Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Knockout of DMT1 in Caco-2 Cells by Using Crispr Cas9
2.3. Measurement of Cell Viability
2.4. Measurement of Labile Iron with Phen Green SK in Wild-Type and DMT1 Knockout Caco-2 Cells
2.5. Live Fluorescence Imaging of Labile Iron in Wild-Type and DMT1 Knockout Caco-2 Cells
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Knockout of DMT1 in Caco-2 Cells by Using Crispr Cas9
3.2. Cell Viability after 2 h of Iron Treatment
3.3. Changes of Labile Iron after Treatment with Different Iron Sources
3.4. Live Cell Fluorescence Imaging of Labile Iron with Phen Green SK
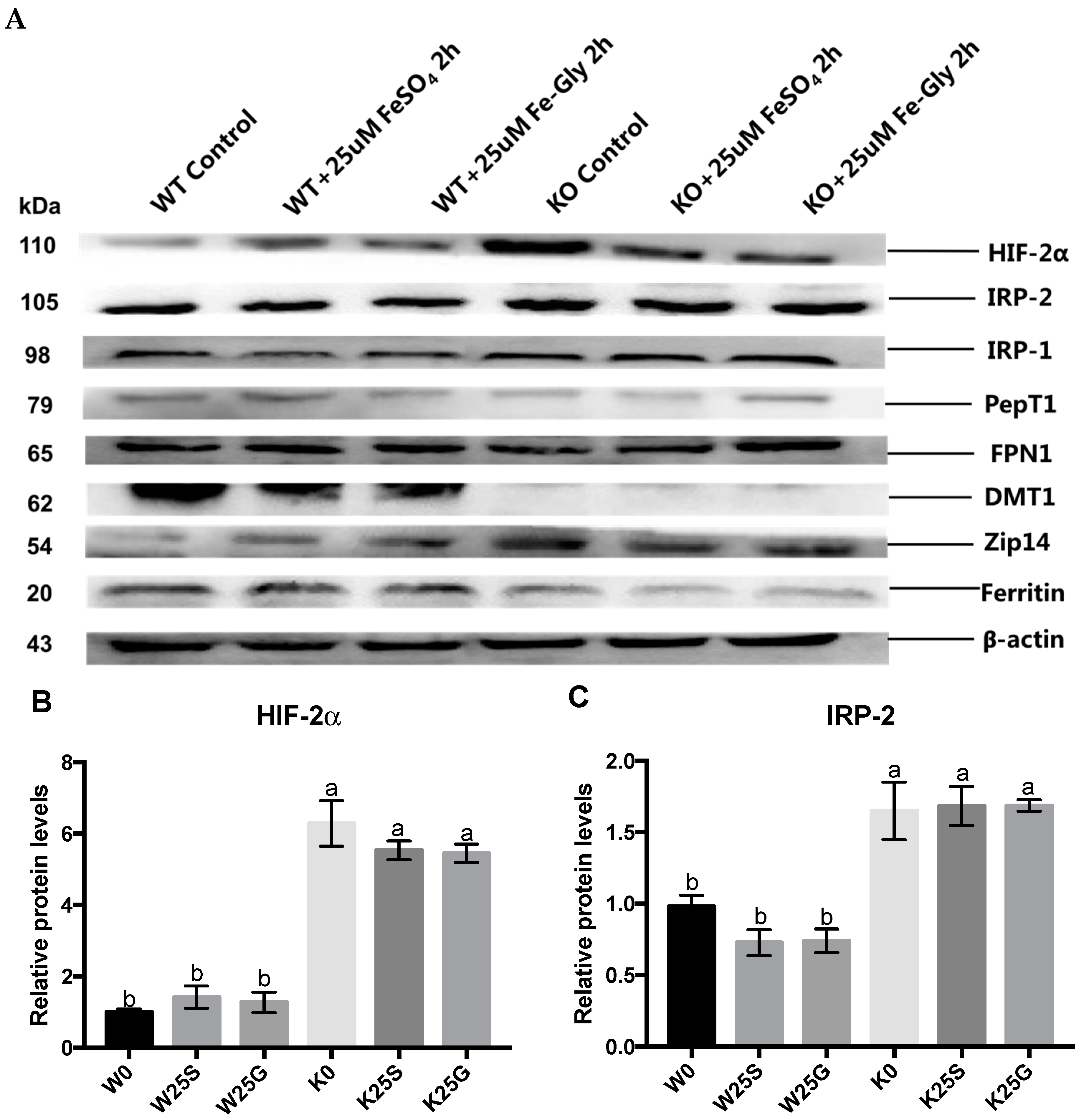
3.5. Expression of Iron Regulators and Transporters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matthias, W.H.; Martina, U.M.; Bruno, G.; Clara, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar]
- Martina, U.M.; Stefano, R.; Matthias, W.H.; Bruno, G. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Creech, B.L.; Spears, J.W.; Flowers, W.L.; Hill, G.M.; Lloyd, K.E.; Armstrong, T.A.; Engle, T.E. Effect of dietary trace mineral concentration and source (inorganic vs. chelated) on performance, mineral status, and fecal mineral excretion in pigs from weaning through finishing. J. Anim. Sci. 2004, 82, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Antonio, P.; Christine, E.; Eugenia, V.; Paul, S.; Florentine, H.; Susan, F. Mechanisms of iron uptake from ferric phosphate nanoparticles in human intestinal Caco-2 cells. Nutrients 2017, 9, 359–372. [Google Scholar]
- Feng, J.; Ma, W.Q.; Xu, Z.R.; He, J.X.; Wang, Y.Z.; Liu, J.X. The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim. Feed Sci. Technol. 2009, 150, 106–113. [Google Scholar] [CrossRef]
- Kwiecien, M.; Samolinska, W.; Bujanowicz-Haras, B. Effects of iron–glycine chelate on growth, carcass characteristic, liver mineral concentrations and haematological and biochemical blood parameters in broilers. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals fromconsuming food grown on sewage irrigated soils and food chain transfer. Ecotox. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, W.Q.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of iron glycine chelate on growth, haematological and immunological characteristics in weaning pigs. Anim. Feed Sci. Technol. 2007, 134, 261–272. [Google Scholar] [CrossRef]
- Ma, W.Q.; Sun, H.; Zhou, Y.; Wu, J.; Feng, J. Effects of iron glycine chelate on growth, tissue mineral concentrations, fecal mineral excretion, and liver antioxidant enzyme activities in broilers. Biol. Trace Elem. Res. 2012, 149, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, X.; Li, S.; Fang, S.; Feng, J. Heme and non-heme iron on growth performances, blood parameters, tissue mineral concentration, and intestinal morphology of weanling pigs. Biol. Trace Elem. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Huang, W.; Chiou, P.W. Bioavailability of iron from amino acid complex in weanling pigs. Anim. Feed Sci. Technol. 2000, 86, 39–52. [Google Scholar] [CrossRef]
- Ashmead, H.D. Comparative Intestinal Absorption and subsequent metabolism of metal amino acid chelates and inorganic metal salts. Biol. Trace Elem. Res. 1993, 445, 306–319. [Google Scholar]
- Liao, Z.C.; Guan, W.T.; Chen, F.; Hou, D.X.; Wang, C.X.; Lv, Y.T.; Qiao, H.Z.; Chen, J.; Han, J.H. Ferrous bisglycinate increased iron transportation through DMT1 and PepT1 in pig intestinal epithelial cells compared with ferrous sulphate. J. Anim. Feed Sci. 2014, 23, 153–159. [Google Scholar] [CrossRef]
- Fernando, P.; Manuel, O.; Eva, H.; Dora, I.M.; Miguel, A.; Angélica, L.; Virginia, G. Iron bis-glycine chelate competes for the nonheme-iron absorption pathway. Am. J. Clin. Nutr. 2002, 76, 577–581. [Google Scholar]
- Yeung, C.K.; Glahn, R.P.; Miller, D.D. Inhibition of Iron Uptake from Iron Salts and Chelates by Divalent Metal Cations in Intestinal Epithelial Cells. J. Agric. Food Chem. 2005, 53, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Fang, S.; Yue, M.; Zhang, Y.; Feng, J. Kinetics absorption characteristics of ferrous glycinate in SD rats and its impact on the relevant transport protein. Biol. Trace Elem. Res. 2014, 158, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Fang, S.; Hu, Q.; Huang, D.; Feng, J. Digital gene expression profiling analysis of duodenum transcriptomes in SD rats administered ferrous sulfate or ferrous glycine chelate by gavage. Sci. Rep. 2016, 6, 37923. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.D.; Trenor, C.C.; Su, M.A.; Foernzler, D.; Beier, D.R.; Dietrich, W.F.; Andrews, N.C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997, 16, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Gasche, C. Iron sensing and signalling. Gut 2012, 61, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.C.; Twan, L.; Stefano, R. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar]
- Salahudeen, A.A.; Thompson, J.W.; Ruiz, J.C.; Ma, H.W.; Kinch, L.N.; Li, Q.; Grishin, N.V.; Bruick, R.K. An E3 ligase possessing an iron- responsive hemerythrin domain is a regulator of iron homeostasis. Science 2009, 326, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, A.A.; Zumbrennen, K.B.; Huang, X.; Powers, D.N.; Durazo, A.; Sun, D.; Bhaskaran, N.; Persson, A.; Uhlen, M.; Sangfelt, O.; et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 2009, 326, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Ferring-Appel, D.; Kaden, S.; Gröne, H.J.; Hentze, M.W. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 2008, 7, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Galy, B.; Schwanhaeusser, B.; Blake, J.; Bähr-Ivacevic, T.; Benes, V.; Selbach, M.; Muckenthaler, M.U.; Hentze, M.W. Iron regulatory protein-1 and -2: Transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood 2011, 118, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Nizzi, C.P.; Chang, Y.I.; Deck, K.M.; Schmidt, P.J.; Galy, B.; Damnernsawad, A.; Broman, A.T.; Kendziorski, C.; Hentze, M.W.; et al. The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 2013, 17, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.R.; Taylor, M.; Xue, X.; Ramakrishnan, S.K.; Martin, A.; Xie, L.; Bredell, B.X.; Gardenghi, S.; Rivella, S.; Shah, Y.M. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc. Natl. Acad. Sci. USA 2013, 110, 4922–4930. [Google Scholar] [CrossRef] [PubMed]
- Anjali, N.; Julio, C.R.; Poorna, S.; Sudipa, G.-R.; Ruth, A.S.; Timothy, L.S.; Richard, K.B.; Caroline, C.P. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011, 14, 647–657. [Google Scholar]
- Shah, Y.M.; Matsubara, T.; Ito, S.; Yim, S.H.; Gonzalez, F.J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009, 9, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005, 579, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, N.; Knutson, M.D.; Enns, C.A. The hereditary hemo-chromatosis protein, HFE, inhibits iron uptake via down-regulation of Zip14 in HepG2 cells. J. Biol. Chem. 2008, 283, 21462–21468. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; Mackenzie, B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Supak, J.; Chia-Yu, W.; Richard, C.; Wei, Z.; Alan, C.; Thomas, B.; Jae-Sung, K.; Shintaro, H.; Toshiyuki, F.; Mitchell, D.K. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 2015, 22, 138–150. [Google Scholar]
- Supak, J.; Adenike, A.; Elizabeth, P.; Ralf, W.; Shintaro, H.; Toshiyuki, F.; Genesys, G.; Jessica, S.; Armin, G.; Christopher, J.; et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 1769–1778. [Google Scholar]
- Aydemir, T.B.; Kim, M.; Kim, J.; Colon-Perez, L.M.; Banan, G.; Mareci, T.H.; Febo, M.; Cousins, R.J. Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J. Neurosci. 2017, 37, 5996–6006. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell- surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Chen, L.; Ding, H.; Zhao, Y.; Feng, J. Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells. Nutrients 2019, 11, 485. https://doi.org/10.3390/nu11030485
Yu X, Chen L, Ding H, Zhao Y, Feng J. Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells. Nutrients. 2019; 11(3):485. https://doi.org/10.3390/nu11030485
Chicago/Turabian StyleYu, Xiaonan, Lingjun Chen, Haoxuan Ding, Yang Zhao, and Jie Feng. 2019. "Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells" Nutrients 11, no. 3: 485. https://doi.org/10.3390/nu11030485
APA StyleYu, X., Chen, L., Ding, H., Zhao, Y., & Feng, J. (2019). Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells. Nutrients, 11(3), 485. https://doi.org/10.3390/nu11030485



