Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Diet
2.4. Oral Glucose Tolerance Test
2.5. Cardiovascular Measurements
2.5.1. Blood Pressure and Heart Rate
2.5.2. Flow-Mediated Dilation
2.5.3. Extracranial Cerebral Blood Flow
2.5.4. Arterial Stiffness
2.6. Plasma Analysis
2.6.1. Glucose and Insulin
2.6.2. EMPs
2.7. Data Analyses
3. Results
3.1. Dietary Intervention
3.2. Oral Glucose Tolerance Test
3.3. Cardiovascular Measures
3.3.1. Blood Pressure and Heart Rate
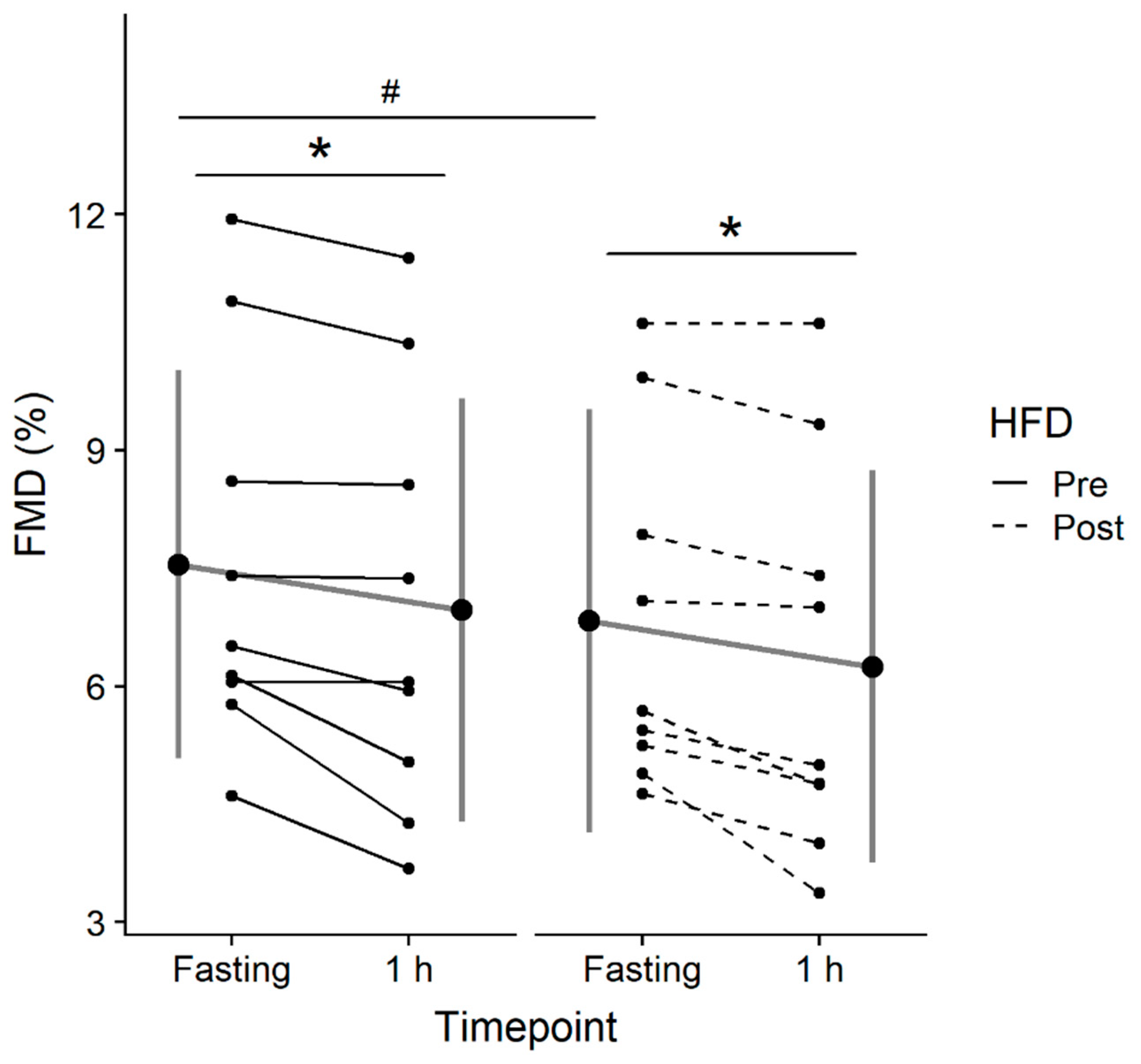
3.3.2. FMD
3.3.3. Extracranial Cerebral Blood Flow
3.3.4. Arterial Stiffness
3.4. EMPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kannel, W.B.; McGee, D.L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care 1979, 2, 120–126. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bell, D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Borch-Johnsen, K.; Neil, A.; Balkau, B.; Larsen, S.; Nissinen, A.; Pekkanen, J.; Tuomilehto, J.; Jousilahti, P.; Lindstrom, J.; Pyorala, M. Glucose tolerance and cardiovascular mortality-Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar]
- Williams, S.B.; Goldfine, A.B.; Timimi, F.K.; Ting, H.H.; Roddy, M.-A.; Simonson, D.C.; Creager, M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Da Ros, R.; Motz, E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: Effects of short-and long-term simvastatin treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Karbach, S.; Jansen, T.; Horke, S.; Heeren, T.; Scholz, A.; Coldewey, M.; Karpi, A.; Hausding, M.; Kröller-Schön, S.; Oelze, M. Hyperglycemia and oxidative stress in cultured endothelial cells—A comparison of primary endothelial cells with an immortalized endothelial cell line. J. Diabetes Complicat. 2012, 26, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Jones, H.; Thijssen, D.; Cable, N.; Atkinson, G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 2011, 57, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.N.; Boulanger, C.M.; Simon, A.; Dignat-George, F.; Freyssinet, J.-M.; Tedgui, A. Endothelial microparticles in diseases. Cell Tissue Res. 2009, 335, 143. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.T.; Padilla, J.; Boyle, L.J.; Credeur, D.P.; Laughlin, M.H.; Fadel, P.J. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 2013, 61, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Windhauser, M.M.; Rood, J.C.; de la Bretonne, J.A. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism 1998, 47, 1520–1524. [Google Scholar] [CrossRef]
- Wan, Z.; Durrer, C.; Mah, D.; Simtchouk, S.; Robinson, E.; Little, J.P. Reduction of AMPK activity and altered MAPKs signalling in peripheral blood mononuclear cells in response to acute glucose ingestion following a short-term high fat diet in young healthy men. Metabolism 2014, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Ketonen, J.; Pilvi, T.; Mervaala, E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessel. 2010, 25, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.R.; Haley-Zitlin, V.; Rosenberger, D.S.; Granholm, A.-C. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr. Neurosci. 2014, 17, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Durrer, C.; Mah, D.; Simtchouk, S.; Little, J.P. One-week high-fat diet leads to reduced toll-like receptor 2 expression and function in young healthy men. Nutr. Res. 2014, 34, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Black, M.A.; Cable, N.T.; Thijssen, D.H.; Green, D.J. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 2008, 51, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Woodman, R.; Playford, D.; Watts, G.; Cheetham, C.; Reed, C.; Taylor, R.; Puddey, I.; Beilin, L.; Burke, V.; Mori, T. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J. Appl. Physiol. 2001, 91, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Tschakovsky, M.E. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J. Appl. Physiol. 2007, 102, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Tschakovsky, M.E. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol. 2005, 568, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Batterham, A.M.; Black, M.A.; Cable, N.T.; Hopkins, N.D.; Dawson, E.A.; Thijssen, D.H.; Jones, H.; Tinken, T.M.; Green, D.J. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J. Appl. Physiol. 2009, 107, 1893–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willie, C.; Macleod, D.; Shaw, A.; Smith, K.; Tzeng, Y.; Eves, N.; Ikeda, K.; Graham, J.; Lewis, N.; Day, T. Regional brain blood flow in man during acute changes in arterial blood gases. J. Physiol. 2012, 590, 3261–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willie, C.K.; Smith, K.J.; Day, T.A.; Ray, L.A.; Lewis, N.C.; Bakker, A.; Macleod, D.B.; Ainslie, P.N. Regional cerebral blood flow in humans at high altitude: gradual ascent and 2 week at 5050 m. J. Appl. Physiol. 2013, 116, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, T.; Ammer, M.; Rammer, M.; Adji, A.; O’rourke, M.F.; Wassertheurer, S.; Rosenkranz, S.; Eber, B. Noninvasive determination of carotid–femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J. Hypertens. 2009, 27, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Durrer, C.; Robinson, E.; Wan, Z.; Martinez, N.; Hummel, M.L.; Jenkins, N.T.; Kilpatrick, M.W.; Little, J.P. Differential impact of acute high-intensity exercise on circulating endothelial microparticles and insulin resistance between overweight/obese males and females. PLoS ONE 2015, 10, e0115860. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Matos, M.E.; Volek, J.S.; Bruno, R.S. Postprandial Hyperglycemia Impairs Vascular Endothelial Function in Healthy Men by Inducing Lipid Peroxidation and Increasing Asymmetric Dimethylarginine: Arginine–3. J. Nutr. 2011, 141, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; San Martin, A.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 2000, 35, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kobayasi, R.; Akamine, E.H.; Davel, A.P.; Rodrigues, M.A.; Carvalho, C.R.; Rossoni, L.V. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J. Hypertens. 2010, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ballard, K.D.; Silvestre, R.; Judelson, D.A.; Quann, E.E.; Forsythe, C.E.; Fernandez, M.L.; Kraemer, W.J. Effects of dietary carbohydrate restriction versus low-fat diet on flow-mediated dilation. Metabolism 2009, 58, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.A.; Jurva, J.W.; Syed, A.Q.; Syed, A.Q.; Kulinski, J.P.; Pleuss, J.; Hoffmann, R.G.; Gutterman, D.D. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension 2008, 51, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Brinkworth, G.D.; Clifton, P.M. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br. J. Nutr. 2007, 98, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Bassenge, E.; Kim, K.-B.; Kim, Y.-N.; Kim, K.-S.; Lee, H.-J.; Moon, K.-C.; Lee, M.-S.; Park, K.-Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.; Thorpe, J.; Testa, R.; Bonfigli, A.R.; Giugliano, D. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res. Clin. Pract. 2008, 82, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Thom, N.; Early, A.; Hunt, B.; Harris, R.; Herring, M. Eating and arterial endothelial function: A meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes. Rev. 2016, 17, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Hargett, L.A.; Bauer, N.N. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm. Circ. 2013, 3, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, F.F.; Nickenig, G.; Werner, N. Microparticles–messengers of biological information. J. Cell. Mol. Med. 2010, 14, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Weber, C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Rautou, P.-E.; Vion, A.-C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, M.; Murphy, R.P.; O’Connor, P.L.; O’Gorman, D.J.; McCaffrey, N.; Cummins, P.M.; Moyna, N.M. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur. J. Appl. Physiol. 2009, 106, 555. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Kanjwal, Y.; Mohanty, P.; Rao, S.; Sung, B.H.; Wilson, M.F.; Dandona, P. Insulin-induced vasodilatation of internal carotid artery. Metabolism 1999, 48, 1470–1473. [Google Scholar] [CrossRef]
- Jansen, R.W.; Penterman, B.J.; Van Lier, H.J.; Hoefnagels, W.H. Blood pressure reduction after oral glucose loading and its relation to age, blood pressure and insulin. Am. J. Cardiol. 1987, 60, 1087–1091. [Google Scholar] [CrossRef]

| Variable | Pre-HFD | Post-HFD |
|---|---|---|
| Energy intake (kcal/day) | 2584 ± 456 | 2417 ± 267 |
| Fat intake (% daily energy) | 37 ± 6 | 71 ± 0.6 |
| Carbohydrate intake (% daily intake) | 46 ± 9 | 11 ± 0.9 |
| Protein intake (% daily intake) | 17 ± 3 | 18 ± 0.6 |
| kcal, kilocalories |
| Pre-HFD | Post-HFD | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Variable | Fasting | 1 h | Fasting | 1 h | Time | Condition | Interaction |
| Systolic Blood Pressure (mmHg) | 120.2 ± 10 | 116.7 ± 11.6 | 117.3 ± 9 | 113.8 ± 7.9 | 0.07 | 0.14 | 1 |
| Diastolic Blood Pressure (mmHg) | 72.2 ± 7.6 | 67.8 ± 8.5 | 66.9 ± 8.5 | 65.3 ± 7.2 | 0.14 | 0.06 | 0.46 |
| Mean Arterial Pressure (mmHg) | 88.2 ± 5.4 | 84.1 ± 6.5 | 83.7 ± 7.8 | 81.5 ± 6.6 | 0.04 | 0.03 | 0.52 |
| Resting Heart Rate (BPM) | 60.0 ± 9.5 | 59.7 ± 12.2 | 56.7 ± 7.7 | 60.8 ± 8.9 | 0.11 | 0.35 | 0.05 |
| Central PWV (m/s) | 5.7 ± 0.5 | 5.8 ± 0.4 | 5.6 ± 0.8 | 5.4 ± 0.5 | 0.59 | 0.16 | 0.34 |
| Peripheral PWV (m/s) | 7.8 ± 1.5 | 7.4 ± 1.2 | 7.1 ± 0.6 | 7.2 ± 1.1 | 0.53 | 0.03 | 0.26 |
| CCA Diameter (cm) | 0.68 ± 0.03 | 0.70 ± 0.03 | 0.69 ± 0.04 | 0.70 ± 0.04 | 0.04 | 0.96 | 0.71 |
| CCA Velocity (cm/s) | 35.52 ± 6.82 | 35.06 ± 4.20 | 34.27 ± 3.86 | 33.48 ± 4.97 | 0.61 | 0.22 | 0.94 |
| CCA Flow (mL/s) | 6.70 ± 1.22 | 6.90 ± 0.80 | 6.54 ± 1.07 | 6.50 ± 1.06 | 0.53 | 0.23 | 0.68 |
| ICA Diameter (cm) | 0.53 ± 0.03 | 0.55 ± 0.04 | 0.52 ± 0.04 | 0.54 ± 0.03 | 0.01 | 0.06 | 0.53 |
| ICA Velocity (cm/s) | 38.61 ± 7.62 | 35.19 ± 3.80 | 38.11 ± 7.79 | 34.86 ± 4.87 | 0.02 | 0.75 | 0.95 |
| ICA Flow (mL/s) | 4.35 ± 1.09 | 4.15 ± 0.82 | 4.00 ± 0.87 | 3.97 ± 0.62 | 0.41 | 0.06 | 0.53 |
| VA Diameter (cm) | 0.40 ± 0.04 | 0.42 ± 0.04 | 0.39 ± 0.04 | 0.41 ± 0.04 | 0.005 | 0.10 | 0.38 |
| VA Velocity (cm/s) | 19.86 ± 4.53 | 19.40 ± 3.94 | 20.33 ± 3.26 | 20.02 ± 3.50 | 0.41 | 0.24 | 0.87 |
| VA Flow (mL/s) | 1.32 ± 0.51 | 1.37 ± 0.53 | 1.26 ± 0.39 | 1.36 ± 0.40 | 0.16 | 0.46 | 0.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durrer, C.; Lewis, N.; Wan, Z.; Ainslie, P.N.; Jenkins, N.T.; Little, J.P. Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis. Nutrients 2019, 11, 489. https://doi.org/10.3390/nu11030489
Durrer C, Lewis N, Wan Z, Ainslie PN, Jenkins NT, Little JP. Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis. Nutrients. 2019; 11(3):489. https://doi.org/10.3390/nu11030489
Chicago/Turabian StyleDurrer, Cody, Nia Lewis, Zhongxiao Wan, Philip N. Ainslie, Nathan T. Jenkins, and Jonathan P. Little. 2019. "Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis" Nutrients 11, no. 3: 489. https://doi.org/10.3390/nu11030489
APA StyleDurrer, C., Lewis, N., Wan, Z., Ainslie, P. N., Jenkins, N. T., & Little, J. P. (2019). Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis. Nutrients, 11(3), 489. https://doi.org/10.3390/nu11030489





