. [Google Scholar] [CrossRef]
- Horn, S.D.; Kinikini, M.; Moore, L.W.; Hammond, F.M.; Brandstater, M.E.; Smout, R.J.; Barrett, R.S. Enteral Nutrition for Patients With Traumatic Brain Injury in the Rehabilitation Setting: Associations With Patient Preinjury and Injury Characteristics and Outcomes. Arch. Phys. Med. Rehabil. 2015, 96, S245–S255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, R.L. Current and evolving management of traumatic brain injury. Crit. Care Nurs. Clin. N. Am. 2009, 21, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zheng, S.Q.; Chen, X.C.; Jiang, S.W.; Chen, H.B. Comparisons between small intestinal and gastric feeding in severe traumatic brain injury: A systematic review and meta-analysis of randomized controlled trials. J. Neurosurg. 2015, 123, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, G.V.; Bochicchio, K.; Nehman, S.; Casey, C.; Andrews, P.; Scalea, T.M. Tolerance and efficacy of enteral nutrition in traumatic brain-injured patients induced into barbiturate coma. J. Parenter. Enteral Nutr. 2006, 30, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Branco, R.G. Neuroprotective measures in children with traumatic brain injury. World J. Crit. Care Med. 2016, 5, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Ansari, M.A. Natural Compounds as a Therapeutic Intervention following Traumatic Brain Injury: The Role of Phytochemicals. J. Neurotrauma 2017, 34, 1491–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frati, A.; Cerretani, D.; Fiaschi, A.I.; Frati, P.; Gatto, V.; La Russa, R.; Pesce, A.; Pinchi, E.; Santurro, A.; Fraschetti, F.; et al. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 2600. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative stress in traumatic brain injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Y.; Yang, W.; Zheng, P.; Zeng, J.; Tong, W. Connexin40 correlates with oxidative stress in brains of traumatic brain injury rats. Restor. Neurol. Neurosci. 2017, 35, 217–224. [Google Scholar] [CrossRef]
- Mei, Z.; Zheng, P.; Tan, X.; Wang, Y.; Situ, B. Huperzine A alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury. Metab. Brain Dis. 2017, 32, 1861–1869. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Dai, Z.; Wu, P.; Shi, H.; Zhao, S. Lack of mitochondrial ferritin aggravated neurological deficits via enhancing oxidative stress in a traumatic brain injury murine model. Biosci. Rep. 2017, 37, BSR20170942. [Google Scholar] [CrossRef] [PubMed]
- Halstrom, A.; MacDonald, E.; Neil, C.; Arendts, G.; Fatovich, D.; Fitzgerald, M. Elevation of oxidative stress indicators in a pilot study of plasma following traumatic brain injury. J. Clin. Neurosci. 2017, 35, 104–108. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Lucke-Wold, B.P.; Nguyen, L.; Matsumoto, R.R.; Turner, R.C.; Rosen, C.L.; Huber, J.D. Salubrinal reduces oxidative stress, neuroinflammation and impulsive-like behavior in a rodent model of traumatic brain injury. Brain Res. 2016, 1643, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Brann, D.W. NADPH Oxidase 2 Regulates NLRP3 Inflammasome Activation in the Brain after Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2017, 2017, 6057609. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, B.Y.; Lee, S.H.; Kho, A.R.; Jeong, J.H.; Hong, D.K.; Suh, S.W. Administration of Protocatechuic Acid Reduces Traumatic Brain Injury-Induced Neuronal Death. Int. J. Mol. Sci. 2017, 18, 2510. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhu, H.; Taheri, S.; Monday, W.L.; Perry, S.; Kindy, M.S. Reduced Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by Prophylactic Diet Supplementation in Mice. Nutrients 2019, 11, 299. https://doi.org/10.3390/nu11020299
Yu J, Zhu H, Taheri S, Monday WL, Perry S, Kindy MS. Reduced Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by Prophylactic Diet Supplementation in Mice. Nutrients. 2019; 11(2):299. https://doi.org/10.3390/nu11020299
Chicago/Turabian StyleYu, Jin, Hong Zhu, Saeid Taheri, William L. Monday, Stephen Perry, and Mark S. Kindy. 2019. "Reduced Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by Prophylactic Diet Supplementation in Mice" Nutrients 11, no. 2: 299. https://doi.org/10.3390/nu11020299





