A Pathophysiological Model of Non-Alcoholic Fatty Liver Disease Using Precision-Cut Liver Slices
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Precision-Cut Liver Slices (PCLSs)
2.3. Culture Media
2.4. Oil Red O Staining
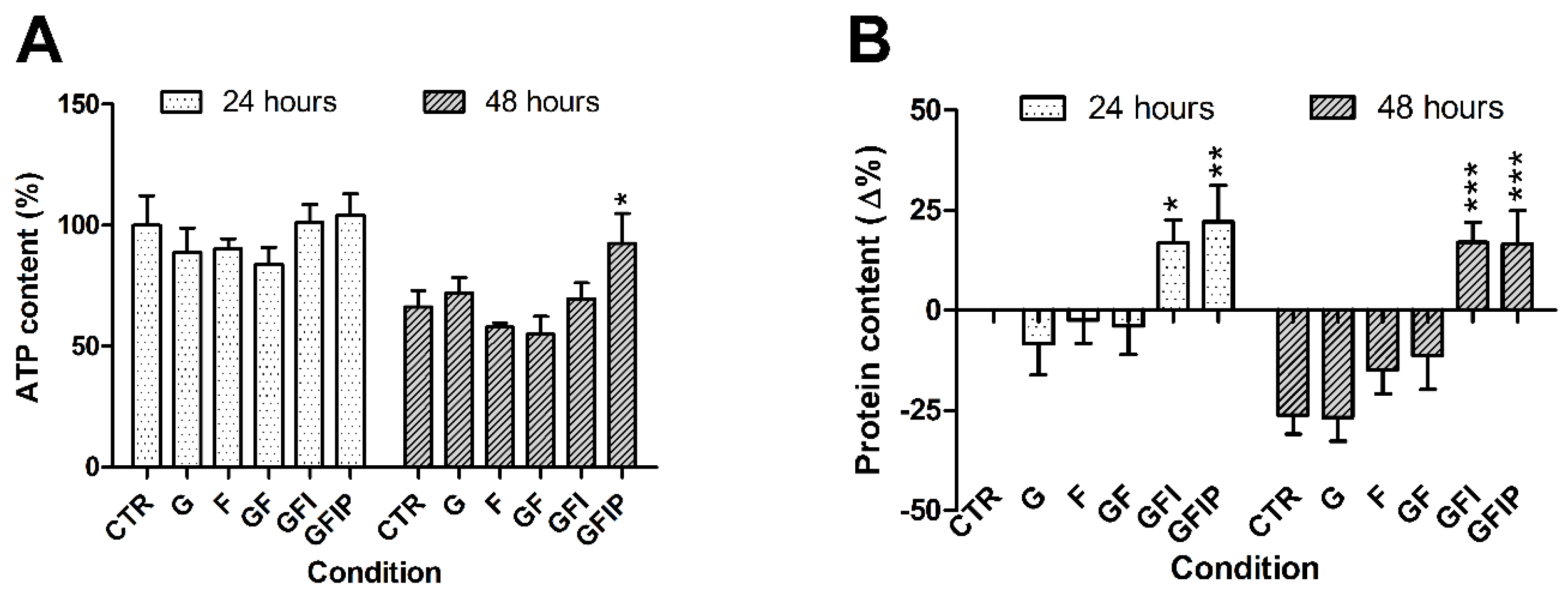
2.5. ATP Determination
2.6. Protein Estimation
2.7. Triglyceride Quantitation
2.8. Quantitative Real-Time PCR
2.9. Statistics
3. Results
3.1. PCLS Characteristics
3.2. Hepatic Steatosis in PCLSs
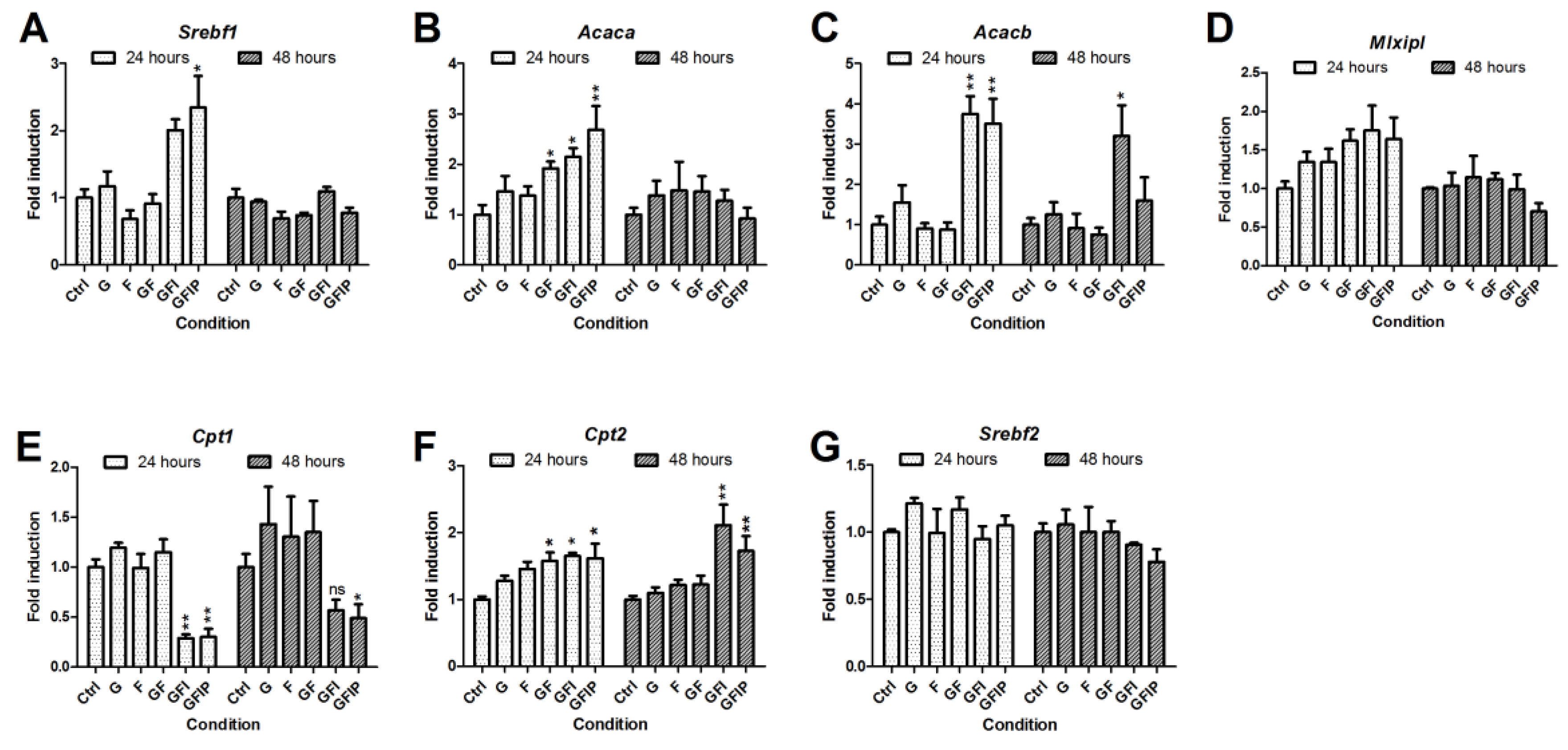
3.3. Lipid Metabolism
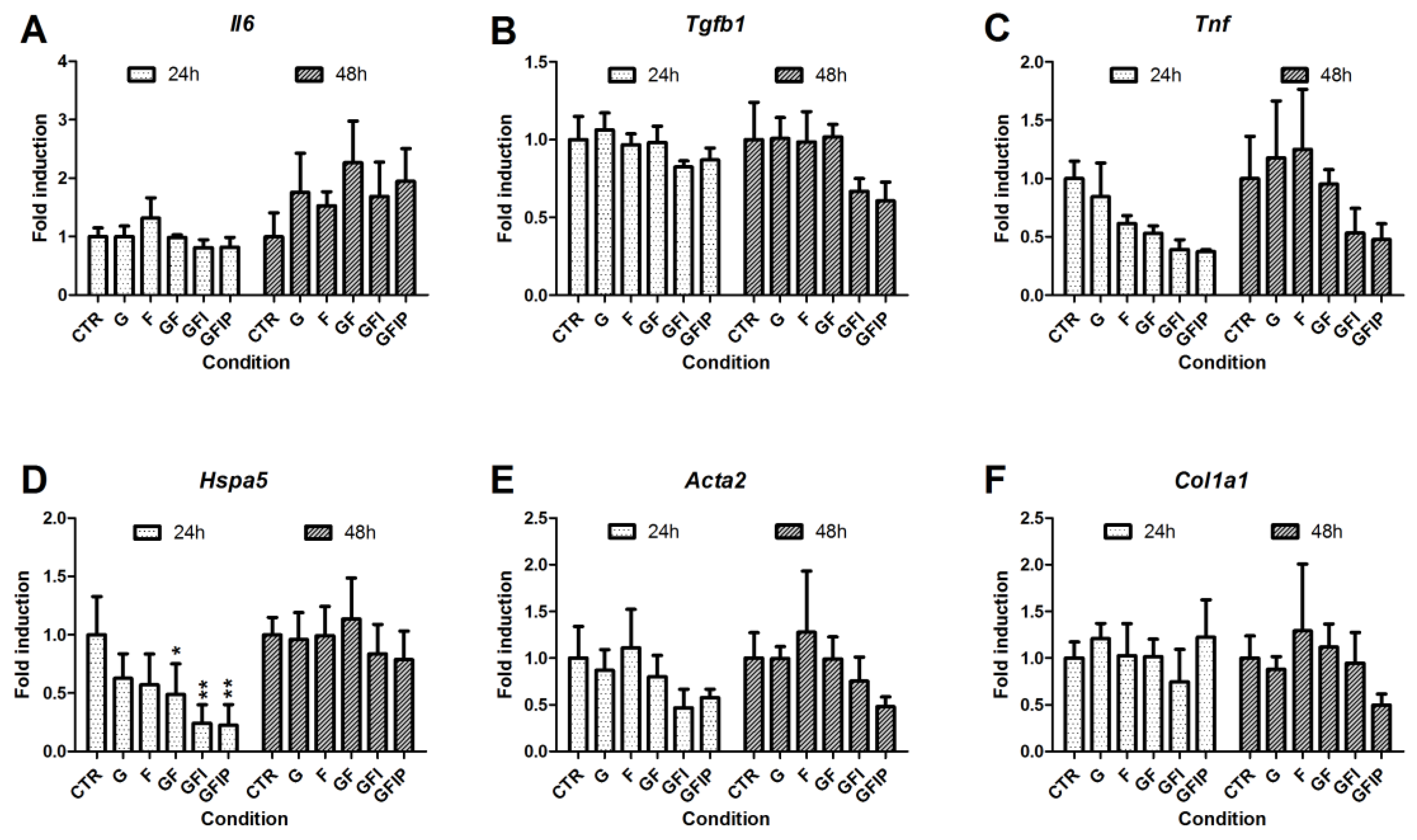
3.4. Inflammation, Endoplasmic Reticulum Stress, and Fibrosis in PCLSs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic Fatty Liver Disease. JAMA 2015, 313, 2263. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sherif, Z.A.; Saeed, A.; Ghavimi, S.; Nouraie, S.-M.; Laiyemo, A.O.; Brim, H.; Ashktorab, H. Global Epidemiology of Nonalcoholic Fatty Liver Disease and Perspectives on US Minority Populations. Dig. Dis. Sci. 2016, 61, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, N.; Erion, D.M.; Zhang, D.; Kahn, M.; Beddow, S.A.; Chu, X.; Still, C.D.; Gerhard, G.S.; Han, X.; Dziura, J.; et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2011, 108, 16381–16385. [Google Scholar] [CrossRef] [PubMed]
- Ress, C.; Kaser, S. Mechanisms of intrahepatic triglyceride accumulation. World J. Gastroenterol. 2016, 22, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Mustafa, G.; Alam, M.; Ahmad, N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J. Gastrointest. Pathophysiol. 2016, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Modulation of hepatic steatosis by dietary fatty acids. World J. Gastroenterol. 2014, 20, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.C.; Tappy, L. Physiological handling of dietary fructose-containing sugars: Implications for health. Int. J. Obes. 2016, 40, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Horst, K.W. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2017, 91, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty infiltration of liver in hyperlipidemic patients. Dig. Dis. Sci. 2000, 45, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Savy, V.; Zara, V. Olive Oil Increases the Hepatic Triacylglycerol Content in Mice by a Distinct Influence on the Synthesis and Oxidation of Fatty Acids. Biosci. Biotechnol. Biochem. 2008, 72, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pardo, V.; González-Rodríguez, Á.; Muntané, J.; Kozma, S.C.; Valverde, Á.M. Role of hepatocyte S6K1 in palmitic acid-induced endoplasmic reticulum stress, lipotoxicity, insulin resistance and in oleic acid-induced protection. Food Chem. Toxicol. 2015, 80, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; Chen, X.M.; Wan, J.M.; Gui, H.B.; Ruan, X.Z.; Du, X.G. Autophagy Protects against Palmitic Acid-Induced Apoptosis in Podocytes in vitro. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.R.; Liu, J.; Plumeri, D.; Cao, Y.B.; He, T.; Lin, L.; Li, Y.; Jiang, Y.Y.; Li, J.; Shang, J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am. J. Transl. Res. 2011, 3, 284–291. [Google Scholar] [PubMed]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef] [PubMed]
- Corey, K.E.; Rinella, M.E. Medical and Surgical Treatment Options for Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2016, 61, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Rustgi, V.K. Current Pharmacologic Therapy for Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2016, 20, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, G.; Bergheim, I. In vitro and in vivo models of non-alcoholic fatty liver disease (NAFLD). Int. J. Mol. Sci. 2013, 14, 11963–11980. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Tapia, N.C.; Rosso, N.; Tiribelli, C. In vitro models for the study of non-alcoholic fatty liver disease. Curr. Med. Chem. 2011, 18, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- MacHado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Magee, N.; Zou, A.; Zhang, Y. Pathogenesis of Nonalcoholic Steatohepatitis: Interactions between Liver Parenchymal and Nonparenchymal Cells. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Westra, I.M.; Hadi, M.; Oosterhuis, D.; de Jong, K.P. Human precision-cut liver slices as an ex vivo model to test antifibrotic drugs in the early onset and end-stage of liver fibrosis. Precision-cut liver slices an ex vivo Model early onset end-stage liver Fibros. 2014, 35, 97. [Google Scholar] [CrossRef]
- Olinga, P.; Schuppan, D. Precision-cut liver slices: A tool to model the liver ex vivo. J. Hepatol. 2013, 58, 1252–1253. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, I.A.M.; Olinga, P.; De Jager, M.H.; Merema, M.T.; De Kanter, R.; Van De Kerkhof, E.G.; Groothuis, G.M.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Sugimoto, K.; Douard, V.; Shah, A.; Inui, H.; Yamanouchi, T.; Ferraris, R.P. Effect of dietary fructose on portal and systemic serum fructose levels in rats and in KHK(−/−) and GLUT5(−/−) mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G779–G790. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.; Rousseau, N.; Demeure, R.; Gallez, B.; Taper, H.; Declerck, B.; Delzenne, N. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 2002, 132, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gauna, C.; Uitterlinden, P.; Kramer, P.; Kiewiet, R.M.; Janssen, J.A.M.J.L.; Delhanty, P.J.D.; Van Aken, M.O.; Ghigo, E.; Hofland, L.J.; Themmen, A.P.N.; et al. Intravenous glucose administration in fasting rats has differential effects on acylated and unacylated ghrelin in the portal and systemic circulation: A comparison between portal and peripheral concentrations in anesthetized rats. Endocrinology 2007, 148, 5278–5287. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Spreckelmeyer, S.; Estrada-Ortiz, N.; Prins, G.G.H.; van der Zee, M.; Gammelgaard, B.; Stürup, S.; de Graaf, I.A.M.; Groothuis, G.M.M.; Casini, A. On the toxicity and transport mechanisms of cisplatin in kidney tissues in comparison to a gold-based cytotoxic agent. Metallomics 2017, 9, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Bronfman, M.; Vanneste, R.; Hers, H.G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem. J. 1977, 162, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Starokozhko, V.; Abza, G.B.; Maessen, H.C.; Merema, M.T.; Kuper, F.; Groothuis, G.M.M. Viability, function and morphological integrity of precision-cut liver slices during prolonged incubation: Effects of culture medium. Toxicol. Vitr. 2015, 30, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation of protein synthesis by insulin. Biochem. Soc. Trans. 2006, 34, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Wolfe, R.R. Insulin action on protein metabolism. Baillieres Clin. Endocrinol. Metab. 1993, 7, 989–1005. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Elias, I.; Ferré, T.; Vilà, L.; Muñoz, S.; Casellas, A.; Garcia, M.; Molas, M.; Agudo, J.; Roca, C.; Ruberte, J.; et al. Alox5ap overexpression in adipose tissue leads to LXA 4 production and protection against diet-induced obesity and insulin resistance. Diabetes 2016, 65, db160040. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Amankwa-Sakyi, M.; Flynn, T.J. Cellular glutathione in fatty liver in vitro models. Toxicol. Vitr. 2011, 25, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell. Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Ritchie, M.G. How might epigenetics contribute to ecological speciation? Curr. Zool. 2013, 59, 686–696. [Google Scholar] [CrossRef]
- Anthérieu, S.; Rogue, A.; Fromenty, B.; Guillouzo, A.; Robin, M.A. Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in heparg cells. Hepatology 2011, 53, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Zhang, J.G.; Wu, X.; Liu, Y.; Gu, S.Y.; Zhu, G.H.; Wang, Y.Z.; Liu, G.L.; Li, X.Y. Nuciferine downregulates Per-Arnt-Sim kinase expression during its alleviation of lipogenesis and inflammation on oleic acid-induced hepatic steatosis in HepG2cells. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Guo, Y.; Ye, H.; Yu, C.; Xu, C.; Xu, L.; Wu, S.; Sun, W.; Wei, H.; et al. Functional proteomic analysis of nonalcoholic fatty liver disease in rat models: Enoyl-coenzyme a hydratase down-regulation exacerbates hepatic steatosis. Hepatology 2010, 51, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Salehi, S.; Cheung, P.; Homko, C.; Song, W.; Loveland-Jones, C.; Jayarajan, S. Comparison of In Vivo Effects of Insulin on SREBP-1c Activation and INSIG-1/2 in Rat Liver and Human and Rat Adipose Tissue. Obesity (Silver Spring) 2013, 21, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Roche, E.; Kim, K.; Prentki, M. Glucose Regulates Acetyl-coA Carboxylase Gene Expression in a Pancreatic B-Cell Line (INS-1)*. J. Biol. Chem. 1993, 268, 18905–18911. [Google Scholar] [PubMed]
- Hurtado Del Pozo, C.; Vesperinas-García, G.; Rubio, M.Á.; Corripio-Sánchez, R.; Torres-García, A.J.; Obregon, M.J.; Calvo, R.M. ChREBP expression in the liver, adipose tissue and differentiated preadipocytes in human obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2011, 1811, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.D.; Guo, G.L. Mechanistic review of drug-induced steatohepatitis. Toxicol. Appl. Pharmacol. 2015, 289, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Yao, D.; Yamaguchi, M.; Chida, J.; Yao, D.; Kido, H. Bezafibrate upregulates carnitine palmitoyltransferase II expression and promotes mitochondrial energy crisis dissipation in fibroblasts of patients with influenza-associated encephalopathy. Mol. Genet. Metab. 2011, 104, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.C.; Esler, W.P.; Patel, R.; Lanba, A.; Vera, N.B.; Pfefferkorn, J.A.; Vernochet, C. Inhibition of Acetyl-CoA carboxylase 1 (ACC1) and 2 (ACC2) reduces proliferation and de novo lipogenesis of EGFRvIII human glioblastoma cells. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. The ER chaperone and signaling regulator GRP78 / BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kaplowitz, N.; Lau, M.Y.; Kao, E.; Petrovic, L.M.; Lee, A.S. Liver-specific loss of GRP78 perturbs the global unfolded protein response and exacerbates a spectrum of acute and chronic liver diseases. Hepatology 2012, 54, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Guan, X.; Liu, W.; Zhang, Y. DAL-1 attenuates epithelial to mesenchymal transition and metastasis by suppressing HSPA5 expression in non-small cell lung cancer. Oncol. Rep. 2017, 38, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.L.; Soto-pantoja, D.R.; Clarke, P.A.G.; Cruz, M.I.; Hilakivi-clarke, L.; Roberts, D.D.; Clarke, R.; Zwart, A.; Anni, W. Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res. 2016, 76, 5657–5671. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.; Green, R.M. The Unfolded Protein Response in Fatty Liver Disease. Semin. Liver Dis. 2014, 33, 321–329. [Google Scholar] [CrossRef]
- Gentile, C.L.; Frye, M.; Pagliassotti, M.J. Endoplasmic Reticulum Stress and the Unfolded Protein Response. Antioxid. Redox. Signal. 2011, 15, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, H.L.; Chabanon, H.; Hainault, I.; Luquet, S.; Magnan, C.; Koike, T.; Ferré, P.; Foufelle, F. GRP78 expression inhibits insulin and ER stress–induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009, 119, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Baye, T.M.; Carless, M.; Wallace, J.; Komorowski, R.; Kleiner, D.E.; Andris, D.; Makladi, B.; Cole, R.; Charlton, M.; et al. Hepatic Gene Networks in Morbidly Obese Patients with Nonalcoholic Fatty Liver Disease. Obes. Surg. 2010, 20, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Hwang, S.; Cherrington, N.J.; Ryu, D. Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget 2017, 8, 63370–63381. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E.; Chalasani, N. Drug-Induced Hepatic Steatosis. Semin. Liver Dis. 2014, 34, 205–214. [Google Scholar] [CrossRef] [PubMed]

| Medium | Additives | Final Concentration of Additives | |||
|---|---|---|---|---|---|
| Glucose | Fructose | Insulin | Palmitic Acid | ||
| CTR | None | 11 mM | |||
| G | Glucose | 25 mM | |||
| F | Fructose | 5 mM | |||
| GF | Glucose and fructose | 25 mM | 5 mM | ||
| GFI | Glucose, fructose, and insulin | 25 mM | 5 mM | 1 nM | |
| GFIP | Glucose, fructose, insulin, and palmitic acid | 25 mM | 5 mM | 1 nM | 240 μM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prins, G.H.; Luangmonkong, T.; Oosterhuis, D.; Mutsaers, H.A.M.; Dekker, F.J.; Olinga, P. A Pathophysiological Model of Non-Alcoholic Fatty Liver Disease Using Precision-Cut Liver Slices. Nutrients 2019, 11, 507. https://doi.org/10.3390/nu11030507
Prins GH, Luangmonkong T, Oosterhuis D, Mutsaers HAM, Dekker FJ, Olinga P. A Pathophysiological Model of Non-Alcoholic Fatty Liver Disease Using Precision-Cut Liver Slices. Nutrients. 2019; 11(3):507. https://doi.org/10.3390/nu11030507
Chicago/Turabian StylePrins, Grietje H., Theerut Luangmonkong, Dorenda Oosterhuis, Henricus A. M. Mutsaers, Frank J. Dekker, and Peter Olinga. 2019. "A Pathophysiological Model of Non-Alcoholic Fatty Liver Disease Using Precision-Cut Liver Slices" Nutrients 11, no. 3: 507. https://doi.org/10.3390/nu11030507
APA StylePrins, G. H., Luangmonkong, T., Oosterhuis, D., Mutsaers, H. A. M., Dekker, F. J., & Olinga, P. (2019). A Pathophysiological Model of Non-Alcoholic Fatty Liver Disease Using Precision-Cut Liver Slices. Nutrients, 11(3), 507. https://doi.org/10.3390/nu11030507






