The Development and Evaluation of a Diet Quality Index for Asian Toddlers and Its Perinatal Correlates: The GUSTO Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
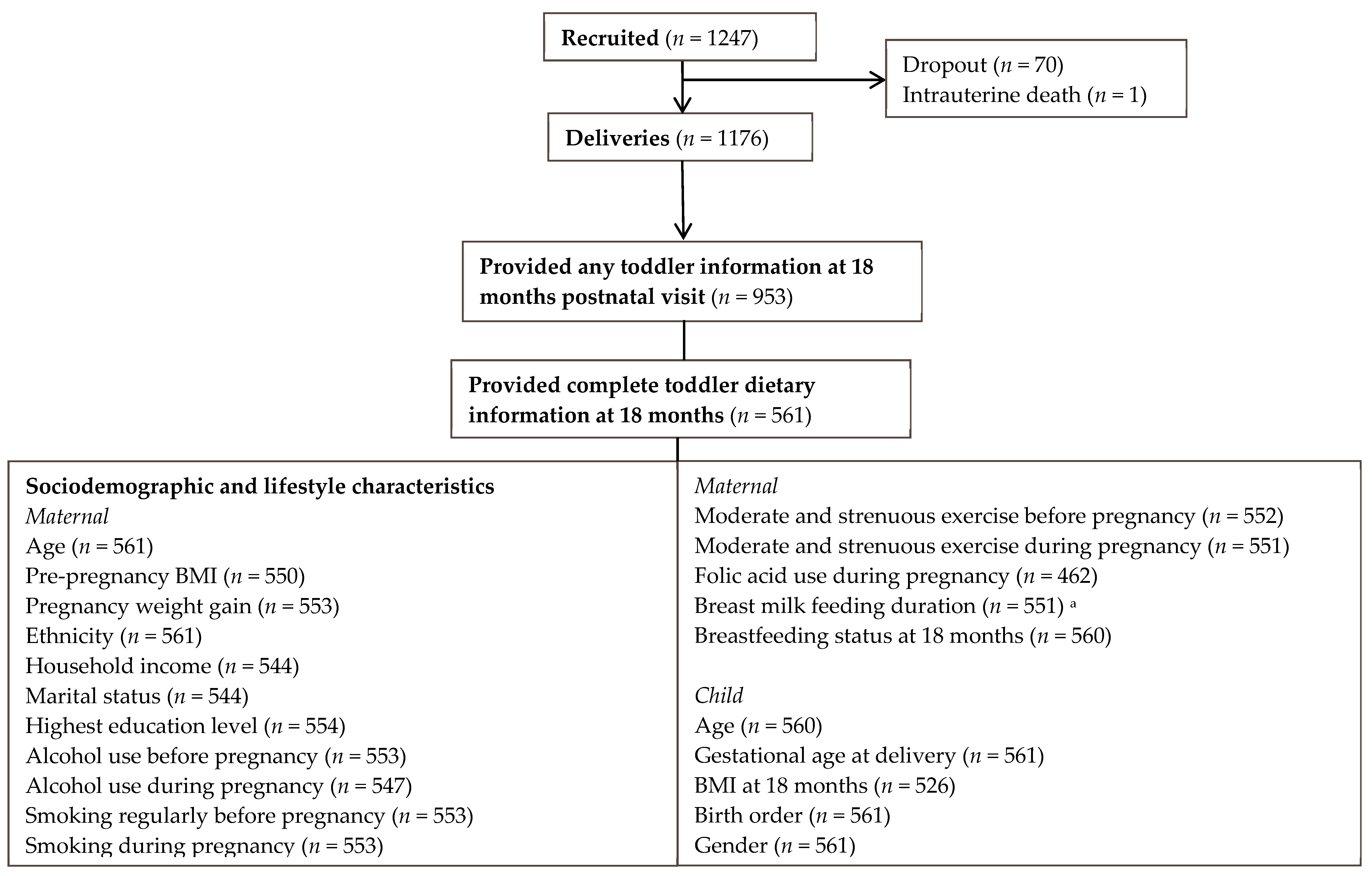
2.1. Study Population
2.2. Data Collection
2.2.1. Maternal Characteristics
2.2.2. Toddler’s Dietary Intake and Characteristics
2.2.3. Structure and Development of the DQI
2.2.4. Weighting and Scoring of DQI Components
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. DQI Characteristics
3.3. Construct Validity
3.4. Sociodemographic and Lifestyle Characteristics and DQI
4. Discussion
4.1. Development and Construct Validity of DQI
4.2. Sociodemographic and Lifestyle Characteristics and DQI
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dewey, K. Guiding Principles for Complementary Feeding of the Breastfed (PAHO and WHO). Pan. Am. Heal. Organ. World Heal. Organ. 2001, 18–25. Available online: https://www.popline.org/node/236830 (accessed on 26 December 2016).
- Harris, G. Development of taste and food preferences in children. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Paes, V.M.; Ong, K.K.; Lakshman, R. Factors influencing obesogenic dietary intake in young children (0–6 years): Systematic review of qualitative evidence. BMJ Open 2015, 5, e007396. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Kant, A.K. Indexes of Overall Diet Quality: A Review. J. Am. Diet. Assoc. 1996, 96, 785–791. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J. Hum. Nutr. Diet. 2014, 27, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-C.; Chen, P.-H. Health Consequences of Nutrition in Childhood and Early Infancy. Pediatr. Neonatol. 2009, 50, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Ríos, E.M.; Sinigaglia, O.; Diaz, B.; Palacios, C. Development of a Diet Quality Score for Infants and Toddlers and its association with weight. J. Nutr. Health Food Sci. 2016, 4. [Google Scholar] [CrossRef]
- Smithers, L.G.; Golley, R.K.; Brazionis, L.; Lynch, J.W. Characterizing whole diets of young children from developed countries and the association between diet and health: A systematic review. Nutr. Rev. 2011, 69, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Kiefte-de Jong, J.C.; Geelen, A.; Villamor, E.; Moll, H.A.; de Jongste, J.C.; Raat, H.; Hofman, A.; Jaddoe, V.W.V.; Franco, O.H.; et al. The development of a diet quality score for preschool children and its validation and determinants in the Generation R Study. J. Nutr. 2015, 145, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.K.; Jansen, E.; Mallan, K.; Magarey, A.M.; Daniels, L. Poor dietary patterns at 1–5 years of age are related to food neophobia and breastfeeding duration but not age of introduction to solids in a relatively advantaged sample. Eat. Behav. 2018, 31, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.K.; Golley, R.K.; Magarey, A.M. A short food-group-based dietary questionnaire is reliable and valid for assessing toddlers’ dietary risk in relatively advantaged samples. Br. J. Nutr. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-X.; Toh, J.-Y.; van Lee, L.; Han, W.-M.; Shek, L.; Tan, K.-H.; Yap, F.; Godfrey, K.M.; Chong, Y.-S.; Chong, M.F.-F. Food Sources of Energy and Macronutrient Intakes among Infants from 6 to 12 Months of Age: The Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Int. J. Environ. Res. Public Health 2018, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.R.; Amin, R.; Taylor-Robinson, D.; Viner, R.; Warner, J.; Stephenson, T. Ethnic Minorities Are at Greater Risk for Childhood-Onset Type 2 Diabetes and Poorer Glycemic Control in England and Wales. J. Adolesc. Health 2016, 59, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C. Rising burden of obesity in Asia. J. Obes. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.-E.; Tint, M.T.; Gluckman, P.D.; Godfrey, K.M.; Rifkin-Graboi, A.; Chan, Y.H.; Stünkel, W.; Holbrook, J.D.; Kwek, K.; Chong, Y.-S.; et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int. J. Epidemiol. 2013, 43, 1401–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Promotion Board Singapore. Energy and Nutrient Composition Search. 2016. Available online: http://www.hpb.gov.sg/HOPPortal/health-article/HPBSUEXTAPP1_4021885 (accessed on 19 December 2016).
- USDA Food Composition Database Home Page [Internet]. Available online: https://ndb.nal.usda.gov/ndb/. (accessed on 26 December 2016).
- Lim, H.X.; Toh, J.Y.; Tan, K.H.; Chong, Y.-S.; Yap, F.; Godfrey, K.M.; Lee, Y.S.; Chong, M.F. Validation of a Semi-Quantitative Food Frequency Questionnaire for 18-month-old Toddlers: The GUSTO Study. Public Health Nutr. 2019, in press. [Google Scholar]
- Lovell, A.; Bulloch, R.; Wall, C.R.; Grant, C.C. Quality of food-frequency questionnaire validation studies in the dietary assessment of children aged 12 to 36 months: A systematic literature review. J. Nutr. Sci. 2017, 6, e16. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Board Singapore. A Healthy Food Foundation—For Kids and Teens; Health Promotion Board Singapore: Singapore, 2015. Available online: https://www.healthhub.sg/live-healthy/578/A (accessed on 26 December 2016).
- Department of Health. Nutrition Guidelines for Children Aged 2 to 6 (Hong Kong); Department of Health: Hong Kong, China, 2014; pp. 1–150. Available online: https://www.startsmart.gov.hk/files/pdf/nutritional_guide_en.pdf (accessed on 26 December 2016).
- Ministry of Health. Malaysian Dietary Guidelines for Children and Adolescents; Ministry of Health: Singapore, 2013; pp. 1–74. Available online: http://www.moh.gov.my/images/gallery/Garispanduan/MDG%20Children%20and%20Adolescents%20Summary.pdf (accessed on 26 December 2016).
- National Institute of Nutrition. Dietary Guidelines for Indians—A Manual; National Institute of Nutrition: Secunderabad, India, 2011; p. 139. Available online: https://www.icmr.nic.in/content/dietary-guidelines-indians-manual-kamala-krishnaswamy-b-sesikeran-second-edition-2011 (accessed on 26 December 2016).
- Nutrition Division Ministry of Health. Food Based Dietary Guidelines for Sri Lankans. 2011. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/sri-lanka/en/ (accessed on 26 December 2016).
- Netherlands Nutrition Centre (Voedingscentrum). Dutch Food-based Dietary Guidelines (Richtlijnen voedselkeuze). 2011. Available online: https://www.voedingscentrum.nl/nl/service/english.aspx (accessed on 26 December 2016).
- Flemish Institute for Health Promotion and Disease Prevention. Available online: https://www.epsa-projects.eu/index.php?title=Flemish_Institute_for_Health_Promotion_and_Disease_Prevention_(VIGEZ) (accessed on 26 December 2016).
- Hilbig, A.; Alexy, U.; Drossard, C.; Kersting, M. GRETA: Ernährung von Kleinkindern in Deutschland. Aktuel. Ernahrungsmed 2011, 36, 224–231. [Google Scholar] [CrossRef]
- Schweizerische Gesellschaft für Ernährung. Available online: https://www.swissveg.ch/SGE (accessed on 26 December 2016).
- United States Department of Agriculture (USDA). 2015–2020 Dietary Guidelines for Americans. In 2015–2020 Diet Guidel. Am., 8th ed.; USDA: Washington, DC, USA, 2015; p. 18. Available online: https://health.gov/dietaryguidelines/2015/ (accessed on 26 December 2016).
- National Medical Health and Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Board Singapore. Recommended Dietary Allowances. 2015. Available online: https://www.healthhub.sg/live-healthy/192/recommended_dietary_allowances (accessed on 26 December 2016).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Arciniega, A.A.; Mendez, M.A.; Baena-Díez, J.M.; Martori, M.A.R.; Soler, C.; Marrugat, J.; Covas, M.-I.; Sanz, H.; Llopis, A.; Schröder, H. Concurrent and construct validity of Mediterranean diet scores as assessed by an FFQ. Public Health Nutr. 2015, 14, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Goh, D.Y.T.; Jacob, A. Children’s consumption of beverages in Singapore: Knowledge, attitudes and practice. J. Paediatr. Child Health 2011, 47, 465–472. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, S.A.; Grimes, C.A.; Lacy, K.E.; Nowson, C.A.; Campbell, K.J. Dietary sources and sodium intake in a sample of Australian preschool children. BMJ Open 2016, 6, e008698. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A.; Rexwinkel, H.; Bulux, J.; Solomons, N.; West, C. Comparison of three methods for estimating daily individual discretionary salt intake: 24 hour recall, duplicate-portion method, and urinary lithium-labelled household salt excretion. Eur. J. Clin. Nutr. 1999, 53, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L. Rethinking dietary cholesterol. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Board Singapore. Birth to Eighteen Years: Dietary Tips for Your Child’s Wellbeing. 2012; pp. 1–27. Available online: https://www.healthhub.sg/sites/assets/Assets/Categories/Food N Nutrition/birth-18 (English)_FINALA4.pdf (accessed on 26 December 2016).
- Manios, Y.; Kourlaba, G.; Kondaki, K.; Grammatikaki, E.; Birbilis, M.; Oikonomou, E.; Roma-Giannikou, E. Diet Quality of Preschoolers in Greece Based on the Healthy Eating Index: The GENESIS Study. J. Am. Diet. Assoc. 2009, 109, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Kleiser, C.; Mensink, G.B.M.; Scheidt-Nave, C.; Kurth, B.-M. HuSKY: A healthy nutrition score based on food intake of children and adolescents in Germany. Br. J. Nutr. 2009, 102, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Hartman, T.; Siega-Riz, A.M.; Herring, A.H. A diet quality index for American preschoolers based on current dietary intake recommendations and an indicator of energy balance. J. Am. Diet. Assoc. 2006, 106, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Colega, M.; Phaik, E.; Quah, L.; Chan, Y.H.; Godfrey, K.M.; Kwek, K.; Saw, S.-M.; Gluckman, P.D.; Chong, Y.-S.; et al. A healthy eating index to measure diet quality in pregnant women in Singapore: A cross-sectional study. BMC Nutr. 2015, 1, 39. [Google Scholar] [CrossRef]
- Turrell, G.; Kavanagh, A.M. Socio-economic pathways to diet: Modelling the association between socio-economic position and food purchasing behaviour. Public Health Nutr. 2006, 9, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Findeis, J.L.; Shrestha, S.S. Use of the Revised Children’s Diet Quality Index to assess preschooler’s diet quality, its sociodemographic predictors, and its association with body weight status. J. Pediatr. 2008, 84, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Naidoo, N.; Ferzacca, S.; Reddy, G.; Van Dam, R.M. The role of women in food provision and food choice decision-making in Singapore: A case study. Ecol. Food Nutr. 2014, 53, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Rezali, F.W.; Chin, Y.S.; Mohd Shariff, Z.; Mohd Yusof, B.N.; Sanker, K.; Woon, F.C. Evaluation of diet quality and its associated factors among adolescents in Kuala Lumpur, Malaysia. Nutr. Res. Pract. 2015, 9, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Jalaludin, M.Y.; Su, T.T.; Dahlui, M.; Azmi Mohamed, M.N.; Abdul Majid, H. Determinants of Diet and Physical Activity in Malaysian Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 603. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Araneta, M.R.G.; Kanaya, A.M.; Chiang, J.L.; Fujimoto, W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015, 38, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.; Donath, S. Maternal diet and breast feeding: A case for rethinking physiological explanations for breastfeeding determinants. Early Hum. Dev. 2011, 88, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.A.; Hesketh, K.D.; Campbell, K.J. Breastfeeding mothers consume more vegetables and a greater variety of fruits and vegetables than non-breastfeeding peers: The influence of socioeconomic position. Nutr. Diet. 2012, 69, 84–90. [Google Scholar] [CrossRef]
- Wall, C.R.; Gammon, C.S.; Bandara, D.K.; Grant, C.C.; Atatoa Carr, P.E.; Morton, S.M.B. Dietary Patterns in Pregnancy in New Zealand-Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients 2016, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Fisk, C.M.; Crozier, S.R.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Influences on the quality of young children’s diets: The importance of maternal food choices. Br. J. Nutr. 2011, 105, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Birch, L. Infant Dietary Experience and Acceptance of Solid Foods. Pediatrics 1994, 93, 271–277. [Google Scholar] [PubMed]
- Mennella, J.A.; Jagnow, C.P.; Beauchamp, G.K. Prenatal and Postnatal Flavor Learning by Human Infants. Pediatrics 2001, 107, e88. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Tsai, H.-J.; Wang, G.; Hong, X.; Zhou, Y.; Zhang, C.; Liu, C.; Liu, R.; Wang, H.; et al. Maternal Preconception Body Mass Index and Offspring Cord Blood DNA Methylation: Exploration of Early Life Origins of Disease Xin. Environ. Mol. Mutagen. 2015, 55, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.C.; Mill, J.; Uher, R.; Schmidt, U. Eating disorders, gene–environment interactions and epigenetics. Neurosci. Biobehav. Rev. 2011, 35, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Foote, J.A.; Wilkens, L.R.; Basiotis, P.P.; Carlson, A.; White, K.K.L.; Yonemori, K.M. Simple Measures of Dietary Variety Are Associated with Improved Dietary Quality. J. Am. Diet. Assoc. 2006, 106, 425–429. [Google Scholar] [CrossRef] [PubMed]
| Component | Recommended Intakes Per Day by Singapore Guidelines | Scoring Criteria Used for DQI | Maximum Score | Example of One Serving | |
|---|---|---|---|---|---|
| Basic components a | Total rice, bread, and alternatives | ≥2–3 serves | ≥2 serves | 10 | 100 g plain rice |
| Total fruit | ≥0.5–1 serve | ≥1 serve | 10 | 130 g apple | |
| Total vegetables * | ≥0.5 serve | ≥1 serve | 10 | 100 g carrot | |
| Total meat and alternatives | ≥0.5 serve | ≥0.5 serve | 10 | 90 g chicken | |
| Total milk and dairy products | ≥1.5 serves | ≥1.5 serves | 10 | 150 g yogurt | |
| Additional components b | Whole grains | A variety is recommended | 1.25 point awarded for consumption of each food item with whole grains (4 items in total) | 5 | - |
| Foods high in sugar | ≤35 g added sugar | ≤35 g added sugar | 10 | - |
| Maternal Characteristics | Mean ± SD or n (%) |
|---|---|
| Age (years) | 31.3 ± 5.0 |
| Pre-pregnancy BMI (kg/m2) | 23.6 ± 4.6 |
| Pregnancy weight gain (kg) | 11.3 ± 4.4 |
| Ethnicity | |
| Indian | 80 (14.3) |
| Malay | 157 (28.0) |
| Chinese | 324 (57.8) |
| Household income category | |
| <S$2000 | 65 (11.6) |
| S$2000–5999 | 295 (52.6) |
| >S$6000 | 169 (30.1) |
| Missing | 32 (5.7) |
| Marital status | |
| Single, not living with husband | 11 (2.0) |
| Married, living with husband | 533 (95.0) |
| Missing | 17 (3.0) |
| Education level | |
| Primary/Secondary | 149 (26.6) |
| Post-secondary | 200 (35.7) |
| University and above | 207 (36.9) |
| Missing | 5 (0.9) |
| Alcohol use before pregnancy | |
| No | 360 (64.2) |
| Yes | 193 (34.4) |
| Missing | 8 (1.4) |
| Alcohol use during pregnancy | |
| No | 535 (95.4) |
| Yes | 12 (2.1) |
| Missing | 14 (2.5) |
| Smoking regularly before pregnancy | |
| No | 488 (87.0) |
| Yes | 65 (11.6) |
| Missing | 8 (1.4) |
| Smoking during pregnancy | |
| No | 542 (96.6) |
| Yes | 11 (2.0) |
| Missing | 8 (1.4) |
| Moderate and strenuous exercise before pregnancy | |
| No | 390 (69.5) |
| Yes | 162 (28.9) |
| Missing | 9 (1.6) |
| Moderate and strenuous exercise during pregnancy | |
| No | 542 (96.6) |
| Yes | 9 (1.6) |
| Missing | 10 (1.8) |
| Folic acid supplement use during pregnancy | |
| No | 52 (9.3) |
| Yes | 410 (73.1) |
| Missing | 99 (17.6) |
| Breast milk feeding duration | |
| Never breastfeed | 16 (2.9) |
| <3 months | 210 (37.4) |
| 3 to <6 months | 99 (17.6) |
| 6 to <12 months | 101 (18.0) |
| ≥12 months | 125 (22.3) |
| Missing | 10 (1.8) |
| Breastfeeding status at 18 months | |
| No | 494 (88.1) |
| Yes | 66 (11.8) |
| Missing | 1 (0.2) |
| Child characteristics | |
| Age (month) | 18.3 ± 0.7 |
| Gestational age at delivery (weeks) | 38.8 ± 1.4 |
| BMI at 18 months old (kg/m2) | 16.2 ± 1.3 |
| Birth order | |
| First child | 267 (47.6) |
| Gender | |
| Male | 289 (51.5) |
| Female | 272 (48.5) |
| Caregiver of child | |
| Parents | 319 (56.9) |
| Other family members | 111 (19.8) |
| External help | 36 (6.4) |
| Responsibility shared | 90 (16.0) |
| Missing | 1 (0.2) |
| Diet Quality Index | |||||
|---|---|---|---|---|---|
| Total n = 561 | Low Tertile n = 187 | Middle Tertile n = 187 | High Tertile n = 187 | p-Trend a | |
| Score range | 15.6–63.1 | 15.6–39.7 | 39.8–47.4 | 47.4–63.1 | |
| Mean ± SD | 43.2 ± 8.5 | 33.5 ± 4.8 | 43.8 ± 2.1 | 52.2 ± 3.7 | |
| % of participants meeting recommended intakes of serving/day of food groups b | |||||
| Total rice, bread, and alternatives | 58.1 | 41.7 | 57.2 | 75.4 | <0.001 |
| Total fruit | 28.0 | 9.1 | 22.5 | 52.4 | <0.001 |
| Total vegetable | 9.8 | 0.0 | 5.3 | 24.1 | <0.001 |
| Total meat and alternatives | 56.9 | 28.9 | 56.1 | 85.6 | <0.001 |
| Total milk and dairy products | 52.6 | 52.4 | 47.1 | 58.3 | 0.26 |
| Consuming whole grains c | 67.0 | 41.7 | 73.8 | 85.6 | <0.001 |
| Foods high in sugar d | 94.8 | 87.2 | 97.9 | 99.5 | <0.001 |
| % of participants meeting AMDR/RDA of nutrients b | |||||
| Carbohydrates (AMDR: 45–65% kcal) * | 83.1 | 84.0 | 82.9 | 82.4 | 0.68 |
| Total fat (AMDR: 30–45% kcal) * | 37.3 | 34.2 | 38.0 | 39.6 | 0.29 |
| Saturated fat (AMDR: <10% kcal) * | 66.7 | 62.0 | 67.4 | 70.6 | 0.08 |
| Protein (RDA: 19 g) + | 98.8 | 96.8 | 99.5 | 100.0 | 0.005 |
| Iron (RDA: 7 mg) + | 90.9 | 89.3 | 90.4 | 93.6 | 0.21 |
| Dietary fibre (RDA: 14 g/1000 kcal) * | 2.3 | 1.1 | 1.1 | 3.7 | 0.016 |
| Calcium (RDA: 500 mg) + | 82.5 | 78.6 | 81.3 | 88.2 | 0.021 |
| Vitamin A (RDA: 250 mcg) + | 95.7 | 92.0 | 96.3 | 98.9 | 0.001 |
| Nutrient intakes (continuous variables) e | |||||
| Carbohydrates (% of total energy) | 53.5 ± 7.2 | 54.8 ± 7.4 | 53.3 ± 7.1 | 52.3 ± 7.1 | 0.001 |
| Protein (% of total energy) | 16.2 ± 3.5 | 14.6 ± 3.0 | 16.2 ± 3.1 | 17.9 ± 3.6 | <0.001 |
| Total fat (% of total energy) | 29.7 ± 6.3 | 30.1 ± 6.3 | 29.9 ± 6.5 | 29.1 ± 6.1 | 0.12 |
| Saturated fat (% of total energy) | 7.6 ± 4.3 | 8.0 ± 4.8 | 7.5 ± 4.1 | 7.2 ± 4.0 | 0.06 |
| Monounsaturated fat (% of total energy) | 7.5 ± 3.7 | 7.6 ± 3.9 | 7.6 ± 3.7 | 7.3 ± 3.6 | 0.50 |
| Polyunsaturated fat (% of total energy) | 8.0 ± 9.8 | 7.9 ± 11.2 | 7.8 ± 9.1 | 8.2 ± 9.0 | 0.76 |
| Iron (mg per 1000 kcal) | 11.4 ± 3.3 | 11.7 ± 3.0 | 11.6 ± 3.9 | 11.0 ± 3.0 | 0.07 |
| Dietary fibre (g per 1000kcal) | 5.9 ± 3.2 | 4.5 ± 2.6 | 5.7 ± 2.4 | 7.5 ± 3.6 | <0.001 |
| Beta-carotene (mg per 1000 kcal) | 2.6 ± 2.2 | 1.5 ± 1.6 | 2.5 ± 2.2 | 3.6 ± 2.3 | <0.001 |
| Cholesterol (mg per 1000 kcal) | 93.1 ± 69.8 | 74.7 ± 74.7 | 92.6 ± 63.8 | 111.8 ± 65.8 | <0.001 |
| Calcium (mg per 1000 kcal) | 756.4 ± 269.3 | 761.5 ± 287.1 | 756.4 ± 266.0 | 751.1 ± 255.3 | 0.71 |
| Sodium (mg per 1000 kcal) | 792.4 ± 252.4 | 761.3 ± 294.1 | 793.4 ± 236.3 | 822.3 ± 218.3 | 0.019 |
| Vitamin A (mcg per 1000 kcal) | 620.1 ± 255.9 | 558.9 ± 251.5 | 619.3 ± 264.2 | 682.2 ± 237.8 | <0.001 |
| Univariable Model | Multivariable Model b | |||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Maternal characteristics | ||||
| Age (years) | 0.21 (0.07, 0.35) | 0.003 | 0.06 (−0.08, 0.21) | 0.39 |
| Pre-pregnancy BMI (kg/m2) | −0.45 (−0.60, −0.30) | <0.001 | −0.22 (−0.38, −0.06) | 0.007 |
| Pregnancy weight gain (kg) | 0.12 (−0.04, 0.28) | 0.15 | − | |
| Ethnicity | ||||
| Indian | 0.45 (−0.05, 0.95) | 0.08 | −0.27 (−2.38, 1.84) | 0.80 |
| Malay | 0.58 (0.20, 0.96) | 0.003 | −1.91 (−3.70, −0.12) | 0.037 |
| Chinese | Ref | Ref | ||
| Household income category | ||||
| <S$2000 | −6.98 (−9.33, −4.64) | <0.001 | −1.29 (−4.18, 1.61) | 0.38 |
| S$2000–5999 | −4.51 (−6.06, −2.96) | <0.001 | −1.96 (−3.90, −0.03) | 0.047 |
| >S$6000 | Ref | Ref | ||
| Marital status | ||||
| Single, not living with husband | −3.22 (−8.32, 1.88) | 0.22 | − | |
| Married, living with husband | Ref | |||
| Highest education level | ||||
| Primary/Secondary | −5.86 (−7.58, −4.13) | <0.001 | −2.53 (−4.81, −0.25) | 0.028 |
| Post-secondary | −4.14 (−5.73, −2.55) | <0.001 | −1.10 (−3.07, 0.87) | 0.25 |
| University and above | Ref | Ref | ||
| Alcohol use before pregnancy | ||||
| No | −1.44 (−2.93, 0.05) | 0.06 | 0.20 (−1.42, 1.81) | 0.81 |
| Yes | Ref | Ref | ||
| Alcohol use during pregnancy | ||||
| No | −0.28 (−5.16, 4.60) | 0.91 | − | |
| Yes | Ref | |||
| Smoking regularly before pregnancy | ||||
| No | 4.32 (2.14, 6.50) | <0.001 | 0.27 (−2.13, 2.67) | 0.83 |
| Yes | Ref | Ref | ||
| Smoking during pregnancy | ||||
| No | 0.37 (−4.73, 5.47) | 0.89 | − | |
| Yes | Ref | |||
| Moderate and strenuous exercise before pregnancy | ||||
| No | −2.43 (−3.98, −0.88) | 0.002 | −0.85 (−2.37, 0.68) | 0.28 |
| Yes | Ref | Ref | ||
| Moderate and strenuous exercise during pregnancy | ||||
| No | 1.35 (−4.28, 6.98) | 0.64 | − | |
| Yes | Ref | |||
| Folic acid supplement use during pregnancy | ||||
| No | 0.49 (−1.89, 2.86) | 0.69 | − | |
| Yes | Ref | |||
| Breast milk feeding duration | ||||
| Never breastfeed | −8.26 (−12.37, −4.15) | <0.001 | −6.89 (−11.98, −1.81) | 0.008 |
| <3 months | −7.22 (−8.97, −5.47) | <0.001 | −5.54 (−8.02, −3.07) | <0.001 |
| 3 to <6 months | −2.64 (−4.72, −0.56) | 0.013 | −2.76 (−5.37, −0.15) | 0.04 |
| 6 to <12 months | −2.32 (−4.39, −0.25) | 0.028 | −3.13 (−5.73, −0.53) | 0.018 |
| ≥12 months | Ref | Ref | ||
| Breastfeeding status at 18 months | ||||
| No | −3.94 (−6.10, −1.77) | <0.001 | 1.41 (−1.40, 4.22) | 0.33 |
| Yes | Ref | Ref | ||
| Child characteristics | ||||
| Age (month) | 0.68 (−0.34, 1.70) | 0.19 | − | |
| Gestational age at delivery (weeks) | 0.34 (−0.16, 0.84) | 0.18 | − | |
| BMI at 18-month-old (kg/m2) | −0.64 (−1.18, 0.10) | 0.021 | −0.31 (−0.86, 0.23) | 0.26 |
| Birth order | ||||
| Not first child | 0.21 (−1.20, 1.62) | 0.77 | − | |
| First child | Ref | |||
| Gender | ||||
| Male | 1.50 (0.09, 2.90) | 0.037 | 0.93 (−0.48, 2.34) | 0.20 |
| Female | Ref | Ref | ||
| Caregiver of child | ||||
| Parents | −1.29 (−3.28, 0.71) | 0.21 | 0.19 (−1.87, 2.24) | 0.86 |
| Other family members | −2.18 (−4.53, 0.16) | 0.07 | −1.56 (−3.90, 0.78) | 0.19 |
| External help | −0.07 (−3.36, 3.22) | 0.97 | 0.22 (−2.95, 3.40) | 0.89 |
| Responsibility shared | Ref | Ref | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-W.; Fung, S.M.; Fok, D.; Leong, L.P.; Toh, J.Y.; Lim, H.X.; Pang, W.W.; Tan, K.H.; Chong, Y.-S.; Yap, F.; et al. The Development and Evaluation of a Diet Quality Index for Asian Toddlers and Its Perinatal Correlates: The GUSTO Cohort Study. Nutrients 2019, 11, 535. https://doi.org/10.3390/nu11030535
Chen L-W, Fung SM, Fok D, Leong LP, Toh JY, Lim HX, Pang WW, Tan KH, Chong Y-S, Yap F, et al. The Development and Evaluation of a Diet Quality Index for Asian Toddlers and Its Perinatal Correlates: The GUSTO Cohort Study. Nutrients. 2019; 11(3):535. https://doi.org/10.3390/nu11030535
Chicago/Turabian StyleChen, Ling-Wei, Si Ming Fung, Doris Fok, Lai Peng Leong, Jia Ying Toh, Hui Xian Lim, Wei Wei Pang, Kok Hian Tan, Yap-Seng Chong, Fabian Yap, and et al. 2019. "The Development and Evaluation of a Diet Quality Index for Asian Toddlers and Its Perinatal Correlates: The GUSTO Cohort Study" Nutrients 11, no. 3: 535. https://doi.org/10.3390/nu11030535
APA StyleChen, L.-W., Fung, S. M., Fok, D., Leong, L. P., Toh, J. Y., Lim, H. X., Pang, W. W., Tan, K. H., Chong, Y.-S., Yap, F., Godfrey, K. M., Lee, Y. S., & Chong, M. F.-F. (2019). The Development and Evaluation of a Diet Quality Index for Asian Toddlers and Its Perinatal Correlates: The GUSTO Cohort Study. Nutrients, 11(3), 535. https://doi.org/10.3390/nu11030535





