Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Green Coffee Bean Extract (GCE)
2.3. Mice, Treatments, and Sample Collection
2.4. Atheroprotective Effect Assessment
2.5. Blood and Urine Biochemistry
2.6. Liver and Adipose Tissue Analyses
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. GCE Chemical Characterization
3.2. GCE Treatment is not Atheroprotective but Improves Metabolic Dysregulation and Insulin Resistance in HFD-Fed ApoE-/- Mice
3.3. Treatment of ApoE-/- Mice with GCE Attenuates HFD-Induced Weight Gain and Adiposity Increase
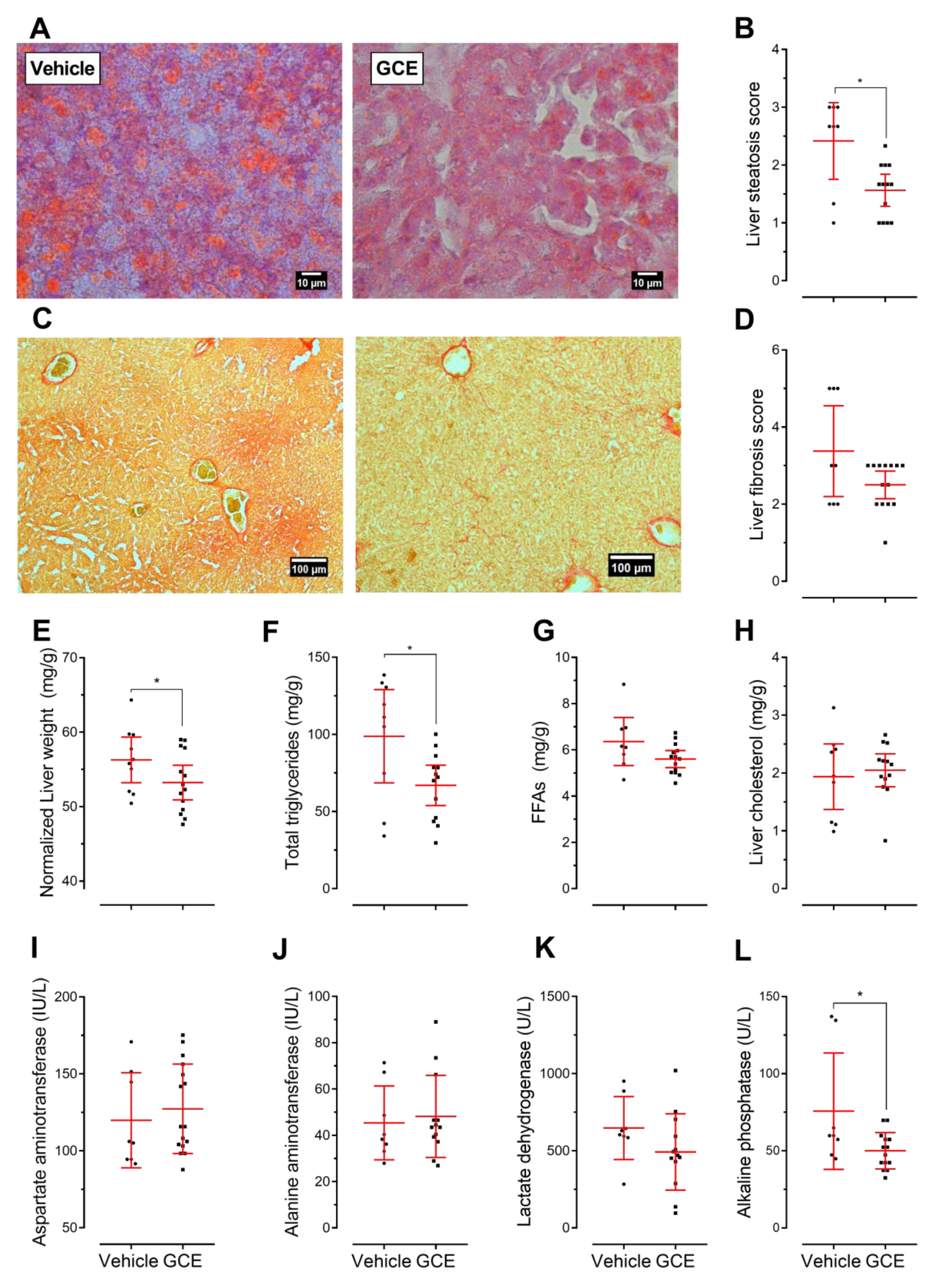
3.4. GCE Improves Nonalcoholic Fatty Liver Disease (NAFLD) and Fibrosis
3.5. In Vivo Protective Effects of GCE are Associated with Immunomodulation
3.6. GCE Contributed Changes in the Gut Microbiotas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998, 1, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholls, S.G. Standards and classification: A perspective on the ‘obesity epidemic’. Soc. Sci. Med. 2013, 87, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Murray, C. The state of global health in 2014. Science 2014, 345, 1275–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampuero, J.; Gallego-Durán, R.; Romero-Gómez, M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: Meta-analysis. Rev. Esp. Enferm. Dig. 2015, 107, 10–16. [Google Scholar] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.; Bugianesi, E.; Gastaldelli, A. Non-Alcoholic Fatty Liver Disease (NAFLD) and Its Connection with Insulin Resistance, Dyslipidemia, Atherosclerosis and Coronary Heart Disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Hansson, G.K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Vinje, S.; Stroes, E.; Nieuwdorp, M.; Hazen, S.L. The gut microbiome as novel cardio-metabolic target: the time has come! Eur. Heart J. 2014, 35, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Sarwer, D.B. Diet, Exercise, and Behavior Therapy in the Treatment of Obesity and Metabolic Syndrome. In Metabolic Syndrome; Springer International Publishing: Salmon Tower Building, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Do Coffee Polyphenols Have a Preventive Action on Metabolic Syndrome Associated Endothelial Dysfunctions? An Assessment of the Current Evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Marventano, S.; Antoci, M.; Cagnetti, A.; Castorina, G.; Galvano, F.; Marranzano, M.; Mistretta, A. Coffee and metabolic impairment: An updated review of epidemiological studies. NFS J. 2016, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puerta Quintero, G.I. Composición química de una taza de café. Av. técnicos Cenicafé 2011, 414, 1–12. [Google Scholar]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Salinardi, T.; Herron-Rubin, K.; Black, R.M. A Weight-Loss Diet Including Coffee-Derived Mannooligosaccharides Enhances Adipose Tissue Loss in Overweight Men but Not Women. Obesity 2012, 20, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lukitasari, M.; Nugroho, D.A.; Rohman, M.S.; Nugrahini, N.I.P.; Sardjono, T.W. Light-roasted Green Coffee Extract improved adiponectin, insulin resistance, and metabolic profile of metabolic syndrome rat model. Asian J. Pharm. Clin. Res. 2017, 10, 279. [Google Scholar] [CrossRef]
- Choi, B.K.; Park, S.B.; Lee, D.R.; Lee, H.J.; Jin, Y.Y.; Yang, S.H.; Suh, J.W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2016, 9, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Gniechwitz, D.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Dietary Fiber from Coffee Beverage: Degradation by Human Fecal Microbiota. J. Agric. Food Chem. 2007, 55, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.J.; Ellacott, K.L.J.; King, V.L.; Hasty, A.H. Mouse models of the metabolic syndrome. Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.C.; Proctor, S.D. Small animal models of cardiovascular disease: Tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc. Pathol. 2006, 15, 318–330. [Google Scholar] [CrossRef] [PubMed]
- King, V.L.; Hatch, N.W.; Chan, H.-W.; de Beer, M.C.; de Beer, F.C.; Tannock, L.R. A Murine Model of Obesity with Accelerated Atherosclerosis. Obesity 2010, 18, 35–41. [Google Scholar] [CrossRef] [PubMed]
- German Institute for Standardization DIN. “DIN 10767 Analysis of Coffee and Coffee Products—Determination of Chlorogenic Acids Content in Roasted Coffee and Soluble Coffee—HPLC Method”. 2015. Available online: https://www.din.de/en/wdc-beuth:din21:234602401 (accessed on 26 January 2019).
- International Organization for Standardization ISO. ISO 20481:2008—Coffee and coffee products—Determination of the caffeine content using high performance liquid chromatography (HPLC)—Reference method. 2008. Available online: https://www.iso.org/standard/34185.html (accessed on 26 January 2019).
- Paigen, B.; Holmes, P.A.; Mitchell, D.; Albee, D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis 1987, 64, 215–221. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rabasa-Lhoret, R.; Bastard, J.-P.; Jan, V.; Ducluzeau, P.-H.; Andreelli, F.; Guebre, F.; Bruzeau, J.; Louche-Pellissier, C.; MaÎtrepierre, C.; Peyrat, J.; et al. Modified Quantitative Insulin Sensitivity Check Index Is Better Correlated to Hyperinsulinemic Glucose Clamp than Other Fasting-Based Index of Insulin Sensitivity in Different Insulin-Resistant States. J. Clin. Endocrinol. Metab. 2003, 88, 4917–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaceau, J.P.; Chambers, E.E.; Fountain, K.J. Waters Application Notes; Waters Corporation: Milford, MA, USA, 2014; pp. 1–7. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Package “vegan” Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 26 January 2019).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Qvalue: Q-Value Estimation for False Discovery Rate Control 2015. Available online: http://master.bioconductor.org/packages/release/bioc/manuals/qvalue/man/qvalue.pdf (accessed on 26 January 2019).
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: a R-based tool for the rapid prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene marker gene sequences. bioRxiv 2018. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Waśkiewicz, A.; Stępień, Ł.; Stanisz, E. Positive and negative aspects of green coffee consumption—antioxidant activity versus mycotoxins. J. Sci. Food Agric. 2017, 97, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Pacífico, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Fungal contamination in green coffee beans samples: A public health concern. J. Toxicol. Environ. Heal. Part A 2017, 80, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, S.; Park, T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evidence-Based Complement. Altern. Med. eCAM 2014, 2014, 718379. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Varghese, M.; Wang, J.; Zhao, W.; Chen, F.; Knable, L.A.; Ferruzzi, M.; Pasinetti, G.M. Dietary supplementation with decaffeinated green coffee improves diet-induced insulin resistance and brain energy metabolism in mice. Nutr. Neurosci. 2012, 15, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luan, H.; Zhang, X.X.; Wang, S.; Zhang, X.X.; Sun, X.; Guo, P. Chlorogenic Acid Protects against Atherosclerosis in ApoE?/? Mice and Promotes Cholesterol Efflux from RAW264.7 Macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef] [PubMed]
- Schierwagen, R.; Maybüchen, L.; Zimmer, S.; Hittatiya, K.; Bäck, C.; Klein, S.; Uschner, F.E.; Reul, W.; Boor, P.; Nickenig, G.; et al. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Karschin, J.; Lagerpusch, M.; Enderle, J.; Eggeling, B.; Müller, M.J.; Bosy-Westphal, A. Endocrine Determinants of Changes in Insulin Sensitivity and Insulin Secretion during a Weight Cycle in Healthy Men. PLoS ONE 2015, 10, e0117865. [Google Scholar] [CrossRef] [PubMed]
- Kocabay, G.; Telci, A.; Tutuncu, Y.; Tiryaki, B.; Ozel, S.; Cevikbas, U.; Okten, A.; Satman, I. Alkaline phosphatase: Can it be considered as an indicator of liver fibrosis in non-alcoholic steatohepatitis with type 2 diabetes? Bratisl. Lek. Listy 2011, 112, 626–629. [Google Scholar] [PubMed]
- Domenico, A.; Benedetti, A.; Marucci, L.; Monache, M.D.; Monterubbianesi, R.; Di Cosimo, E.; Perego, L.; Macarri, G.; Glaser, S.; Le Sage, G.; et al. The Function of Alkaline Phosphatase in the Liver: Regulation of Intrahepatic Biliary Epithelium Secretory Activities in the Rat. Hepatology 2000, 32, 174–184. [Google Scholar] [CrossRef]
- Willerson, J.T.; Ridker, P.M. Inflammation as a Cardiovascular Risk Factor. Circulation 2004. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, T.; Watzenböck, M.L.; Walenbergh, S.M.A.; Amir, S.; Gruber, S.; Kozma, M.O.; Grabsch, H.I.; Koek, G.H.; Pierik, M.J.; Staufer, K.; et al. Low levels of IgM antibodies recognizing oxidation-specific epitopes are associated with human non-alcoholic fatty liver disease. BMC Med. 2016, 14, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, D.B.; Srikakulapu, P.; Kaplan, J.L.; Oldham, S.N.; McSkimming, C.; Garmey, J.C.; Perry, H.M.; Kirby, J.L.; Prohaska, T.A.; Gonen, A.; et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin ResistanceSignificance. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, S.M.; Perry, H.M.; Gonen, A.; Prohaska, T.A.; Srikakulapu, P.; Grewal, S.; Das, D.; McSkimming, C.; Taylor, A.M.; Tsimikas, S.; et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ. Res. 2015, 117, e29–e39. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific Dietary Polyphenols Attenuate Atherosclerosis in Apolipoprotein E–Knockout Mice by Alleviating Inflammation and Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Insull, W. The Pathology of Atherosclerosis: Plaque Development and Plaque Responses to Medical Treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based. Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Farah, B.L.; Singh, B.K.; Siddique, M.M.; Li, Y.; Wu, Y.; Ilkayeva, O.R.; Gooding, J.; Ching, J.; Zhou, J.; et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 2014, 59, 1366–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, C.E.G.; Dórea, J.G.; da Costa, T.H.M. Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials. J. Tradit. Complement. Med. 2018. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Li, Y.-F.; Wang, G.-E.; Tan, R.-R.; Tsoi, B.; Mao, G.-W.; Zhai, Y.-J.; Cao, L.-F.; Chen, M.; Kurihara, H.; et al. Caffeine ameliorates high energy diet-induced hepatic steatosis: sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015, 6, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Alsabri, S.G.; Mari, W.O.; Younes, S.; Alsadawi, M.A.; Oroszi, T.L. Kinetic and Dynamic Description of Caffeine. J. Caffeine Adenosine Res. 2018, 8, 3–9. [Google Scholar] [CrossRef]
- Jung, R.T.; Shetty, P.S.; James, W.P.; Barrand, M.A.; Callingham, B.A. Caffeine: Its effect on catecholamines and metabolism in lean and obese humans. Clin. Sci. (London, Engl. 1979) 1981, 60, 527–535. [Google Scholar] [CrossRef]
- Hartmann, M.; Czok, G. Pharmacokinetics of caffeine in mice and its modification by ethanol. Z. Ernahrungswiss. 1980, 19, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of Caffeine on Plasma Renin Activity, Catecholamines and Blood Pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K. Leptin-Induced Sympathetic Nerve Activation: Signaling Mechanisms and Cardiovascular Consequences in Obesity. Curr. Hypertens. Rev. 2010, 6, 104–209. [Google Scholar] [CrossRef] [PubMed]
- Mowers, J.; Uhm, M.; Reilly, S.M.; Simon, J.; Leto, D.; Chiang, S.H.; Chang, L.; Saltiel, A.R. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKK?? and TBK1. Elife 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gao, Y.-Q.; Zhang, Y.; Wang, H.; Liu, G.-S.; Lei, J.-Y. Chlorogenic acid alleviates autophagy and insulin resistance by suppressing JNK pathway in a rat model of nonalcoholic fatty liver disease. J. Biosci. 2018, 43, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Stål, P. Liver fibrosis in non-alcoholic fatty liver disease—diagnostic challenge with prognostic significance. World J. Gastroenterol. 2015, 21, 11077. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, F.; Coilly, A.; Kadar, H.; Gual, P.; Tran, A.; Desterke, C.; Samuel, D.; Duclos-Vallée, J.-C.; Touboul, D.; Bertrand-Michel, J.; et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci. Rep. 2017, 7, 46658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahmohammadi Jebel, F.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Picardi, A.; Vespasiani-Gentilucci, U. Association Between Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A First Message Should Pass. Am. J. Gastroenterol. 2008, 103, 3036–3038. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Lotersztajn, S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsukamoto, H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Detrimental and Beneficial Effects of Inflammation. Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Itoh, Y.; Yokomizo, C.; Nishimura, T.; Niimi, T.; Fujii, H.; Okanoue, T.; Yoshikawa, T. Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab. Investig. 2010, 90, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Gao, M.; Sun, H.; Liu, D. Interleukin-6 gene transfer reverses body weight gain and fatty liver in obese mice. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Fang, C.; Hayashi, S.; Hao, S.; Zhao, M.; Tsutsui, H.; Nishiguchi, S.; Sheng, J. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J. Gastroenterol. 2016, 51, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Benkdane, M.; Alons, E.; Lotersztajn, S.; Pavoine, C. M2 Kupffer Cells Promote Hepatocyte Senescence. Am. J. Pathol. 2014, 184, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Chng, M.H.Y.; Roskin, K.M.; Jackson, K.J.L.; Nguyen, K.D.; Glanville, J.; Lee, J.-Y.; Engleman, E.G.; Boyd, S.D. High-fat diet induces systemic B-cell repertoire changes associated with insulin resistance. Mucosal Immunol. 2017, 10, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chng, M.H.Y.; Alonso, M.N.; Yuan, R.; Winer, D.A.; Engleman, E.G. B-1a Lymphocytes Attenuate Insulin Resistance. Diabetes 2015, 64, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, A.; Boekholdt, S.M.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.P.; Wareham, N.J.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L.; et al. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: Results from the EPIC-Norfolk Study. J. Lipid Res. 2011, 52, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, M.A.; Schloss, P.D. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. MBio 2016. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.S.; Jonkers, D.M.A.E.; Savelkoul, P.H.; Masclee, A.A.; Pierik, M.J.; Penders, J. Fecal Microbial Composition of Ulcerative Colitis and Crohn’s Disease Patients in Remission and Subsequent Exacerbation. PLoS ONE 2014, 9, e90981. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.M.; Rogers, M.A.M.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. MBio 2014. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.L.; Wood, D.L.A.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.O.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family247 S–S inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Mueller, N.; Álvarez-Quintero, R.; Velásquez-Mejía, E.; Sierra, J.; Corrales-Agudelo, V.; Carmona, J.; Abad, J.; Escobar, J. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R. Hydrogen Sulfide Signaling in the Gastrointestinal Tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, G.D.; Micheletti, T.O.; Pace, F. Nutritional Targets for Modulation of the Microbiota in Obesity. Drug Dev. Res. 2013, 74, 393–402. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef] [PubMed]





| Composition | mg/g | mg/g | |
|---|---|---|---|
| Total carbohydrates | 620 | Ash | 147 |
| Soluble dietary fiber precipitable | <0.136 | Calcium | 0.635 |
| Low molecular weight soluble dietary fiber | <0.0183 | Copper | 0.0176 |
| Insoluble dietary fiber | <0.136 | Iron | 0.0265 |
| High molecular weight soluble dietary fiber | <0.136 | Magnesium | 4.02 |
| Total soluble dietary fiber | <0.136 | Manganese | 0.0119 |
| Total dietary fiber | <0.136 | Phosphorous | 2.45 |
| Crude protein | 242 | Potassium | 67.8 |
| Total cis-unsaturated fatty acids | 0.00007 | Zinc | 0.0416 |
| Polyunsaturated fatty acids | 0.00007 | ||
| Omega-6 fatty acids | 0.00007 | Aflatoxin B1 | <0.00000909 |
| Cholesterol | <0.182 | Aflatoxin B2 | <0.00000909 |
| Caffeine | 88.9 | Aflatoxin G1 | <0.00000909 |
| Total chlorogenic acids | 159.7 ± 5.01 | Aflatoxin G2 | <0.00000909 |
| 3,4-dicaffeoylquinic acid | 1.10 ± 0.00 | Ochratoxin A | <0.000018 |
| 3,5-dicaffeoylquinic acid | 73.06 ± 2.58 | Zearalenone | <0.000182 |
| 3-O-caffeoylquinic acid | 0.06 ± 0.01 | Acrylamide | 0.0000956 |
| 4,5-dicaffeoylquinic acid | 0.58 ± 0.00 | ||
| 4-O-caffeoylquinic acid | 47.84 ± 2.24 | Calories | |
| 5-O-caffeoylquinic acid | 36.74 ± 2.59 | 3.42 kcal/g | |
| Caffeic acid | 0.18 ± 0.02 |
| Parameter | Vehicle | GCE | p-Value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 923.0 (688.0–1158.0) | 839.3 (687.3–991.3 | 0.49 |
| HDL (mg(dL) | 169.1 (99.5–238.7) | 142.1 (92.1–192.1) | 0.79 |
| LDL (mg(dL) | 390.9 (226.8–555.0) | 310.7 (199.4–422.0) | 0.53 |
| Triglycerides (mg/dL) | 173.7 (140.9–206.5) | 176.6 (137.7–215.6) | 0.81 |
| Glucose (mg/dL) | 297.3 (252.8–341.8) | 227.1 (184.9–269.3) | 0.02 |
| Insulin (ng/ml) | 0.54 (0.27–0.80) | 0.43 (0.21–0.65) | 0.49 |
| HOMA-IR | 11.49 (5.29–17.69) | 4.61 (2.87–6.34) | 0.004 |
| HOMA-B | 23.57 (10.63–36.51) | 40.77 (10.76–70.78) | 0.24 |
| QUICKI | 0.28 (0.26–0.30) | 0.31 (0.30–0.33) | 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caro-Gómez, E.; Sierra, J.A.; Escobar, J.S.; Álvarez-Quintero, R.; Naranjo, M.; Medina, S.; Velásquez-Mejía, E.P.; Tabares-Guevara, J.H.; Jaramillo, J.C.; León-Varela, Y.M.; et al. Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice. Nutrients 2019, 11, 497. https://doi.org/10.3390/nu11030497
Caro-Gómez E, Sierra JA, Escobar JS, Álvarez-Quintero R, Naranjo M, Medina S, Velásquez-Mejía EP, Tabares-Guevara JH, Jaramillo JC, León-Varela YM, et al. Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice. Nutrients. 2019; 11(3):497. https://doi.org/10.3390/nu11030497
Chicago/Turabian StyleCaro-Gómez, Erika, Jelver A. Sierra, Juan S. Escobar, Rafael Álvarez-Quintero, Mauricio Naranjo, Sonia Medina, Eliana P. Velásquez-Mejía, Jorge H. Tabares-Guevara, Julio C. Jaramillo, Yudy M. León-Varela, and et al. 2019. "Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice" Nutrients 11, no. 3: 497. https://doi.org/10.3390/nu11030497







