Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
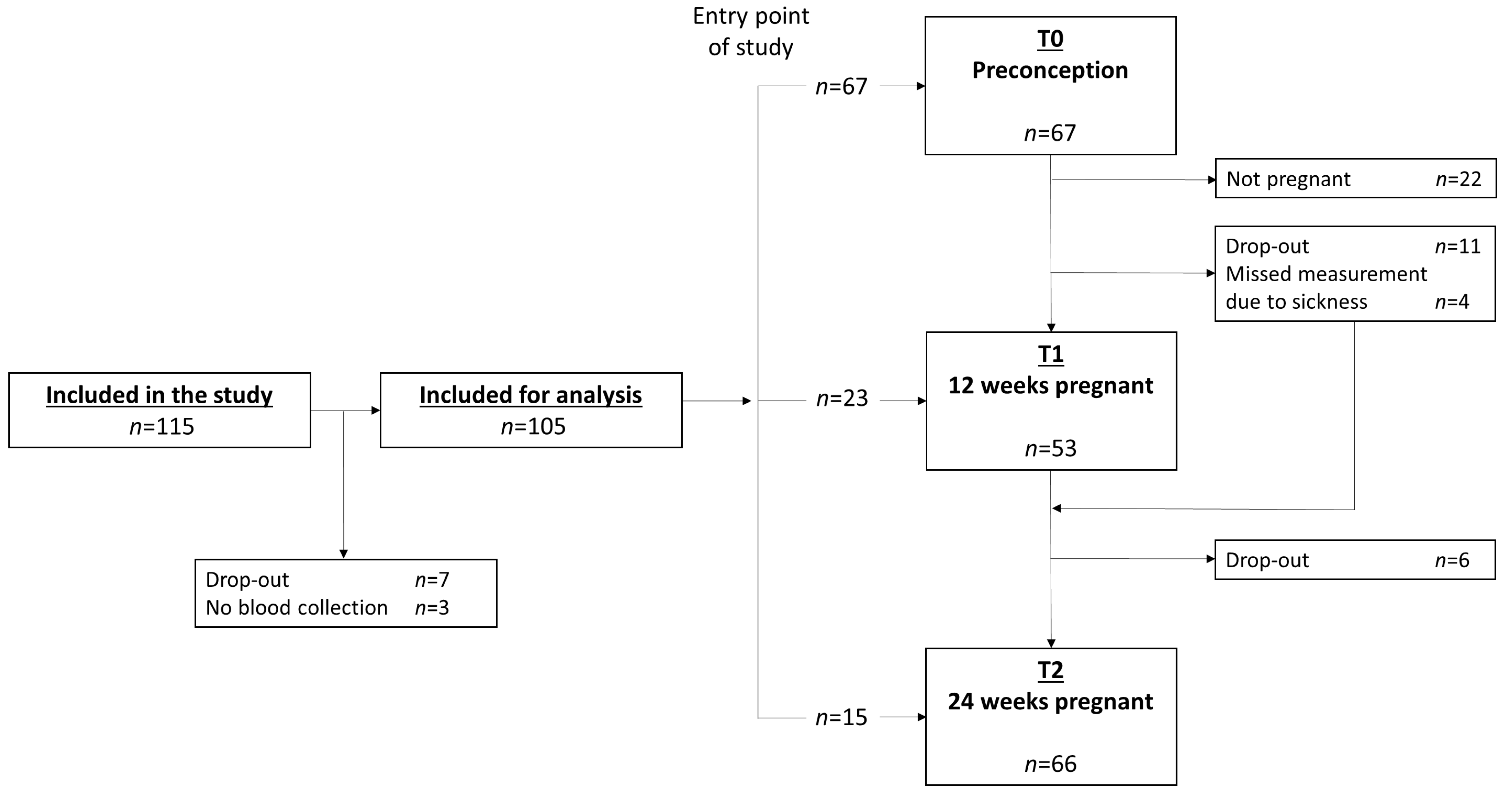
2.2. Analysis Sample
2.3. Dietary Assessment
2.3.1. Food Frequency Questionnaire (FFQ)
2.3.2. Supplement Use
2.3.3. Dutch Healthy Diet 2015 (DHD15) Index Score
2.4. Biochemical Analysis
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Changes in Micronutrient Intake, Micronutrient Status and Diet Quality Throughout Pregnancy
3.2. Associations of Micronutrient Intake, Micronutrient Status and Diet Quality with Glucose Tolerance During Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Berti, C.; Biesalski, H.K.; Gartner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in pregnancy: Current knowledge and unresolved questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in fetal growth and development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Bailey, L.B.; Pietrzik, K.; Shane, B.; Holzgreve, W. Micronutrients and women of reproductive potential: Required dietary intake and consequences of dietary deficiency or excess. Part ii—vitamin d, vitamin a, iron, zinc, iodine, essential fatty acids. J. Matern. Fetal Neonatal Med. 2011, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F.; Group, D.C.I. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef]
- Koivusalo, S.B.; Rono, K.; Klemetti, M.M.; Roine, R.P.; Lindstrom, J.; Erkkola, M.; Kaaja, R.J.; Poyhonen-Alho, M.; Tiitinen, A.; Huvinen, E.; et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: The finnish gestational diabetes prevention study (radiel): A randomized controlled trial. Diabetes Care 2016, 39, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the upbeat study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Donazar-Ezcurra, M.; Lopez-del Burgo, C.; Bes-Rastrollo, M. Primary prevention of gestational diabetes mellitus through nutritional factors: A systematic review. BMC Pregnancy Childbirth 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Song, Y.; Bertrand, K.A.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.E.; Mills, J.L.; Hu, F.B.; Zhang, C. Prepregnancy habitual intake of vitamin d from diet and supplements in relation to risk of gestational diabetes mellitus: A prospective cohort study. J. Diabetes 2018, 10, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: A systematic review of observational studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S. Dietary iron intake, iron status, and gestational diabetes. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 1672S–1680S. [Google Scholar] [CrossRef]
- Burris, H.H.; Camargo, C.A., Jr. Vitamin d and gestational diabetes mellitus. Curr. Diab. Rep. 2014, 14, 451. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.; Yajnik, C.S.; Fall, C.H. Low plasma vitamin b12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.; Shek, L.P.; Yap, F.K.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High folate and low vitamin b12 status during pregnancy is associated with gestational diabetes mellitus. Clin. Nutr. 2017, 37, 940–947. [Google Scholar] [CrossRef]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin b12 status among pregnant women in the UK and its association with obesity and gestational diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef]
- Cikot, R.J.; Steegers-Theunissen, R.P.; Thomas, C.M.; de Boo, T.M.; Merkus, H.M.; Steegers, E.A. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br. J. Nutr. 2001, 85, 49–58. [Google Scholar] [CrossRef]
- Hure, A.J.; Collins, C.E.; Smith, R. A longitudinal study of maternal folate and vitamin b12 status in pregnancy and postpartum, with the same infant markers at 6 months of age. Matern Child Health J 2012, 16, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Agger, A.O.; Nielsen, O.J. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan. Med. Bull. 1991, 38, 471–476. [Google Scholar]
- Milman, N.; Bergholt, T.; Byg, K.E.; Eriksen, L.; Hvas, A.M. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur. J. Haematol. 2007, 79, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miao, W.; Li, C.; Yu, X.; Shan, Z.; Guan, H.; Teng, W. Dynamic changes in serum 25-hydroxyvitamin d during pregnancy and lack of effect on thyroid parameters. PLoS ONE 2014, 9, e90161. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; de Vries, J.H. Self-reported energy intake by ffq compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; de Vries, J.H.; Meijboom, S.; Beekman, M.; de Craen, A.J.; Slagboom, P.E.; Feskens, E.J. Relative validity of the food frequency questionnaire used to assess dietary intake in the leiden longevity study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Verkleij-Hagoort, A.C.; de Vries, J.H.; Stegers, M.P.; Lindemans, J.; Ursem, N.T.; Steegers-Theunissen, R.P. Validation of the assessment of folate and vitamin b12 intake in women of reproductive age: The method of triads. Eur. J. Clin. Nutr. 2007, 61, 610–615. [Google Scholar] [CrossRef]
- NEVO-Tabel. Dutch Food Composition Table 2011/Version 3; National Institute for Publich Health: Bilthoven, The Netherlands, 2011. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin b6, Folate, Vitamin b12, Pantothenic Acid, Biotin, and Choline; Institute of Medicine: Washington, DC, USA, 1998. [Google Scholar]
- Health Council of the Netherlands. Dutch Dietary Guidelines 2015; Publication No. 2015/24; Health Council of the Netherlands: The Hague, The Netherlands, 2015.
- Looman, M.; Feskens, E.J.; de Rijk, M.; Meijboom, S.; Biesbroek, S.; Temme, E.H.; de Vries, J.; Geelen, A. Development and evaluation of the dutch healthy diet index 2015. Public Health Nutr. 2017, 20, 2289–2299. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Vaes, A.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Swart, K.M.; Ham, A.C.; van Dijk, S.C.; Enneman, A.W.; Sohl, E.; van Schoor, N.M. Relative importance of summer sun exposure, vitamin d intake, and genes to vitamin d status in dutch older adults: The b-proof study. J. Steroid Biochem. Mol. Biol. 2016, 164, 168–176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Cnaan, A.; Laird, N.M.; Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997, 16, 2349–2380. [Google Scholar] [CrossRef]
- Crozier, S.R.; Robinson, S.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Women’s dietary patterns change little from before to during pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: The australian longitudinal study on women’s health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef]
- Dubois, L.; Diasparra, M.; Bedard, B.; Colapinto, C.K.; Fontaine-Bisson, B.; Morisset, A.S.; Tremblay, R.E.; Fraser, W.D. Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in quebec, canada: The 3d cohort study (design, develop, discover). Am. J. Clin. Nutr. 2017, 106, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; van den Berg, C.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Klein Gunnewiek, J.M.T.; Balvers, M.G.J.; Leendertz-Eggen, C.L.; Wijnberger, L.D.E.; Feskens, E.J.M.; et al. Supplement use and dietary sources of folate, vitamin d, and n-3 fatty acids during preconception: The glimp2 study. Nutrients 2018, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.S. Nutrition in pregnancy. Nutr. Bull. 2006, 31, 28–59. [Google Scholar] [CrossRef]
- Blondin, J.H.; LoGiudice, J.A. Pregnant women’s knowledge and awareness of nutrition. Appl. Nurs. Res. 2018, 39, 167–174. [Google Scholar] [CrossRef]
- Stephenson, J.; Patel, D.; Barrett, G.; Howden, B.; Copas, A.; Ojukwu, O.; Pandya, P.; Shawe, J. How do women prepare for pregnancy? Preconception experiences of women attending antenatal services and views of health professionals. PLoS ONE 2014, 9, e103085. [Google Scholar] [CrossRef]
- Szwajcer, E.; Hiddink, G.J.; Maas, L.; Koelen, M.; van Woerkum, C. Nutrition awareness before and throughout different trimesters in pregnancy: A quantitative study among dutch women. Fam. Pract. 2012, 29 (Suppl. 1), i82–i88. [Google Scholar] [CrossRef]
- Livock, M.; Anderson, P.J.; Lewis, S.; Bowden, S.; Muggli, E.; Halliday, J. Maternal micronutrient consumption periconceptionally and during pregnancy: A prospective cohort study. Public Health Nutr. 2017, 20, 294–304. [Google Scholar] [CrossRef]
- McKenna, E.; Hure, A.; Perkins, A.; Gresham, E. Dietary supplement use during preconception: The australian longitudinal study on women’s health. Nutrients 2017, 9, 1119. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the medical research council vitamin study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Valera-Gran, D.; Garcia de la Hera, M.; Navarrete-Munoz, E.M.; Fernandez-Somoano, A.; Tardon, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef]
- Ortiz-Andrellucchi, A.; Doreste-Alonso, J.; Henriquez-Sanchez, P.; Cetin, I.; Serra-Majem, L. Dietary assessment methods for micronutrient intake in pregnant women: A systematic review. Br. J. Nutr. 2009, 102 (Suppl. 1), S64–86. [Google Scholar] [CrossRef]
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Coad, J.; Conlon, C. Iron deficiency in women: Assessment, causes and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 625–634. [Google Scholar] [CrossRef]
- Barebring, L.; Schoenmakers, I.; Glantz, A.; Hulthen, L.; Jagner, A.; Ellis, J.; Barebring, M.; Bullarbo, M.; Augustin, H. Vitamin d status during pregnancy in a multi-ethnic population-representative swedish cohort. Nutrients 2016, 8, 655. [Google Scholar] [CrossRef]
- Davies-Tuck, M.; Yim, C.; Knight, M.; Hodges, R.; Doery, J.C.; Wallace, E. Vitamin d testing in pregnancy: Does one size fit all? Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 149–155. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lucey, A.J.; Horgan, R.; Kenny, L.C.; Kiely, M. Impact of pregnancy on vitamin d status: A longitudinal study. Br. J. Nutr. 2014, 112, 1081–1087. [Google Scholar] [CrossRef]
- Park, H.; Wood, M.R.; Malysheva, O.V.; Jones, S.; Mehta, S.; Brannon, P.M.; Caudill, M.A. Placental vitamin d metabolism and its associations with circulating vitamin d metabolites in pregnant women. Am. J. Clin. Nutr. 2017, 106, 1439–1448. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin d effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Dawodu, A.; Wagner, C.L. Mother-child vitamin d deficiency: An international perspective. Arch. Dis. Child. 2007, 92, 737–740. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; McClain, D.; Manco, M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Crandall, J.P.; Wylie-Rosett, J.; Kabat, G.C.; Rohan, T.E.; Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta 2009, 1790, 671–681. [Google Scholar] [CrossRef]
- Faerch, K.; Borch-Johnsen, K.; Holst, J.J.; Vaag, A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: Does it matter for prevention and treatment of type 2 diabetes? Diabetologia 2009, 52, 1714–1723. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Towards an Optimal Use of Folic Acid; Publication no. 2008/02E; Health Council of the Netherlands: The Hague, The Netherlands, 2008.

| Total N = 105 | T0 N = 67 | T1 N = 53 | T2 N = 66 | p-Value a | |
|---|---|---|---|---|---|
| Gestational age | - | −13 (−25; −1) | 13 (12.3; 14.9) | 25 (24; 26) | <0.001 |
| Age (years) | 31.9 (29.8; 34.3) | 31.7 (29.0; 34.1) | 31.9 (29.7; 33.8) | 32.6 (30.7; 34.7) | 0.840 |
| Educational level (%) | 0.940 | ||||
| Low | 4.7 | 4.5 | 5.7 | 6.1 | |
| Moderate | 34.3 | 32.8 | 26.4 | 31.8 | |
| High | 61.0 | 62.7 | 67.9 | 62.1 | |
| Western ethnicity (%) | 94.3 | 95.5 | 98.1 | 95.5 | 0.697 |
| Smokers (%) | 9.5 | 11.9 | 1.9 | 1.5 | 0.012 |
| Parity (% ≥1 child) | 92.4 | 91.0 | 96.2 | 94.0 | 0.511 |
| Nausea during pregnancy (%) | - | - | 90.0 | 53.9 | - |
| Vomitting during pregnancy (%) | - | - | 32.0 | 20.0 | - |
| BMI (kg/m2) | 25.0 (22.6; 28.9) | 24.4 (22.2; 28.4) | 24.7 (22.5; 29.2) | 27.0 (24.6; 31.4) | 0.007 |
| Waist circumference (cm) | 88.5 (79.0; 95.5) | 84.8 (78.0; 92.5) | 88.4 (80.8; 96.4) | 98.5 (90.5; 106.0) | <0.001 |
| Hip circumference (cm) | 105.5 (100.0; 113.0) | 105.3 (98.5; 112.0) | 105.9 (99.0; 113.8) | 108.4 (101.4; 116.8) | 0.135 |
| Physical activity (MET min/week) | 1200 (750; 1860) | 1320 (870; 2029) | 853 (671; 1358) | 978 (390; 1459) | 0.0234 |
| Energy intake (kJ) | 8583 (6885; 9623) | 8583 (6713; 9462) | 8465 (7009; 9975) | 9189 (7432; 10541) | 0.133 |
| Carbohydrates (E%) | 46.5 (43.2; 49.7) | 45.4 (42.3; 48.6) | 46.5 (45.2; 50.3) | 48.1 (44.8; 50.3) | 0.030 |
| Fat (E%) | 35.4 (32.4; 37.7) | 36.2 (32.5; 38.8) | 35.2 (32.7; 37.5) | 34.1 (31.8; 38.2) | 0.343 |
| Protein (E%) | 15.1 (13.8; 16.7) | 15.6 (14.3; 16.9) | 14.9 (13.7; 16.6) | 14.5 (13.3; 15.8) | 0.045 |
| Alcohol (%) | <0.001 | ||||
| Abstainers | 80.1 | 55.2 | 96.2 | 92.4 | |
| 0–1 standard glass/day | 17.7 | 38.8 | 3.8 | 7.6 | |
| >1 stanard glass/day | 2.2 | 6.0 | 0.0 | 0.0 | |
| Blood sampling between December and April (%) | 23.1 | 17.9 | 26.4 | 25.8 | 0.448 |
| Outcome | Characteristic | β | 95% CI | p-Value |
|---|---|---|---|---|
| Folate status b (nmol/l) | Time—12 weeks pregnancy a | 3.14 | −1.33; 7.62 | 0.166 |
| Time—24 weeks pregnancy a | 0.20 | −3.69; 4.10 | 0.917 | |
| Supplemental folate intake (FE μg) | 0.026 | 0.020; 0.031 | <0.001 | |
| Dietary folate intake (FE μg) | −0.015 | −0.044; 0.014 | 0.297 | |
| Vitamin B6 status c (nmol/L) | Time—12 weeks pregnancy a | −1.70 | −9.10; 5.70 | 0.647 |
| Time—24 weeks pregnancy a | −10.3 | −17.5; −3.25 | 0.005 | |
| Supplemental vitamin B6 intake (mg) | 2.15 | 0.89; 3.43 | 0.001 | |
| Dietary vitamin B6 intake (mg) | 1.17 | −9.42; 11.8 | 0.826 | |
| Vitamin B12 status d (pmol/L) | Time—12 weeks pregnancy a | −55.2 | −74.0; −36.4 | <0.001 |
| Time—24 weeks pregnancy a | −91.1 | −109; −73.1 | <0.001 | |
| Supplemental vitamin B12 intake (μg) | 0.52 | −0.55; 1.59 | 0.336 | |
| Dietary vitamin B12 intake (μg) | 5.06 | −1.76; 11.9 | 0.143 | |
| 25(OH)D status e (nmol/L) | Time—12 weeks pregnancy a | 17.0 | 9.90; 24.0 | <0.001 |
| Time—24 weeks pregnancy a | 33.5 | 25.7; 41.3 | <0.001 | |
| Supplemental vitamin D intake (μg) | 1.03 | 0.51; 1.54 | <0.001 | |
| Dietary vitamin D intake (μg) | 6.66 | 3.78; 9.55 | <0.001 | |
| Ferritin status f (µg/L) | Time—12 weeks pregnancy a | −5.75 | −12.4; 0.88 | 0.088 |
| Time—24 weeks pregnancy a | −23.0 | −28.2; −17.8 | <0.001 | |
| Supplemental iron intake (mg) | 0.12 | −0.12; 0.36 | 0.328 | |
| Dietary iron intake (mg) | −0.22 | −1.45; 1.01 | 0.723 |
| Outcome | Model | Exposure Variable | β | 95% CI | p-Value |
|---|---|---|---|---|---|
| Fasting glucose | (1) b | Time—12 weeks pregnancy a | −0.252 | −0.386; −0.117 | 0.001 |
| Time—24 weeks pregnancy a | −0.425 | −0.570; −0.281 | <0.001 | ||
| (2) c | DHD-15 index score | −0.006 | −0.011; −0.001 | 0.017 | |
| (3) c | Total folate intake (FE μg) | 0.000 | −0.001; 0.001 | 0.929 | |
| Total vitamin B6 intake (mg) | −0.005 | −0.064; 0.055 | 0.869 | ||
| Total vitamin B12 intake (μg) | −0.014 | −0.221; 0.193 | 0.893 | ||
| Total vitamin D intake (μg) | 0.013 | −0.037; 0.062 | 0.612 | ||
| Total iron intake (mg) | −0.069 | −0.124; 0.015 | 0.013 | ||
| (4) d | Serum folate | −0.003 | −0.007; 0.002 | 0.261 | |
| Serum 25(OH)D | 0.001 | −0.001; 0.003 | 0.310 | ||
| Whole blood vitamin B6 | 0.000 | 0.000; 0.000 | 0.843 | ||
| Serum vitamin B12 | 0.000 | −0.001; 0.001 | 0.960 | ||
| Serum ferritin | 0.000 | −0.004; 0.004 | 0.966 | ||
| 2 h glucose | (1) b | Time—12 weeks pregnancy a | 0.375 | 0.041; 0.71 | 0.029 |
| Time—24 weeks pregnancy a | 1.079 | 0.653; 1.505 | <0.001 | ||
| (2) c | DHD-15 index score | −0.011 | −0.025; 0.002 | 0.100 | |
| (3) c | Total folate intake (FE μg) | −0.001 | −0.004; 0.002 | 0.590 | |
| Total vitamin B6 intake (mg) | −0.046 | −0.209; 0.118 | 0.580 | ||
| Total vitamin B12 intake (μg) | 0.121 | −0.444; 0.686 | 0.671 | ||
| Total vitamin D intake (μg) | 0.030 | −0.094; 0.154 | 0.635 | ||
| Total iron intake (mg) | −0.064 | −0.226; 0.097 | 0.429 | ||
| (4) d | Serum folate | −0.002 | −0.013; 0.008 | 0.656 | |
| Serum 25(OH)D | 0.000 | −0.007; 0.007 | 0.996 | ||
| Whole blood vitamin B6 | −0.001 | −0.003; 0.002 | 0.660 | ||
| Serum vitamin B12 | 0.000 | −0.002; 0.002 | 0.788 | ||
| Serum ferritin | 0.005 | −0.005; 0.014 | 0.325 | ||
| HbA1c | (1) b | Time—12 weeks pregnancy a | −1.963 | −2.571; −1.355 | <0.001 |
| Time—24 weeks pregnancy a | −3.360 | −4.004; −2.716 | <0.001 | ||
| (2) c | DHD-15 index score | −0.016 | −0.044; 0.012 | 0.248 | |
| (3) c | Total folate intake (FE μg) | 0.001 | −0.006; 0.007 | 0.847 | |
| Total vitamin B6 intake (mg) | −0.167 | −0.478; 0.144 | 0.288 | ||
| Total vitamin B12 intake (μg) | −0.138 | −1.231; 0.954 | 0.801 | ||
| Total vitamin D intake (μg) | 0.087 | −0.172; 0.346 | 0.505 | ||
| Total iron intake (mg) | −0.484 | −0.783; −0.184 | 0.002 | ||
| (4) d | Serum folate | 0.021 | −0.001; 0.044 | 0.066 | |
| Serum 25(OH)D | −0.006 | −0.018; 0.007 | 0.364 | ||
| Whole blood vitamin B6 | −0.008 | −0.023; 0.006 | 0.249 | ||
| Serum vitamin B12 | 0.002 | −0.003; 0.006 | 0.506 | ||
| Serum ferritin | −0.011 | −0.032; 0.010 | 0.288 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Looman, M.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Klein Gunnewiek, J.M.T.; Balvers, M.G.J.; Wijnberger, L.D.E.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy. Nutrients 2019, 11, 460. https://doi.org/10.3390/nu11020460
Looman M, Geelen A, Samlal RAK, Heijligenberg R, Klein Gunnewiek JMT, Balvers MGJ, Wijnberger LDE, Brouwer-Brolsma EM, Feskens EJM. Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy. Nutrients. 2019; 11(2):460. https://doi.org/10.3390/nu11020460
Chicago/Turabian StyleLooman, Moniek, Anouk Geelen, Rahul A. K. Samlal, Rik Heijligenberg, Jacqueline M. T. Klein Gunnewiek, Michiel G. J. Balvers, Lia D. E. Wijnberger, Elske M. Brouwer-Brolsma, and Edith J. M. Feskens. 2019. "Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy" Nutrients 11, no. 2: 460. https://doi.org/10.3390/nu11020460
APA StyleLooman, M., Geelen, A., Samlal, R. A. K., Heijligenberg, R., Klein Gunnewiek, J. M. T., Balvers, M. G. J., Wijnberger, L. D. E., Brouwer-Brolsma, E. M., & Feskens, E. J. M. (2019). Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy. Nutrients, 11(2), 460. https://doi.org/10.3390/nu11020460





