Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell ROS Determination by DCFH-DA
2.5. Western Blot Analysis
2.6. Immunofluorescence Assay
2.7. Comet Assay
2.8. Molecular Docking Analysis
2.9. Statistical Analysis
3. Results
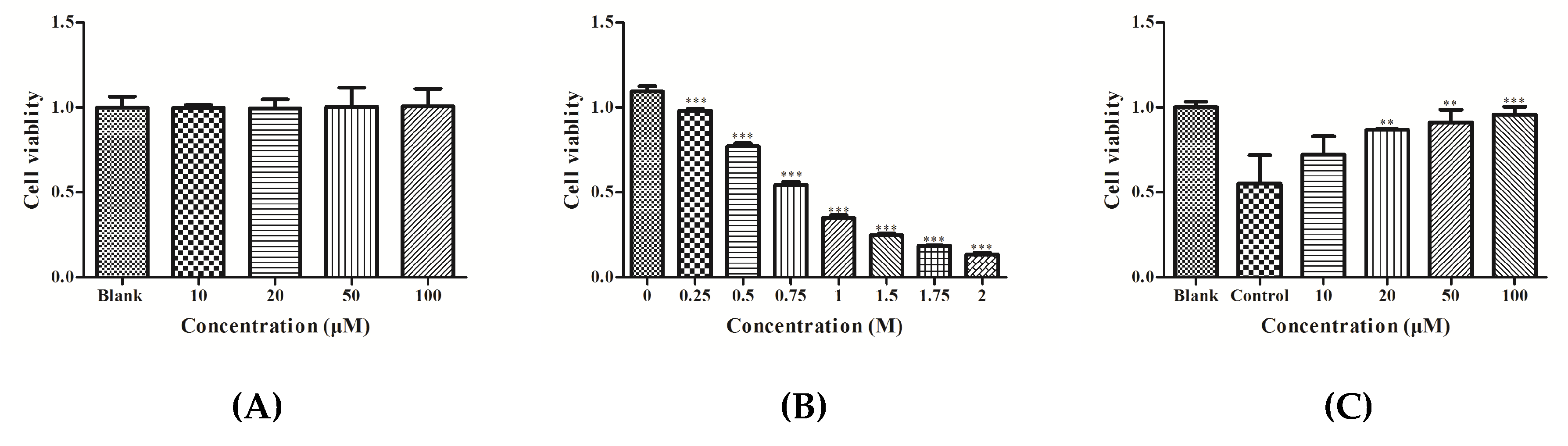
3.1. Effect of YGDEY on Cell Viability of HepG2 Cells
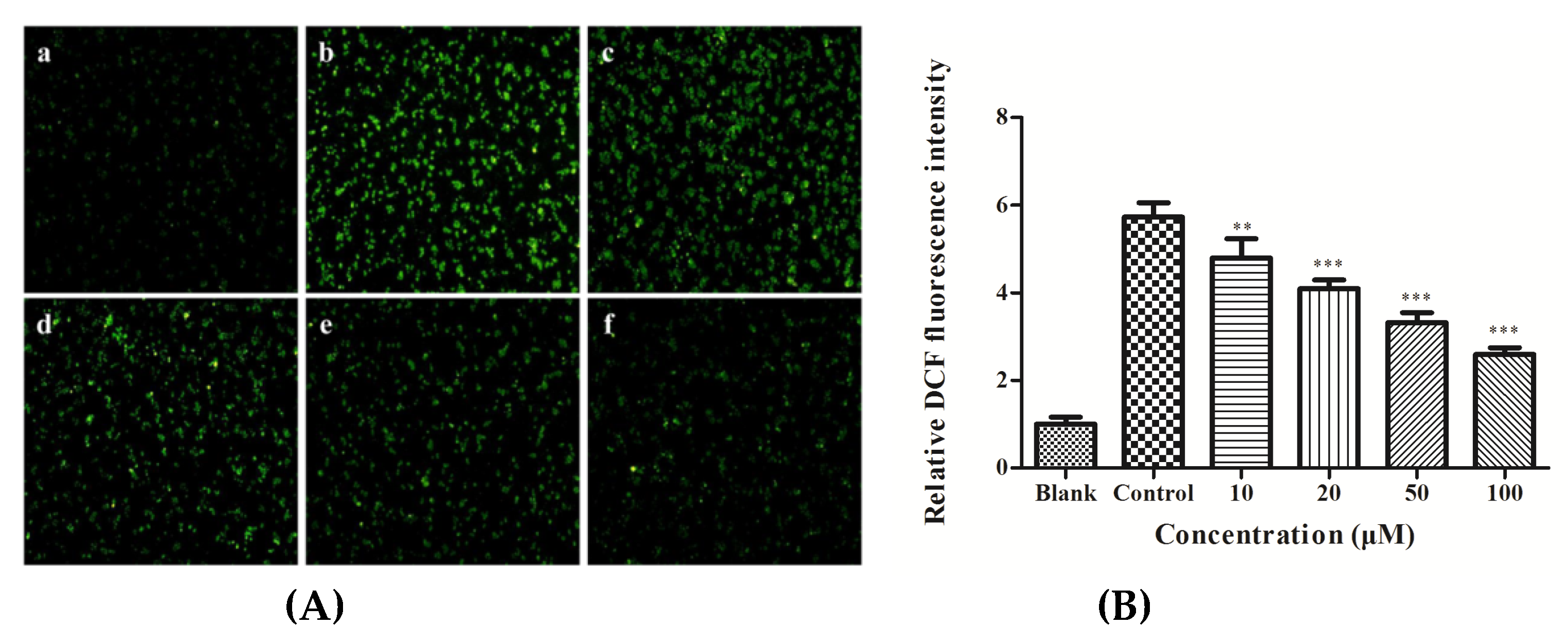
3.2. ROS Production in HepG2 Cells
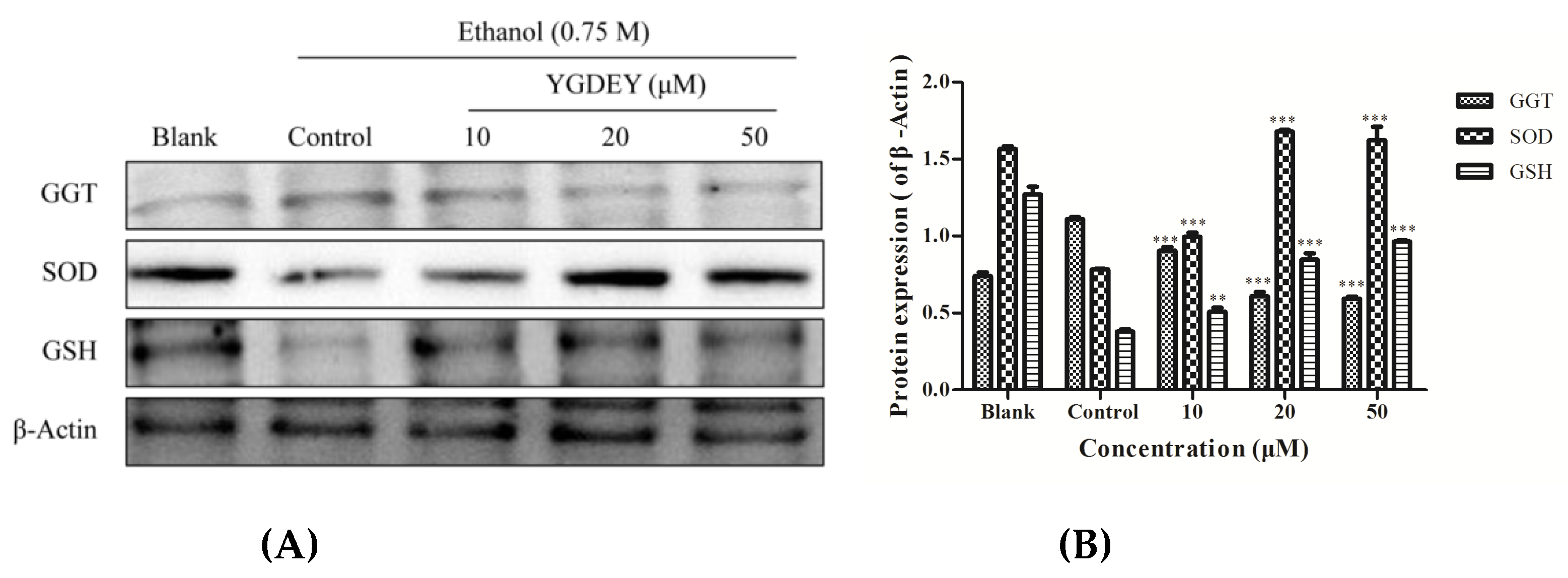
3.3. Effect of YGDEY on Antioxidant Enzymes SOD, GSH, and GGT
3.4. Effect of YGDEY on the Expression of bcl-2, bax, and caspase-3
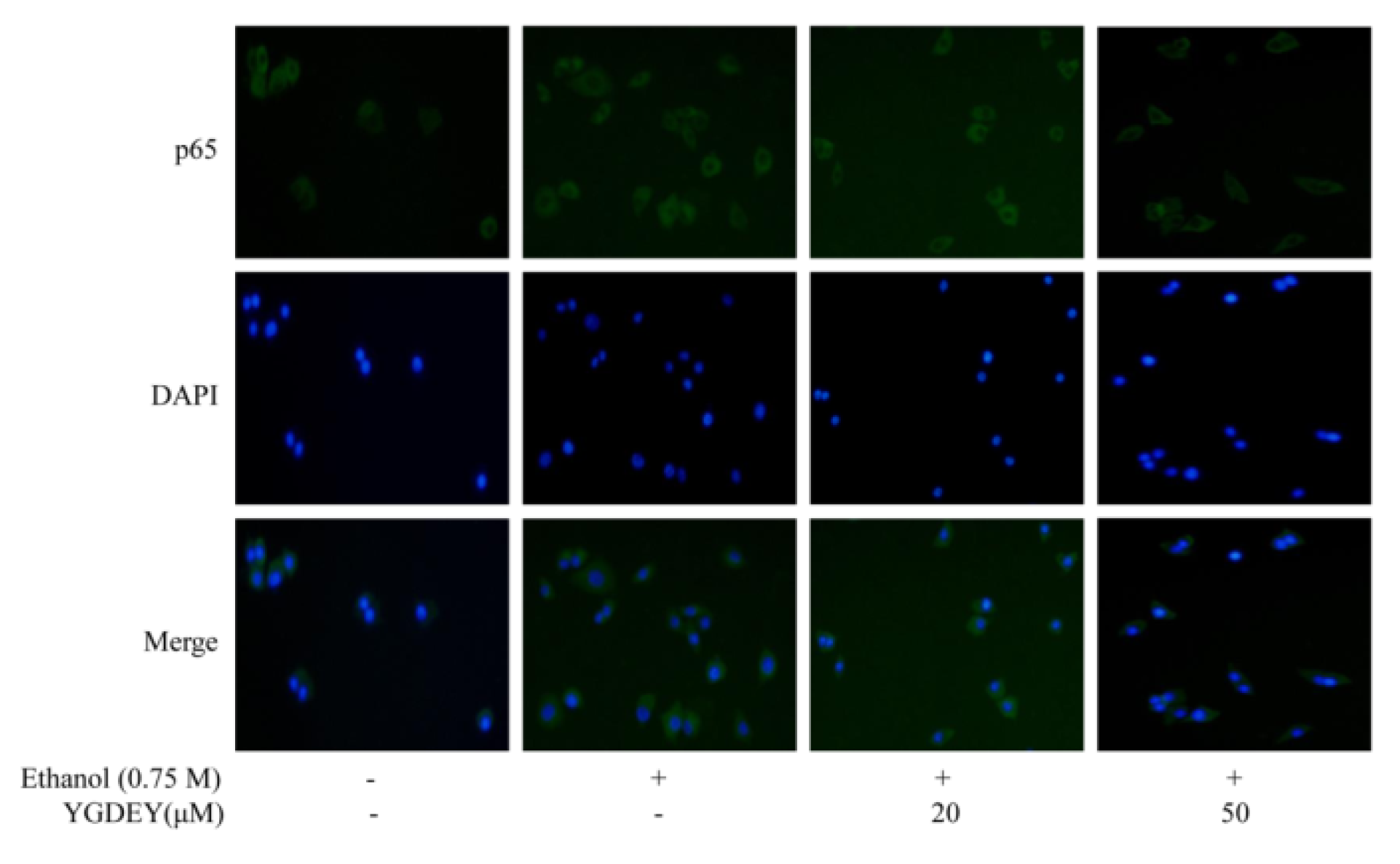
3.5. Effect of YGDEY on the Activation of Akt and NF κB
3.6. Immunofluorescence Analysis
3.7. Regulatory Effect of YGDEY on MAPK Cell Signal Transduction
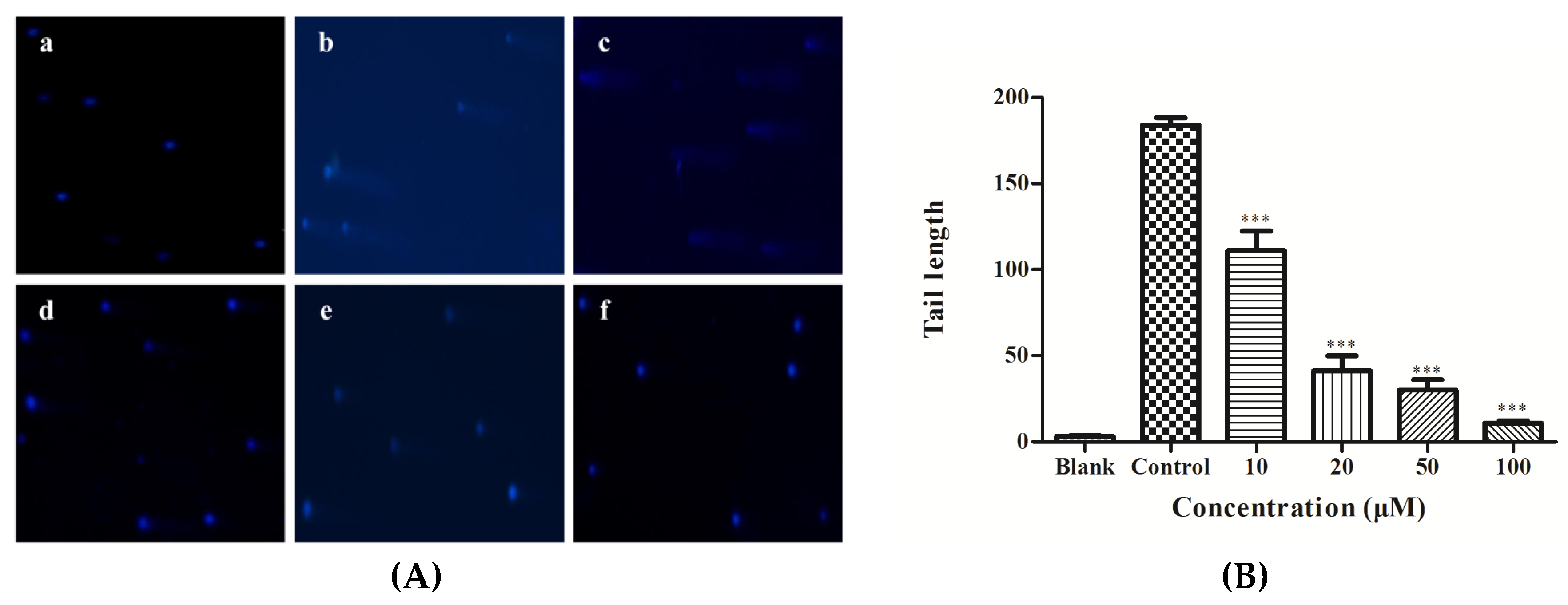
3.8. DNA Damage Analysis
3.9. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Gupta, S.; Singhal, U.; Pandey, R.; Aggarwal, S.K. Evaluation of the oxidative stress in chronic alcoholics. J. Clin. Diagn. Res. 2013, 7, 1568–1571. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Swaminathan, K.; Kumar, S.M.; Clemens, D.L.; Dey, A. In vitro evidence for chronic alcohol and high glucose mediated increased oxidative stress and hepatotoxicity. Alcohol. Clin. Exp. Res. 2012, 36, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Wang, J.; Li, L.Z.; Hu, W.J.; Qu, Y.D.; Ding, Y.P.; Meng, L.N.; Teng, L.R.; Wang, D. Hepatoprotective effects of Antrodia cinnamomea: The modulation of oxidative stress signaling in a mouse model of alcohol-induced acute liver injury. Oxid. Med. Cell. Longev. 2017, 7841823. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Technol. 2015, 52, 6194–6205. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, M.; Piątkowska-Chmiel, I.; Korolczuk, A.; Herbet, M.; Gawrońska-Grzywacz, M.; Gieroba, R.; Sysa, M.; Czajkowska-Bania, K.; Cygal, M.; Korga, A.; et al. The beneficial effects of resveratrol on the steatosis and mitochondrial oxidative stress in HepG2 cells. Can. J. Physiol. Pharm. 2017, 95, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.C.; Lai, S.H.; Yao, J.H.; Zhang, C.; Yin, H.; Li, W.; Han, B.J.; Liu, Y.J. The induction of apoptosis in HepG-2 cells by ruthenium(II) complexes through an intrinsic ROS-mediated mitochondrial dysfunction pathway. Eur. J. Med. Chem. 2016, 122, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Y.H.; Lin, Z.F.; Zhao, M.Q.; Xu, T.T.; Wang, C.B.; Deng, N. Silver nanoparticles induce HepG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res. Lett. 2016, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.B.; Shen, C.; Wang, Z.G.; Li, S.T.; Zhang, X.M.; Song, Z.Y. Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice. J. Nutr. Biochem. 2013, 24, 1520–1528. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.J.; Ryu, B.; Park, J.W.; Kim, S.K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci. 2006, 79, 2056–2068. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, J.A.; Ryu, B.; Kim, S.K. An antihypertensive peptide from tilapia gelatin diminishes free radical formation in murine microglial cells. J. Agric. Food Chem. 2011, 59, 12193–12197. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Dongare, S.; Mathur, R.; Mohanty, I.R.; Srivastava, S.; Mathur, S.; Nag, T.C. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol. Cell. Biochem. 2015, 408, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Yang, C.; Park, S.; Bazer, F.W.; Song, G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through PI3K/AKT and MAPK signal transduction cascades. J. Cell. Physiol. 2017, 232, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Choonpicharn, S.; Tateing, S.; Jaturasitha, S.; Rakariyatham, N.; Suree, N.; Niamsup, H. Identification of bioactive peptide from Oreochromis niloticus skin gelatin. J. Food Sci. Technol. 2016, 53, 1222–1229. [Google Scholar] [CrossRef]
- Lin, H.C.; Alashi, A.M.; Aluko, R.E.; Pan, B.S.; Chang, Y.W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Korczek, K.; Tkaczewska, J.; Migdal, W. Antioxidant and antihypertensive protein hydrolysates in fish products: A review. Czech. J. Food Sci. 2018, 36, 195–207. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, L.P.; Zhuang, Y.L. Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food Funct. 2018, 9, 5251–5259. [Google Scholar] [CrossRef]
- Toopcham, T.; Roytrakul, S.; Yongsawatdigul, J. Characterization and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from tilapia using Virgibacillus halodenitrificans SK1-3-7 proteinases. J. Funct. Foods 2015, 14, 435–444. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Wang, X.X.; Zhou, J.; Cui, H.Y.; Xu, W.D.; He, Y.Q.; Ma, H.L.; Gao, R.C. Effect of oral administration of collagen hydrolysates from Nile tilapia on the chronologically aged skin. J. Funct. Foods 2018, 44, 112–117. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of dietary yeast nucleotide on antioxidant activity, non-specific immunity, intestinal cytokines, and disease resistance in Nile Tilapia. Fish. Shellfish Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Zhang, R.L.; Chen, J.; Jiang, X.W.; Yin, L.S.; Zhang, X.W. Antioxidant and hypoglycaemic effects of tilapia skin collagen peptide in mice. Int. J. Food Sci. Tech. 2016, 51, 2157–2163. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Liu, Q.M.; Yuan, L.; Zhuang, Y.L. Protective effects of LSGYGP from fish skin gelatin hydrolysates on UVB-induced MEFs by regulation of oxidative stress and matrix metalloproteinase activity. Nutrients 2018, 10, 420. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Duan, X.; Zhuang, Y.L. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef]
- Lu, Y.X.; Liu, Y.; Yang, C.Z. Evaluating in vitro DNA damage using comet assay. J. Vis. Exp. 2017, e56450. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Chen, M.H.; May, B.H.; Liao, X.Z.; Liu, J.H.; Tao, L.T.; Sze, D.M.; Zhang, A.L.; Mo, S.L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via bcl-2 and caspase-3. Phytomedicine 2018, 51, 214–225. [Google Scholar] [CrossRef]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.A.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotox. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef]
- Valdés-Arzate, A.; Luna, A.; Bucio, L.; Licona, C.; Clemens, D.L.; Souza, V.; Hernandez, E.; Kershenobich, D.; Gutiérrez-Ruiz, M.C.; Gómez-Quiroz, L.E. Hepatocyte growth factor protects hepatocytes against oxidative injury induced by ethanol metabolism. Free Radical Biol. Med. 2009, 47, 424–430. [Google Scholar] [CrossRef]
- Sueyoshi, S.; Sawai, S.; Satoh, M.; Seimiya, M.; Sogawa, K.; Fukumura, A.; Tsutsumi, M.; Nomura, F. Fractionation of gamma-glutamyltransferase in patients with nonalcoholic fatty liver disease and alcoholic liver disease. World J. Hepatol. 2016, 8, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Sun, B.C.; Zhao, X.L.; Zhao, N.; Dong, X.Y.; Che, N.; Yao, Z.; Ma, Y.M.; Gu, Q.; Zong, W.K.; et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by bcl-2 and twist1: A study of hepatocellular carcinoma. Hepatology 2011, 54, 1690–1706. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Ma, J.; Wang, J.J. Cytotoxicity, oxidative stress, and apoptosis in HepG2 cells induced by ionic liquid 1-methyl-3-octylimidazolium bromide. Ecotox. Environ. Saf. 2015, 120, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Li, J.; Huang, S.X.; Bai, Z.L. Apoptosis induced by low-concentration ethanol in hepatocellular carcinoma cell strains and down-regulated AFP and surviving analysis by proteomic technology. World Acad. Sci. Eng. Technol. 2010, 37, 1080–1086. [Google Scholar]
- Zhang, Y.; An, Y.; Jiang, L.P.; Geng, C.Y.; Cao, J.; Jiang, L.J.; Zhong, L.F. The role of oxidative stress in Sudan IV-induced DNA damage in human liver-derived HepG2 cells. Environ. Toxicol. 2011, 26, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S. Afr. J. Bot. 2017, 112, 70–78. [Google Scholar] [CrossRef]
- Saidi, S.A.; Azaza, M.S.; van Pelt, J.; EI Feki, A. Evaluation of the oxidative status in Oreochromis niloticus fed with tuna by-product meal: Possible human health impact. Arch. Physiol. Biochem. 2014, 120, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Taoufiq, Z.; Pino, P.; Dugas, N.; Conti, M.; Tefit, M.; Mazier, D.; Vouldoukis, I. Transient supplementation of superoxide dismutase protects endothelial cells against Plasmodium falciparum-induced oxidative stress. Mol. Biochem. Parasit. 2006, 150, 166–173. [Google Scholar] [CrossRef]
- Mikulski, D.; Jankowski, J.; Zduńczyk, Z.; Wróblewska, M.; Sartowska, K.; Majewska, T. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J. Anim. Feed Sci. 2009, 18, 518–530. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Rekha, R.; Vijayakumar, S.; Faggio, C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotox. Environ. Saf. 2018, 162, 147–159. [Google Scholar] [CrossRef]
- Bai, Y.T.; Jiang, L.P.; Liu, X.F.; Wang, D.; Yang, G.; Geng, C.Y.; Li, Q.J.; Zhong, L.F.; Sun, Q.H.; Chen, M. The role of oxidative stress in citreoviridin-induced DNA damage in human liver-derived HepG2 cells. Environ. Toxicol. 2015, 30, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Zal, F.; Bolouki, A. Protective effects of quercetin on nicotine induced oxidative stress in ‘HepG2 cells’. Toxicol. Mech. Methods 2017, 27, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Jiang, Z.; Yang, Q.; Mi, Y.; Liu, Y.; Shi, R.; Zhou, Y.; Wang, J.; Lu, W.; et al. Diagnostic value of alcoholic liver disease (ALD)/nonalcoholic fatty liver disease (NAFLD) index combined with gamma-glutamyltransferase in differentiating ALD and NAFLD. Korean J. Intern. Med. 2016, 31, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Wei, H.K.; Yu, H.C.; Xing, Q.; Zou, Y.; Zhou, Y.F.; Peng, J. Fish skin gelatin hydrolysate produced by ginger powder induces glutathione synthesis to prevent hydrogen peroxide-induced intestinal oxidative stress via Pept1-p62-Nrf2 cascade. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Lee, J.H.; Samarakoon, K.; Kim, J.S.; Jeon, Y.J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Yang, C.F.; Ong, C.N. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int. J. Cancer 1999, 81, 820–828. [Google Scholar] [CrossRef]
- Shin, J.H.; Min, S.H. Novel functional roles of caspase-related genes in the regulation of apoptosis and autophagy. Korean J. Physiol. Pharmacol. 2016, 20, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhao, X.H. The growth proliferation, apoptotic prevention, and differentiation induction of the gelatin hydrolysates from three sources to human fetal osteoblasts (hFOB 1.19 cells). Molecules 2018, 23, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Ma, H.G.; Sun, P.; Wang, Q.L.; Zhang, T.T.; Shen, Y.M.; Zhu, W.M.; Li, X. Bisindolylmaleimide alkaloid BMA-155Cl induces autophagy and apoptosis in human hepatocarcinoma HepG-2 cells through the NF-κB p65 pathway. Acta Pharmacol. Sin. 2017, 38, 524–538. [Google Scholar] [CrossRef]
- Ye, W.; Chen, Y.C.; Li, H.H.; Zhang, W.M.; Liu, H.H.; Sun, Z.H.; Liu, T.M.; Li, S.N. Two trichothecene mycotoxins from Myrothecium roridum induce apoptosis of HepG-2 cells via caspase activation and disruption of mitochondrial membrane potential. Molecules 2016, 21, 781. [Google Scholar] [CrossRef]
- Anguiano-Sevilla, L.A.; Lugo-Cervantes, E.; Ordaz-Pichardo, C.; Rosas-Trigueros, J.L.; Jaramillo-Flores, M.E. Apoptosis induction of Agave lechuguilla torrey extract on human lung adenocarcinoma cells (SK-LU-1). Int. J. Mol. Sci. 2018, 19, 3765. [Google Scholar] [CrossRef]
- Wu, C.L.; Geng, X.P.; Wan, S.Y.; Hou, H.; Yu, F.Z.; Jia, B.L.; Wang, L. Cecropin-P17, an analog of Cecropin B, inhibits human hepatocellular carcinoma cell HepG-2 proliferation via regulation of ROS, caspase, bax, and bcl-2. J. Pept. Sci. 2015, 21, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018, 8, 15787. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Masumoto, J.; Tada, T.; Nomiyama, T.; Hongo, K.; Nakayama, J. Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin. Cancer Res. 2007, 13, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Kim, J.E.; Koh, E.K.; Song, S.H.; Kang, H.G.; Lee, Y.H.; Kim, H.D.; Hong, J.T.; Hwang, D.Y. Hepatoprotective effect of gallotannin-enriched extract isolated from gall on hydrogen peroxide-induced cytotoxicity in HepG2 cells. Pharmacogn. Mag. 2017, 13, S294–S300. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.L.; Ding, W.Q.; Zeng, X.N.; Kong, H.; Wang, H.; Xie, W.P. Deguelin induces the apoptosis of lung cancer cells through regulating a ROS driven Akt pathway. Cancer Cell Int. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Tang, Z.Y.; Chu, P.; Song, Y.L.; Yang, Y.; Sun, B.; Niu, M.Y.; Qaed, E.; Shopit, A.; Han, G.Z.; et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: Involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radical Biol. Med. 2018, 120, 228–238. [Google Scholar] [CrossRef]
- Li, N.; Xin, W.Y.; Yao, B.R.; Cong, W.; Wang, C.H.; Hou, G.G. N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Starkel, P.; De Saeger, C.; de Bravo, M.G.; Crespo, R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018, 199, 48–59. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ren, Z.; Chen, S.; Guo, X.Q.; Liu, F.; Guo, L.; Mei, N. ROS generation and JNK activation contribute to 4-methoxy-TEMPO-induced cytotoxicity, autophagy, and DNA damage in HepG2 cells. Arch. Toxicol. 2018, 92, 717–728. [Google Scholar] [CrossRef]
- Lin, C.L.; Lee, C.H.; Chen, C.M.; Cheng, C.W.; Chen, P.N.; Ying, T.H.; Hsieh, Y.H. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell. Physiol. Biochem. 2018, 46, 322–334. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Frohlich, K.U.; Wissing, S.; Buttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Han, B.J.; Li, W.; Jiang, G.B.; Lai, S.H.; Zhang, C.; Zeng, C.C.; Liu, Y.J. Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and bcl-2 family proteins. Oncol. Rep. 2015, 34, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, T.; Nagiah, S.; Tiloke, C.; Abdul, N.S.; Chuturgoon, A.A. Fusaric acid induces DNA damage and post-translational modifications of p53 in human hepatocellular carcinoma (HepG2) cells. J. Cell. Biochem. 2017, 118, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.F.; Wang, L.; He, Y.Q.; Wang, Z.B.; Xu, J.M.; Ma, H.L. Hydrolysis kinetics and antioxidant activity of collagen under simulated gastrointestinal digestion. J. Funct. Foods 2014, 11, 493–499. [Google Scholar] [CrossRef]



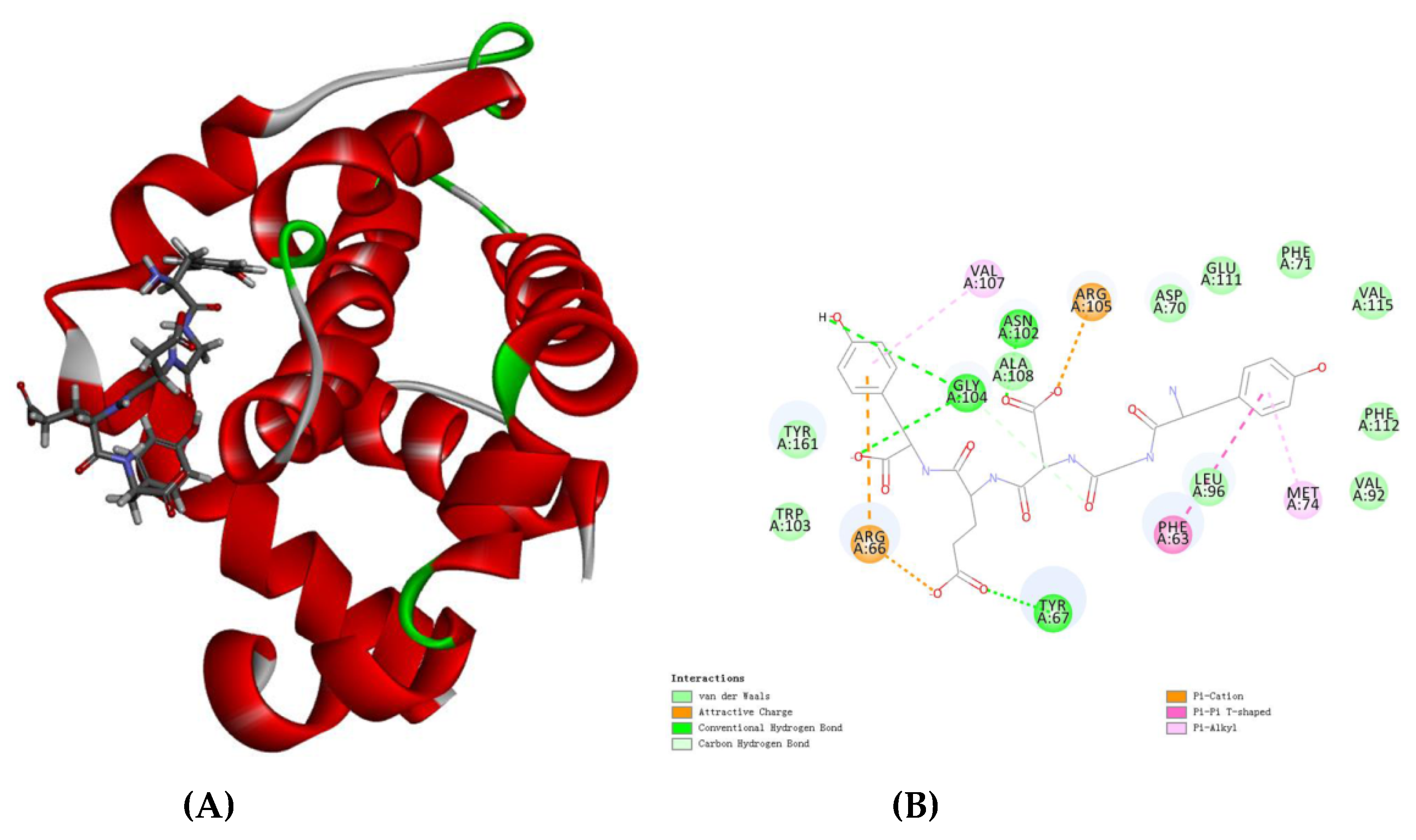
| Number | Receptor | Ligand | -CDOCKER_INTERACTION_ENERGY (kcal/mol) |
|---|---|---|---|
| 1 | 71.4138 | ||
| 2 | 66.7418 | ||
| 3 | 66.3802 | ||
| 4 | 65.9307 | ||
| 5 | bcl-2 | YGDEY | 65.7639 |
| 6 | 61.4377 | ||
| 7 | 61.2073 | ||
| 8 | 60.5086 | ||
| 9 | 60.1290 | ||
| 10 | 58.2131 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-F.; Gong, F.; Zhang, Y.Y.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.-J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients 2019, 11, 392. https://doi.org/10.3390/nu11020392
Chen M-F, Gong F, Zhang YY, Li C, Zhou C, Hong P, Sun S, Qian Z-J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients. 2019; 11(2):392. https://doi.org/10.3390/nu11020392
Chicago/Turabian StyleChen, Mei-Fang, Fang Gong, Yuan Yuan Zhang, Chengyong Li, Chunxia Zhou, Pengzhi Hong, Shengli Sun, and Zhong-Ji Qian. 2019. "Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways" Nutrients 11, no. 2: 392. https://doi.org/10.3390/nu11020392





