Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Extraction Procedure and Chemical Characterisation
2.2. Caco-2 Cell Culture
2.2.1. Cytotoxicity Evaluation
2.2.2. In Vitro Interleukin 8 (CXCL8) Detection using Enzyme Linked Immunosorbent Assay (ELISA)
2.3. Ex Vivo Porcine Colonic Tissue Collection and Treatment
2.3.1. Ex Vivo Interleukin 8 (CXCL8) Detection using ELISA
2.3.2. RNA Extraction and cDNA Synthesis
2.3.3. Quantitative Real-Time PCR (QPCR)
2.4. Statistical Analysis
3. Results
3.1. Polyphenolic Composition of PPE
3.2. Experimental Results on Caco-2 cells
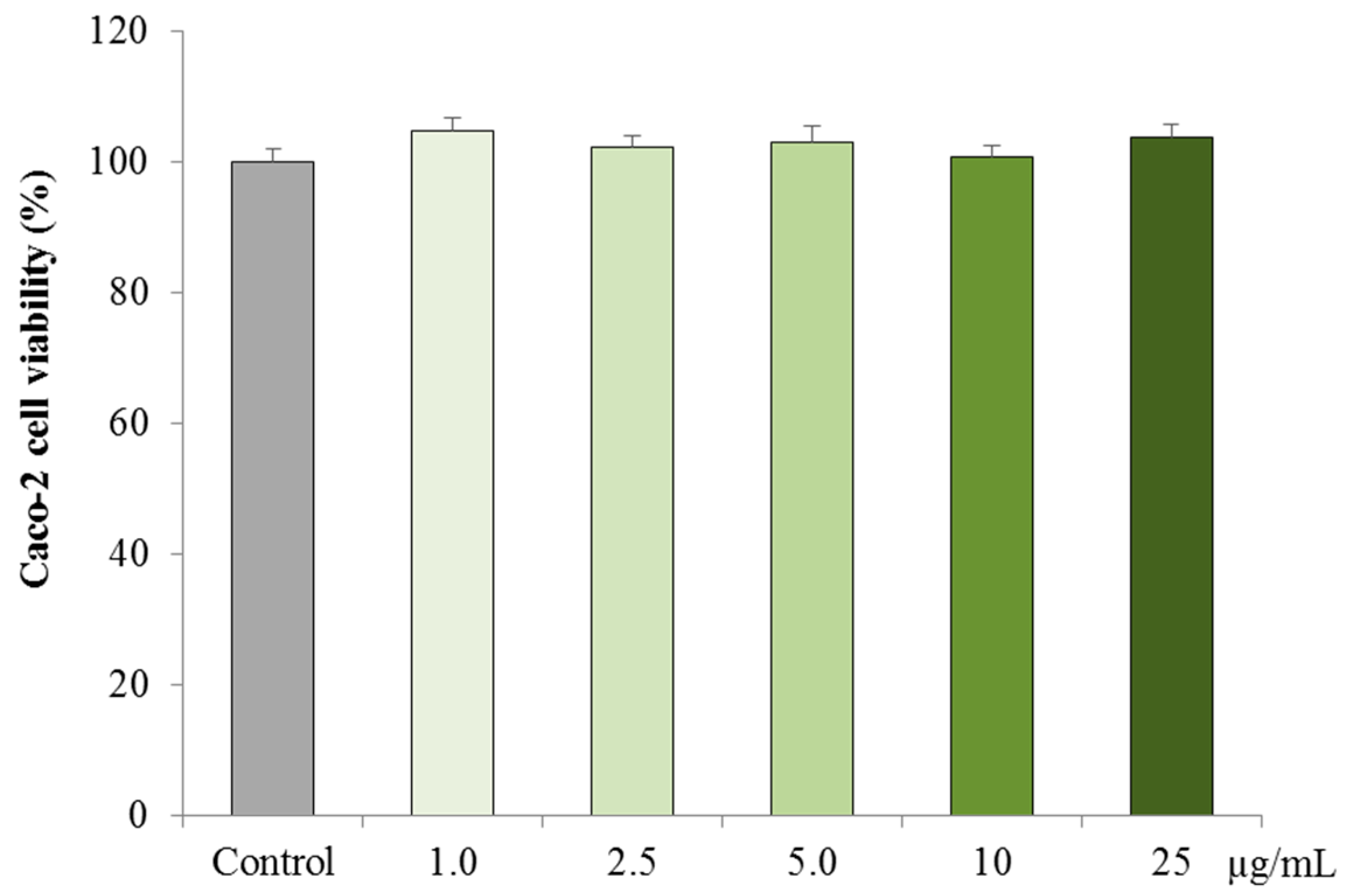
3.2.1. Cytotoxicity on Caco-2 cells
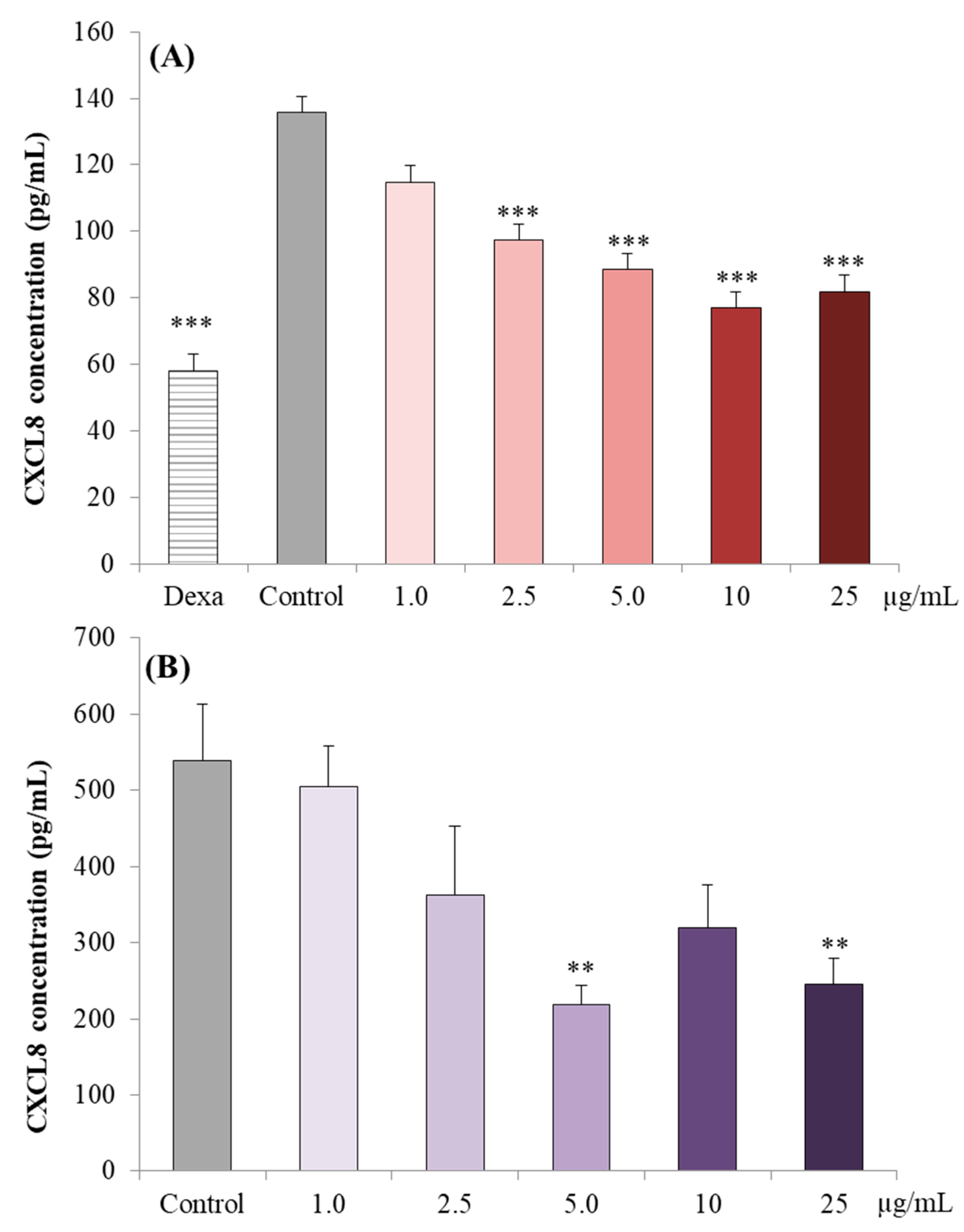
3.2.2. CXCL8 Concentrations in Caco-2 cell Supernatants
3.3. Experimental Results on Ex-Vivo Porcine Colonic Tissue Explants
3.3.1. CXCL8 Concentrations in the Supernatant
3.3.2. Effect of LPS on Cytokine Expression in Porcine Colonic Tissues
3.3.3. Effect of PPE on Cytokine Expression in LPS Challenged Porcine Colonic Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salas-Salvado, J.; Becerra-Tomas, N.; Garcia-Gavilan, J.F.; Bullo, M.; Barrubes, L. Mediterranean diet and cardiovascular disease prevention: What do we know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Sergio Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Craig Willcox, D.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Barontini, M.; Bernini, R.; Carastro, R.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-derived compounds: A basis for the creation of new pharmacological agents for cancer prevention and therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Dachineni, R.; Ai, G.; Kumar, D.R.; Sadhu, S.S.; Tummala, H.; Bhat, G.J. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: A potential role in cancer prevention. Mol. Cancer Res. 2016, 14, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dachineni, R.; Kumar, D.R.; Callegari, E.; Kesharwani, S.S.; Sankaranaray, A.; Seefeldt, T.; Tummala, H.; Bhat, G.J. Salicylic acid metabolites and derivatives inhibit CDK activity: Novel insights into aspirin’s chemopreventive effects against colorectal cancer. Int. J. Oncol. 2017, 51, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Amanda Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of hydroxytyrosol-enriched fractions from Olea europaea L. by-products and evaluation of the in vitro effects on a model of colorectal cancer cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2018, 233, 41–46. [Google Scholar] [CrossRef]
- Achraf Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of pomegranate juice supplementation on oxidative stress biomarkers following weightlifting exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Manandhar, B.; Paudel, R.K. Anticancer activity of Punica granatum (pomegranate): A review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci. Hort. 2014, 179, 36–45. [Google Scholar] [CrossRef]
- Romier, C.B.; Van De Walle, J.; During, A.; Joly, A.; Rousseau, C.; Henry, O.; Schneider, Y.J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009, 47, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Romier, C.B.; Schneider, Y.J.; Larondelle, Y.; During, A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Yaidikar, L.; Thakur, S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of pro-inflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol. Cell. Biochem. 2015, 402, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Tian, H.; Li, W.; Hung, H.; Sun, F. Potential role of punicalagin against oxidative stress induced testicular damage. Asian J. Androl. 2016, 18, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in inflammatory bowel disease: A review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Omar, U.; Aloqbi, A.; Yousr, M.; Yousr, N. Protective effects of punicalagin on Caco-2 intestine cell line under oxidative stress caused by tert-butyl hydroperoxide. J. Pharmacol. Nutr. Sci. 2016, 5, 249–256. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Polyak, S.; Draganov, P.V. Colorectal cancer surveillance in inflammatory bowel disease: The search continues. World J. Gastroenterol. 2009, 15, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Kaplan, G.G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surgery 1998, 22, 382–389. [Google Scholar] [CrossRef]
- Rossi, R.E.; Whyand, T.; Murray, C.D.; Hamilton, M.I.; Conte, D.; Caplin, M.E. The role of dietary supplements in inflammatory bowel disease: A systematic review. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Kesharwania, S.S.; Ahmadb, R.; Bakkaria, M.A.; Rajputa, M.K.S.; Dachineni, R.; Valivetia, C.K.; Kapurb, S.; Bhata, G.J.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control Release 2018, 290, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Brit. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahar, B.; O’Doherty, J.V.; Maher, S.; McMorrow, J.; Sweeney, T. Chitooligosaccharide elicits acute inflammatory cytokine response through AP-1 pathway in human intestinal epithelial-like (Caco-2) cells. Mol. Immunol. 2012, 51, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, A.; Noronha, N.; Bahar, B.; Ryan, M.T.; Murray, B.A.; Kelly, P.M.; O’Loughlin, I.B.; O’Doherty, J.V.; Sweeney, T. Anti-inflammatory effects of a casein hydrolysate and its peptide-enriched fractions on TNFα-challenged Caco-2 cells and LPS-challenged porcine colonic explants. Food Sci. Nutr. 2014, 2, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, A.; Sweeney, T. Milk Proteins: Processing of Bioactive Fractions and Effects on Gut Health. In milk proteins-from structure to biological properties and health aspects. InTech 2016. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Sweeney, T. Assessment of RNA integrity in the post-mortem pig colonic tissue ex vivo. J. Anim. Sci. 2012, 90, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, F.; Barlozzari, G.; Scardigli, A.; Romani, A.; Macrì, G.; Polinori, N.; Bernini, R.; Santi, L. Leishmanicidal activity of green tea leaves and pomegranate peel extracts on L. infantum. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, F.; Bernini, R.; Basiricò, L.; Bernabucci, U.; Campo, M.; Romani, A.; Santi, L.; Lacetera, N. Antioxidant and anti-inflammatory effects of pomegranate peel extracts on bovine mammary epithelial cells BME-UV1. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: Challenges, potentialities, and perspectives on emerging approaches. Topics Curr. Chem. 2018, 376, 229–282. [Google Scholar] [CrossRef] [PubMed]
- Jerenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Bento, A.F.; Leite, D.F.P.; Marcon, R.; Claudino, R.F.; Dutra, R.C.; Cola, M.; Martini, C.A.; Calixto, J.B. Evaluation of chemical mediators and cellular response during acute and chronic gut inflammatory response induced by dextran sodium sulfate in mice. Biochem. Pharmacol. 2012, 84, 1459–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnier, D.I.; Bailey, S.R.; Schuster, R.M.; Lentsch, A.B.; Pritts, T.A. TNF-α induces vectorial secretion of IL-8 in Caco-2 cells. J. Gastrointest. Sur. 2012, 14, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leuk. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Hollebeeck, S.; Winand, J.; Hérent, M.F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012, 3, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models. An indispensable tool in food science and nutrition. Trends Food Sci. Technol. 2001, 22, S11–S20. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Noronha, N.; Bahar, B.; Ryan, M.T.; Murray, B.A.; Kelly, P.M.; O’Loughlin, I.B.; O’Doherty, J.V.; Sweeney, T. The anti-inflammatory potential of a moderately hydrolysed casein and its 5 kDa fraction in in vitro and ex vivo models of the gastrointestinal tract. Food Funct. 2015, 6, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Moue, M.; Tohno, M.; Shimazu, T.; Kido, T.; Aso, H.; Saito, T.; Kitazawa, H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kusugami, K.; Fukatsu, A.; Tanimoto, M.; Shinoda, M.; Haruta, J.I.; Kuroiwa, A.; Ina, K.; Kanayama, K.; Ando, T.; Matsuura, T.; et al. Elevation of interleukin-6 in inflammatory bowel disease is macrophage-and epithelial cell-dependent. Dig. Dis. Sci. 1995, 40, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Daig, R.; Andus, T.; Aschenbrenner, E.; Falk, W.; Schölmerich, J.; Gross, V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 1998, 38, 216–222. [Google Scholar] [CrossRef]
- Scherl, E.J.; Longman, R.S. Biomarkers stratify disease phenotype and therapeutic response in inflammatory bowel disease. Eur. Gastroenterol. Hepatol. Rev. 2012, 7, 224–228. [Google Scholar]
- Libermann, T.A.; Zerbini, L.F. Targeting transcription factors for cancer gene therapy. Curr. Gene Ther. 2006, 6, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harbor Perspect. Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opi. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Xu, X.; Yin, P.; Wan, C.; Chong, X.; Liu, M.; Cheng, P.; Chen, J.; Liu, F.; Xu, J. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation 2014, 37, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Bisignano, C.; Gugliandolo, E.; Carughi, A.; Esposito, E.; Mandalari, G.; Cuzzocrea, S. The anti-inflammatory and antioxidant potential of pistachios (Pistacia vera L.) in vitro and in vivo. Nutrients 2017, 9, 915. [Google Scholar] [CrossRef] [PubMed]




| Accession Number | Forward Primer (5′–3′) | Tm (°C) | Reverse Primer (5′–3′) | Tm (°C) | Product Length (bp) | Efficiency (%) | |
|---|---|---|---|---|---|---|---|
| Reference gene | |||||||
| ACTB | XM_001928093.1 | GCACGGCATCATCACCAA | 52.75 | CCGGAGCTCGTTGTAGAAGGT | 55.99 | 70 | 95.02 |
| B2M | NM_213978.1 | CGGAAAGCCAAATTACCTGAAC | 62.10 | TCTCCCCGTTTTTCAGCAAA | 62.20 | 83 | 103.8 |
| Cytokine gene | |||||||
| IL1A | NM_214029.1 | CAGCCAACGGGAAGATTCTG | 63.0 | ATGGCTTCCAGGTCGTCAT | 60.49 | 76 | 106.6 |
| IL1B | NM_001005149.1 | TTGAATTCGAGTCTGCCCTGT | 60.59 | CCCAGGAAGACGGGCTTT | 60.94 | 76 | 104 |
| IL6 | AB194100 | AGACAAAGCCACCACCCCTAA | 55.27 | CTCGTTCTGTGACTGCAGCTTATC | 59.92 | 69 | 99.99 |
| CXCL8 | NM_213867.1 | TGCACTTACTCTTGCCAGAACTG | 61.9 | CAAACTGGCTGTTGCCTTCTT | 61.7 | 82 | 95.7 |
| IL10 | NM_214041.1 | GCCTTCGGCCCAGTGAA | 63.4 | AGAGACCCGGTCAGCAACAA | 63.1 | 71 | 95.7 |
| IL17A | NM_001005729.1 | CCCTGTCACTGCTGCTTCTG | 60.57 | TCATGATTCCCGCCTTCAC | 60.40 | 57 | 101.2 |
| IFNG | NM_213948.1 | TCTAACCTAAGAAAGCGGAAGAGAA | 61.12 | TTGCAGGCAGGATGACAATTA | 61.54 | 81 | 94.4 |
| TNF | NM_214022.1 | TGGCCCCTTGAGCATCA | 62.5 | CGGGCTTATCTGAGGTTTGAGA | 62.8 | 68 | 91.5 |
| TGFB1 | NM_214015.1 | AGGGCTACCATGCCAATTTCT | 60.63 | CGGGTTGTGCTGGTTGTACA | 61.68 | 101 | 93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients 2019, 11, 548. https://doi.org/10.3390/nu11030548
Mastrogiovanni F, Mukhopadhya A, Lacetera N, Ryan MT, Romani A, Bernini R, Sweeney T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients. 2019; 11(3):548. https://doi.org/10.3390/nu11030548
Chicago/Turabian StyleMastrogiovanni, Fabio, Anindya Mukhopadhya, Nicola Lacetera, Marion T. Ryan, Annalisa Romani, Roberta Bernini, and Torres Sweeney. 2019. "Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants" Nutrients 11, no. 3: 548. https://doi.org/10.3390/nu11030548
APA StyleMastrogiovanni, F., Mukhopadhya, A., Lacetera, N., Ryan, M. T., Romani, A., Bernini, R., & Sweeney, T. (2019). Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients, 11(3), 548. https://doi.org/10.3390/nu11030548







