Optimal Time and Target for Evaluating Energy Delivery after Adjuvant Feeding with Small Bowel Enteral Nutrition in Critically Ill Patients at High Nutrition Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient, ICU Setting and Nutrition Routine
2.3. Data Collection, Assessment, and Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APACHE II | acute physiology and chronic health evaluation II |
| SBEN | small bowel enteral nutrition |
| mNUTRIC | modified nutrition risk in the critically ill |
| PN | parenteral nutrition |
References
- Wang, C.Y.; Huang, C.T.; Chen, C.H.; Chen, M.F.; Ching, S.L.; Huang, Y.C. Optimal Energy Delivery, Rather than the Implementation of a Feeding Protocol, May Benefit Clinical Outcomes in Critically Ill Patients. Nutrients 2017, 9, 527. [Google Scholar] [CrossRef]
- Ladopoulos, T.; Giannaki, M.; Alexopoulou, C.; Proklou, A.; Pediaditis, E.; Kondili, E. Gastrointestinal dysmotility in critically ill patients. Ann. Gastroenterol. 2018, 31, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W. Gut Motility Issues in Critical Illness. Crit. Care Clin. 2016, 32, 191–201. [Google Scholar] [CrossRef]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2018, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- Heyland, D.K.; Stephens, K.E.; Day, A.G.; McClave, S.A. The success of enteral nutrition and ICU-acquired infections: A multicenter observational study. Clin. Nutr. 2011, 30, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Fu, P.K.; Huang, C.T.; Chen, C.H.; Lee, B.J.; Huang, Y.C. Targeted Energy Intake Is the Important Determinant of Clinical Outcomes in Medical Critically Ill Patients with High Nutrition Risk. Nutrients 2018, 10, 1731. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Drover, J.W.; Gramlich, L.; Dodek, P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN. J. Parenter. Enter. Nutr. 2003, 27, 355–373. [Google Scholar] [CrossRef]
- Marik, P.E.; Zaloga, G.P. Early enteral nutrition in acutely ill patients: A systematic review. Crit. Care Med. 2001, 29, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Heighes, P.T.; Simpson, F.; Sweetman, E.A.; Davies, A.R. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: A meta-analysis of randomised controlled trials. Intensive Care Med. 2009, 35, 2018–2027. [Google Scholar] [CrossRef]
- Dhaliwal, R.; Cahill, N.; Lemieux, M.; Heyland, D.K. The Canadian critical care nutrition guidelines in 2013: An update on current recommendations and implementation strategies. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2014, 29, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.R.; Morrison, S.S.; Bailey, M.J.; Bellomo, R.; Cooper, D.J.; Doig, G.S.; Finfer, S.R.; Heyland, D.K. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit. Care Med. 2012, 40, 2342–2348. [Google Scholar] [CrossRef]
- Acosta-Escribano, J.; Fernandez-Vivas, M.; Grau Carmona, T.; Caturla-Such, J.; Garcia-Martinez, M.; Menendez-Mainer, A.; Solera-Suarez, M.; Sanchez-Paya, J. Gastric versus transpyloric feeding in severe traumatic brain injury: A prospective, randomized trial. Intensive Care Med. 2010, 36, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Sun, S.F.; Lin, S.L.; Kang, S.P.; Chu, K.A.; Lin, C.H.; Huang, H.H. Duodenal versus gastric feeding in medical intensive care unit patients: A prospective, randomized, clinical study. Crit. Care Med. 2009, 37, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Sosnowski, K.; Tran, K.; Reeves, A.; Jones, M. A randomised controlled comparison of early post-pyloric versus early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care 2009, 13, R187. [Google Scholar] [CrossRef] [PubMed]
- Montejo, J.C.; Grau, T.; Acosta, J.; Ruiz-Santana, S.; Planas, M.; Garcia-De-Lorenzo, A.; Mesejo, A.; Cervera, M.; Sanchez-Alvarez, C.; Nunez-Ruiz, R.; et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit. Care Med. 2002, 30, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Hong, S.B.; Lim, C.M.; Koh, Y.; Seo, J.; Kim, Y.; Min, J.Y.; Huh, J.W. Comparison of Accuracy of NUTRIC and Modified NUTRIC Scores in Predicting 28-Day Mortality in Patients with Sepsis: A Single Center Retrospective Study. Nutrients 2018, 10, 911. [Google Scholar] [CrossRef]
- Jung, Y.T.; Park, J.Y.; Jeon, J.; Kim, M.J.; Lee, S.H.; Lee, J.G. Association of Inadequate Caloric Supplementation with 30-Day Mortality in Critically Ill Postoperative Patients with High Modified NUTRIC Score. Nutrients 2018, 10, 1589. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Al-Dorzi, H.M.; Tamim, H.M.; Haddad, S.H.; Jones, G.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; Sadat, M.; et al. Permissive Underfeeding or Standard Enteral Feeding in High- and Low-Nutritional-Risk Critically Ill Adults. Post Hoc Analysis of the PermiT Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef] [PubMed]
| Variables | Median | (IQR) |
|---|---|---|
| Age (year) | 69.9 | (60–79.1) |
| Sex (Female/Male) | 21/34 | |
| Body mass index (kg/m2) | 23.9 | (21.5–27.6) |
| Albumin (g/dL) | 2.5 | (2.2–2.9) |
| mNUTRIC score | 7 | (5–8) |
| APACHE II score | 27 | (24–33) |
| SOFA day 1 | 10 | (8–13) |
| SOFA day 3 (n = 54) | 9 | (6–12) |
| SOFA day 7 (n = 53) | 8 | (5–11.5) |
| Length of ICU stay (days) | 21 | (15–32) |
| Length of Hospital stay (days) | 49 | (28–72) |
| Length of ventilatory dependency (days) | 27 | (17–58) |
| Day from ICU admission to small bowel feeding | 8 | (5–13) |
| Comorbidities (n, %) | ||
| Cardiovascular disease | 37 | (67.3%) |
| Cancer | 24 | (43.6%) |
| Chronic obstructive lung disease | 18 | (32.7%) |
| Diabetes mellitus | 16 | (29.1%) |
| Uremia | 11 | (20.0%) |
| Liver cirrhosis | 11 | (20.0%) |
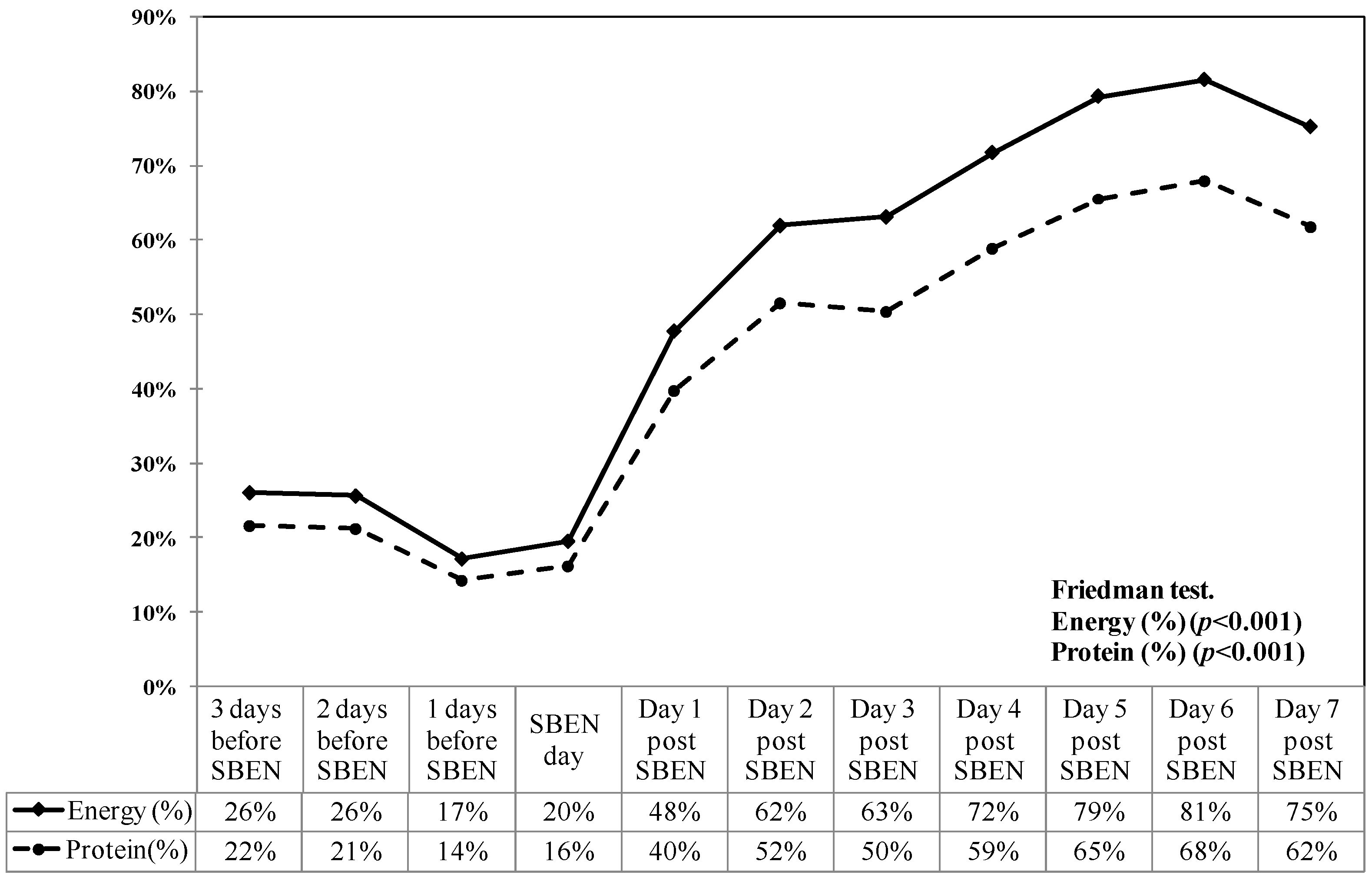
| Average Energy intake achievement rate (%) post SBEN | ||
| Day 2 | 57.6 | (35.8–70.9) |
| Day 3 | 55.9 | (41.4–76.7) |
| Day 4 | 61.6 | (41.2–75.3) |
| Day 5 | 65.0 | (42.9–78.6) |
| Day 6 | 63.8 | (45.3–81) |
| Day 7 | 66.8 | (48.9–83.4) |
| ICU mortality (n, %) | 18 | (32.7%) |
| In-hospital mortality (n, %) | 25 | (45.5%) |
| Variables | Before SBEN | Average Post-SBEN Day 3 |
|---|---|---|
| Actual feeding volume (mL/day) | 454.0 (244.3–637.3) | 948.0 (660.7–1188) ** |
| Actual energy intakes (kcal/day) | 409.2 (205.8–559.2) | 907.2 (594.6–1187.6) ** |
| Actual protein intakes (g/day) | 16.4 (8.2–22.4) | 36.3 (23.8–47.7) ** |
| Energy intake achievement rate (%) | 25.5 (16–33.9) | 55.9 (41.4–76.7) ** |
| Protein intake achievement rate (%) | 21.3 (13.3–28.2) | 46.6 (33.9–63.9) ** |
| Variables | Non-Survival (n = 25) | Survival (n = 30) |
|---|---|---|
| Age (year) | 72.9 (59.1–78.1) | 67.3 (60.2–79.6) |
| Male (n, %) | 14 (56.0%) | 20 (66.7%) |
| Body mass index (kg/m2) | 25.6 (21.1–29.8) | 23.6 (21.5–26.1) |
| Albumin (g/dL) | 2.4 (2.1–2.6) | 2.5 (2.3–3.1) |
| mNUTRIC score | 7.0 (7–8) | 6.5 (5–8) * |
| APACHE II score | 31.0 (24.5–36) | 26.0 (23.8–31) |
| SOFA day 1 | 11.0 (8.5–13.5) | 10.0 (6–12.3) |
| SOFA-day 3 (n = 54) | 11.0 (6–12.5) | 8.0 (6–11.5) |
| SOFA-day 7 (n = 53) | 10.0 (5.5–15) | 7.5 (4–10) ** |
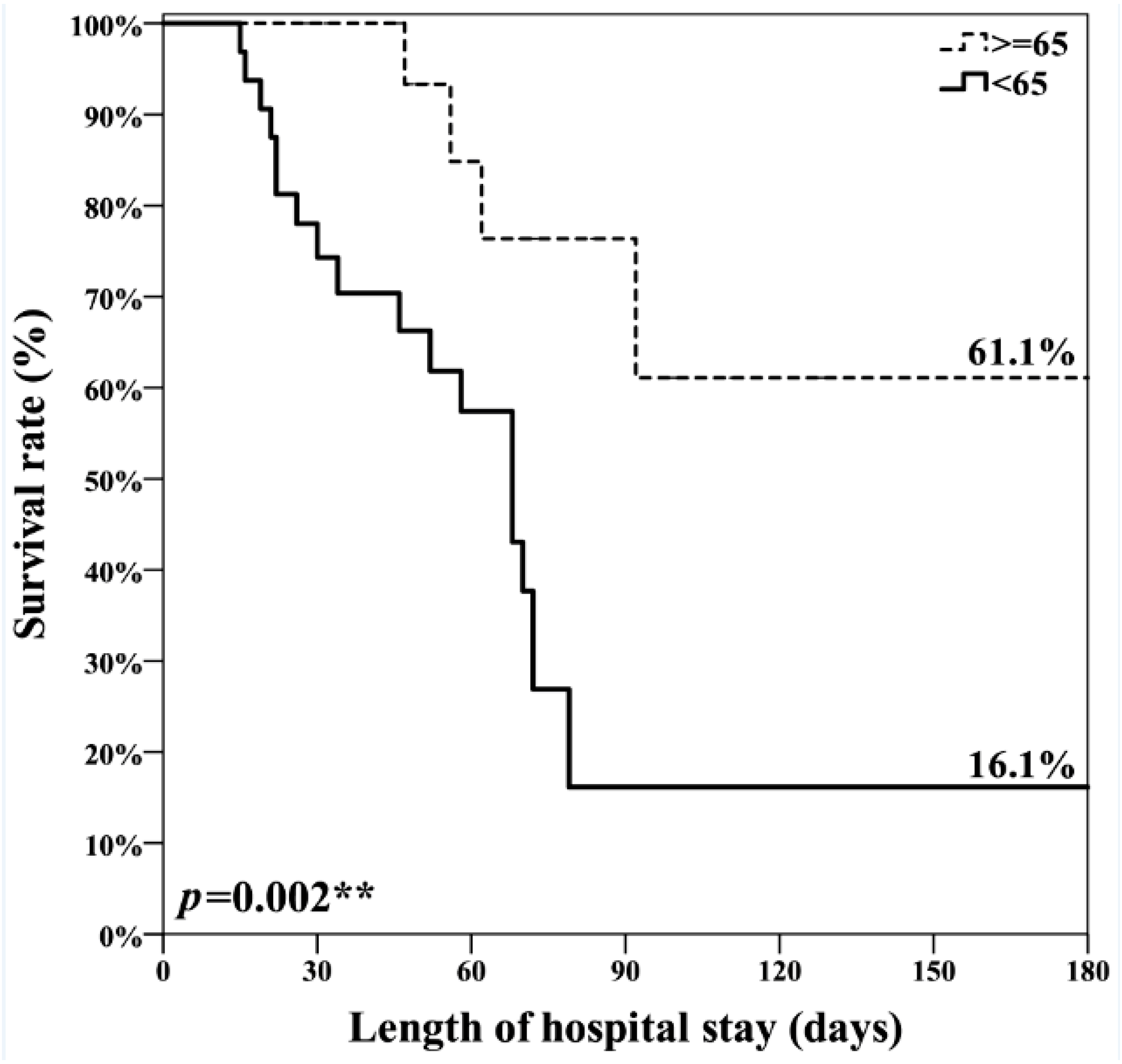
| Length of ICU stay (days) | 26.0 (19–38) | 18.0 (13.8–25.5) * |
| Length of Hospital stay (days) | 56.0 (24–71) | 48.0 (28.8–76) |
| Length of ventilatory dependency (days) | 27.0 (19.5–56.5) | 23.0 (13.8–65.3) |
| Day from ICU admission to small bowel feeding | 9.0 (5–15.5) | 7.0 (4.8–13) |
| Comorbidities (n, %) | ||
| Cardiovascular disease | 15 (60.0%) | 22 (73.3%) |
| Cancer | 8 (32.0%) | 16 (53.3%) |
| Chronic obstructive lung disease | 10 (40.0%) | 8 (26.7%) |
| Diabetes mellitus | 5 (20.0%) | 11 (36.7%) |
| Uremia | 7 (28.0%) | 4 (13.3%) |
| Liver cirrhosis | 7 (28.0%) | 4 (13.3%) |
| Average of Energy intake achievement rate (%) post SBEN | ||
| 2nd day | 45.9 (26–65.4) | 65.2 (43.2–74) * |
| 3rd day | 48.2 (27–64.1) | 67.1 (49.3–82.5) ** |
| 4th days | 43.5 (31.5–67) | 73.0 (52–86.2) ** |
| 5th days | 52.4 (29.7–68.9) | 73.6 (58.7–88.8) ** |
| 6th days | 53.1 (30.9–66.6) | 72.5 (63.1–91.3) ** |
| 7th days | 50.3 (31.7–65.2) | 75.4 (61.7–92.5) ** |
| Energy intake achievement rate (%) ≥65 (Post SBEN 3rd day) (n, %) | 5 (20.0%) | 18 (60.0%) ** |
| Title 1 | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| Age (year) | 1.01 (0.98–1.05) | |
| Gender (Female vs. Male) | 1.34 (0.60–3.01) | |
| Body mass index (kg/m2) | 0.97 (0.88–1.06) | |
| Albumin (mg/dL) | 0.51 (0.25–1.05) | |
| mNUTRIC score | 1.16 (0.85–1.59) | 0.95 (0.70–1.30) |
| APACHE II score | 1.02 (0.96–1.07) | |
| SOFA day 1 | 1.00 (0.91–1.09) | |
| SOFA day 3 | 1.04 (0.94–1.15) | |
| SOFA day 7 | 1.11 (1.00–1.24) * | 1.02 (0.91–1.15) |
| Energy intake achievement rate (%) (Post-SBEN day 3) (<65% vs. ≥65%) | 4.58 (1.55–13.53) ** | 4.97 (1.44–17.07) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-N.; Yang, M.-F.; Wang, C.-Y.; Hsu, C.-Y.; Lee, B.-J.; Fu, P.-K. Optimal Time and Target for Evaluating Energy Delivery after Adjuvant Feeding with Small Bowel Enteral Nutrition in Critically Ill Patients at High Nutrition Risk. Nutrients 2019, 11, 645. https://doi.org/10.3390/nu11030645
Wang W-N, Yang M-F, Wang C-Y, Hsu C-Y, Lee B-J, Fu P-K. Optimal Time and Target for Evaluating Energy Delivery after Adjuvant Feeding with Small Bowel Enteral Nutrition in Critically Ill Patients at High Nutrition Risk. Nutrients. 2019; 11(3):645. https://doi.org/10.3390/nu11030645
Chicago/Turabian StyleWang, Wei-Ning, Mei-Fang Yang, Chen-Yu Wang, Chiann-Yi Hsu, Bor-Jen Lee, and Pin-Kuei Fu. 2019. "Optimal Time and Target for Evaluating Energy Delivery after Adjuvant Feeding with Small Bowel Enteral Nutrition in Critically Ill Patients at High Nutrition Risk" Nutrients 11, no. 3: 645. https://doi.org/10.3390/nu11030645
APA StyleWang, W.-N., Yang, M.-F., Wang, C.-Y., Hsu, C.-Y., Lee, B.-J., & Fu, P.-K. (2019). Optimal Time and Target for Evaluating Energy Delivery after Adjuvant Feeding with Small Bowel Enteral Nutrition in Critically Ill Patients at High Nutrition Risk. Nutrients, 11(3), 645. https://doi.org/10.3390/nu11030645






