The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Overview
2.3. Plasma Parameters and Tolerance Tests in Cohorts 1 and 2
2.3.1. Fasting Blood Glucose and Insulin
2.3.2. Glucose and Insulin Tolerance Tests
2.3.3. Mixed Meal Tolerance Test
2.4. Measurements in Cohort 3 (RYGB Mice)
2.4.1. RYGB, Sham Surgery, and Weight Matching
2.4.2. Measurement of Body Weight, Body Composition, and Food Intake
2.4.3. Measurement of Energy Expenditure, Respiratory Exchange Rate (RER), and Locomotor Activity
2.4.4. Glucose and Insulin Tolerance Tests, Fasting Insulin and Leptin
2.4.5. Final Plasma and Tissue Harvest
2.5. Statistical Analysis
3. Results
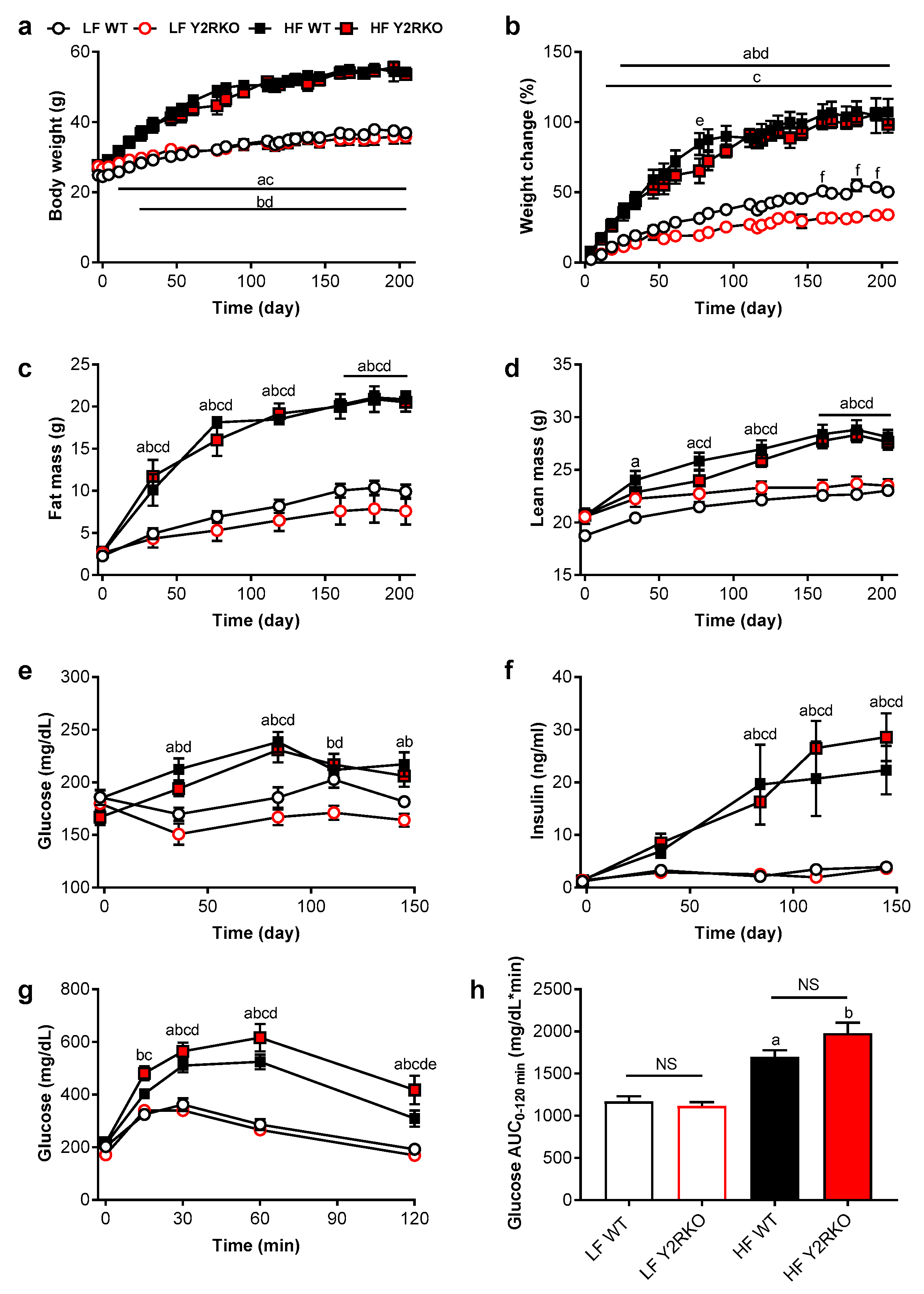
3.1. Y2R-Deficient Mice Grow Normally on Regular Chow Diet and Respond Normally to High-Fat Diet
3.2. Similar Effects of RYGB on Body Weight and Body Composition in Y2RKO and WT Mice
3.3. Similar Effects of RYGB on Food Intake And Metabolism in Y2RKO and WT Mice
3.4. Similar RYGB-Induced Improvements of Glycemic Control in Y2RKO and WT Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, D.P.; Johnson, E.; Haneuse, S.; Arterburn, D.; Coleman, K.J.; O’Connor, P.J.; O’Brien, R.; Bogart, A.; Theis, M.K.; Anau, J.; et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA 2018, 320, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kashyap, S.R. bariatric surgery or intensive medical therapy for diabetes after 5 years. N. Engl. J. Med. 2018, 376, 1997. [Google Scholar]
- Wiggins, T.; Antonowicz, S.S.; Markar, S.R. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schulman, A.R.; Thompson, C.C. Complications of bariatric surgery: What you can expect to see in your gi practice. Am. J. Gastroenterol. 2017, 112, 1640–1655. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Myung, S.K.; Cho, K.H.; Park, Y.G.; Kim, S.G.; Kim do, H.; Kim, S.M. Relationship Between Bariatric Surgery and Bone Mineral Density: A Meta-analysis. Obes. Surg. 2016, 26, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Chen, J.Y.; Courcoulas, A.P.; Dakin, G.F.; Engel, S.G.; Flum, D.R.; Hinojosa, M.W.; Kalarchian, M.A.; Mattar, S.G.; Mitchell, J.E.; et al. Alcohol and other substance use after bariatric surgery: Prospective evidence from a U.S. multicenter cohort study. Surg. Obes. Relat. Dis. 2017, 13, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lonroth, H.; Fandriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Aylwin, S.J.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Mun, E.C.; Stoyneva, V.; Mantzoros, C.S.; Goldfine, A.B. Peptide YY levels are elevated after gastric bypass surgery. Obesity 2006, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.B.; Dirksen, C.; Bojsen-Moller, K.N.; Jacobsen, S.H.; Worm, D.; Hansen, D.L.; Kristiansen, V.B.; Naver, L.; Madsbad, S.; Holst, J.J. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013, 62, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T.K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Korner, J.; Bessler, M.; Cirilo, L.J.; Conwell, I.M.; Daud, A.; Restuccia, N.L.; Wardlaw, S.L. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab. 2005, 90, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Laferrere, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Morinigo, R.; Moize, V.; Musri, M.; Lacy, A.M.; Navarro, S.; Marin, J.L.; Delgado, S.; Casamitjana, R.; Vidal, J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2006, 91, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, D.J.; Osborne, A.; Hawkins, S.C.; Mahon, D.; Ghatei, M.A.; Bloom, S.R.; Welbourn, R.; le Roux, C.W. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes. Surg. 2010, 20, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Bueter, M.; Theis, N.; Lutz, T.; Paulsen, S.; Dalboge, L.S.; Vrang, N.; Jelsing, J. Hypertrophy dependent doubling of l-cells in roux-en-y gastric bypass operated rats. PLoS ONE 2013, 8, e65696. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Zheng, H.; Townsend, R.L.; Sigalet, D.L.; Berthoud, H.R. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010, 151, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.K.; Haver, A.C.; Reidelberger, R.D. Intermittent intraperitoneal infusion of peptide YY(3-36) reduces daily food intake and adiposity in obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R39–R46. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R., Jr.; Mullins, D.E.; Pines, J.M.; Hoos, L.M.; France, C.F.; Compton, D.S.; Graziano, M.P.; Sybertz, E.J.; Strader, C.D.; Van Heek, M. Effect of chronic central administration of glucagon-like peptide-1 (7-36) amide on food consumption and body weight in normal and obese rats. Obes. Res. 1998, 6, 147–156. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Kanoski, S.E.; Alhadeff, A.L.; Grill, H.J. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011, 19, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.B.; Gregersen, N.T.; Pedersen, S.D.; Arentoft, J.L.; Ritz, C.; Schwartz, T.W.; Holst, J.J.; Astrup, A.; Sjodin, A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1248–E1256. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 22–33. [Google Scholar] [CrossRef] [Green Version]
- Fenske, W.K.; Bueter, M.; Miras, A.D.; Ghatei, M.A.; Bloom, S.R.; le Roux, C.W. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int. J. Obes. (Lond) 2012, 36, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ten Kulve, J.S.; Veltman, D.J.; Gerdes, V.E.A.; van Bloemendaal, L.; Barkhof, F.; Deacon, C.F.; Holst, J.J.; Drent, M.L.; Diamant, M.; IJzerman, R.G. Elevated Postoperative Endogenous GLP-1 Levels Mediate Effects of Roux-en-Y Gastric Bypass on Neural Responsivity to Food Cues. Diabetes Care 2017, 40, 1522–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokadem, M.; Zechner, J.F.; Margolskee, R.F.; Drucker, D.J.; Aguirre, V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2014, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Patterson, L.M.; Stylopoulos, N.; Munzberg, H.; Morrison, C.D.; Drucker, D.J.; Berthoud, H.R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R352–R362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson-Perez, H.E.; Chambers, A.P.; Ryan, K.K.; Li, B.; Sandoval, D.A.; Stoffers, D.; Drucker, D.J.; Perez-Tilve, D.; Seeley, R.J. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 2013, 62, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Niida, A.; Kanematsu-Yamaki, Y.; Asakawa, T.; Ishimura, Y.; Fujita, H.; Matsumiya, K.; Nishizawa, N.; Adachi, Y.; Mochida, T.; Tsuchimori, K.; et al. Antiobesity and emetic effects of a short-length peptide YY analog and its PEGylated and alkylated derivatives. Bioorg. Med. Chem. 2018, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, N.; Niida, A.; Masuda, Y.; Kumano, S.; Yokoyama, K.; Hirabayashi, H.; Amano, N.; Ohtaki, T.; Asami, T. Antiobesity Effect of a Short-Length Peptide YY Analogue after Continuous Administration in Mice. ACS Med. Chem. Lett. 2017, 8, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, K.; Gelegen, C.; Karra, E.; Choudhury, A.I.; Drew, M.E.; Fauveau, V.; Viollet, B.; Andreelli, F.; Withers, D.J.; Batterham, R.L. Diet and gastrointestinal bypass-induced weight loss: The roles of ghrelin and peptide YY. Diabetes 2011, 60, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, K.; Gelegen, C.; Irvine, E.E.; Choudhury, A.I.; Amouyal, C.; Andreelli, F.; Withers, D.J.; Batterham, R.L. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol. Metab. 2013, 2, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhao, Z.; Berthoud, H.R.; Ye, J. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE 2013, 8, e52922. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Small, C.J.; Kennedy, A.R.; Neary, N.M.; Sajedi, A.; Ghatei, M.A.; Bloom, S.R. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res. 2005, 1043, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Ffytche, D.H.; Rosenthal, J.M.; Zelaya, F.O.; Barker, G.J.; Withers, D.J.; Williams, S.C. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007, 450, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Naveilhan, P.; Hassani, H.; Canals, J.M.; Ekstrand, A.J.; Larefalk, A.; Chhajlani, V.; Arenas, E.; Gedda, K.; Svensson, L.; Thoren, P.; et al. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat. Med. 1999, 5, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, A.; Schwarzer, C.; Couzens, M.; Fetissov, S.; Furtinger, S.; Jenkins, A.; Cox, H.M.; Sperk, G.; Hokfelt, T.; Herzog, H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8938–8943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.C.; Ip, C.K.; Reed, F.; Sarruf, D.A.; Wulff, B.S.; Herzog, H. Y5 receptor signalling counteracts the anorectic effects of PYY3-36 in diet-induced obese mice. J. Neuroendocrinol 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Guida, C.; McCulloch, L.J.; Godazgar, M.; Stephen, S.D.; Baker, C.; Basco, D.; Dong, J.; Chen, D.; Clark, A.; Ramracheya, R.D. Sitagliptin and Roux-en-Y gastric bypass modulate insulin secretion via regulation of intra-islet PYY. Diabetes Obes. Metab. 2018, 20, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Burdyga, G.; de Lartigue, G.; Raybould, H.E.; Morris, R.; Dimaline, R.; Varro, A.; Thompson, D.G.; Dockray, G.J. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J. Neurosci. 2008, 28, 11583–11592. [Google Scholar] [CrossRef] [PubMed]
- Whited, K.L.; Tso, P.; Raybould, H.E. Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. Endocrinology 2007, 148, 4695–4703. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Townsend, R.L.; Mumphrey, M.B.; Patterson, L.M.; Ye, J.; Berthoud, H.R. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes. Surg. 2014, 24, 2145–2151. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boland, B.; Mumphrey, M.B.; Hao, Z.; Gill, B.; Townsend, R.L.; Yu, S.; Münzberg, H.; Morrison, C.D.; Trevaskis, J.L.; Berthoud, H.-R. The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery. Nutrients 2019, 11, 585. https://doi.org/10.3390/nu11030585
Boland B, Mumphrey MB, Hao Z, Gill B, Townsend RL, Yu S, Münzberg H, Morrison CD, Trevaskis JL, Berthoud H-R. The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery. Nutrients. 2019; 11(3):585. https://doi.org/10.3390/nu11030585
Chicago/Turabian StyleBoland, Brandon, Michael B. Mumphrey, Zheng Hao, Benji Gill, R. Leigh Townsend, Sangho Yu, Heike Münzberg, Christopher D. Morrison, James L. Trevaskis, and Hans-Rudolf Berthoud. 2019. "The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery" Nutrients 11, no. 3: 585. https://doi.org/10.3390/nu11030585
APA StyleBoland, B., Mumphrey, M. B., Hao, Z., Gill, B., Townsend, R. L., Yu, S., Münzberg, H., Morrison, C. D., Trevaskis, J. L., & Berthoud, H.-R. (2019). The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery. Nutrients, 11(3), 585. https://doi.org/10.3390/nu11030585




