Implications of the Westernized Diet in the Onset and Progression of IBD
Abstract
:1. IBD and Environmental Risk Factors
2. The Microbiota and IBD
3. Evolution of the Human Diet
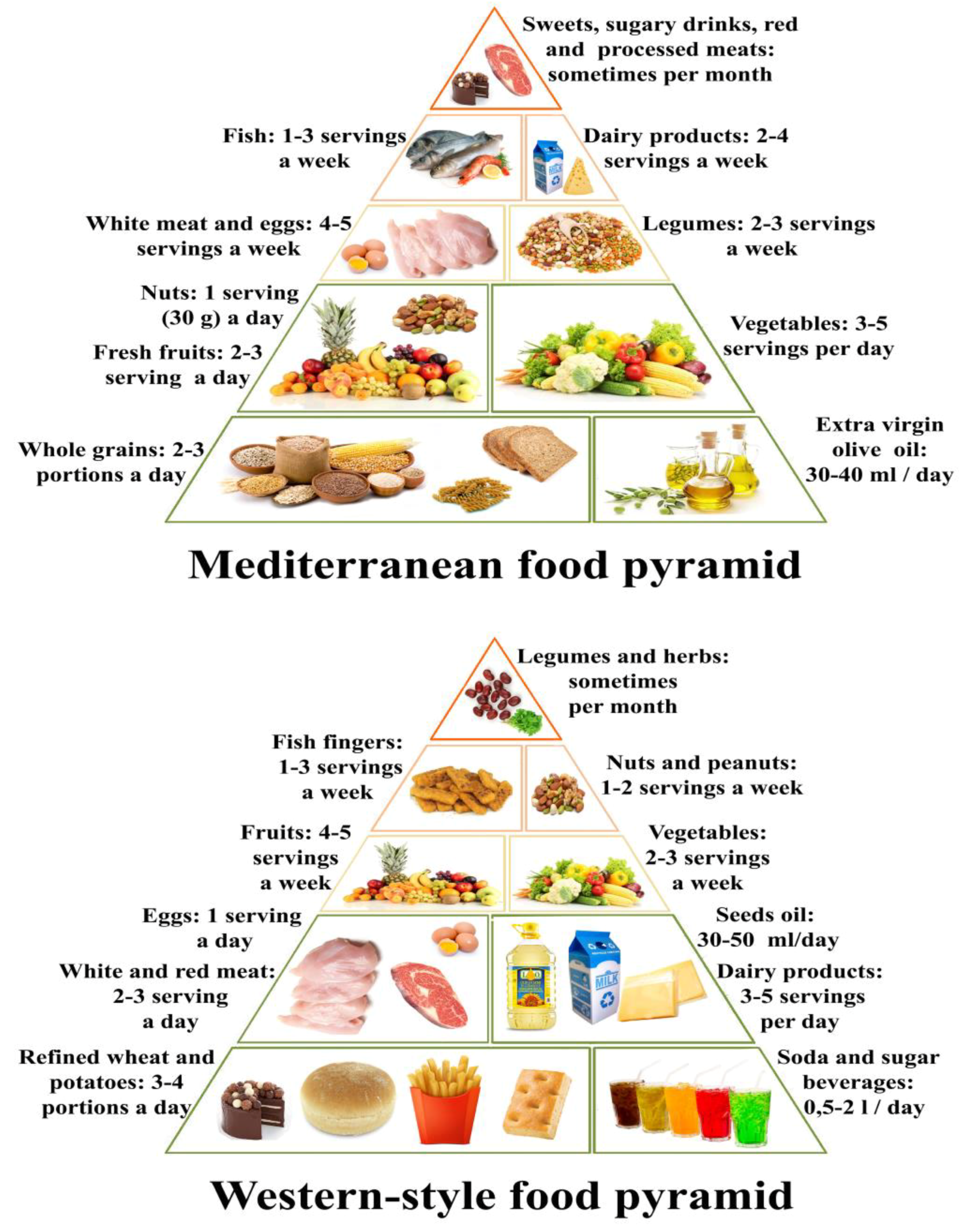
4. The Western-Style Diet and Its Pro-Inflammatory Foods
5. Carbohydrates
6. Proteins
7. Fats
8. Dietary Fibers
9. Micronutrients
10. Food Additives and Ultra-Processed Food
11. Alcohol
12. Nutritional approach in IBD
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leso, V.; Ricciardi, W.; Iavicoli, I. Occupational risk factors in inflammatory bowel disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2838–2851. [Google Scholar]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Carroll, M.W.; Singh, H.; Otley, A.R.; Vutcovici, M.; El-Matary, W.; Nguyen, G.C.; Griffiths, A.M.; et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: Distributed network analysis of multiple population-based provincial health administrative databases. Am. J. Gastroenterol. 2017, 112, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; Kaplan, G.G. Alberta Inflammatory Bowel Disease Consortium. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 1792–1799. [Google Scholar] [CrossRef]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Kamm, M.A.; Colombel, J.F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. The Gut microbiome of pediatric Crohn’s disease patients differs from healthy controls in genes that can influence the balance between a healthy and dysregulated immune response. Inflamm. Bowel Dis. 2016, 22, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ. 2016, 6, e009892. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; De Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [Green Version]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, Gut microbiome and epigenetics: Emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients 2017, 9, E962. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? Wolrd J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Park, S.M.; Opitz, N.; Lyte, M.; Gaykema, R.P. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 2008, 22, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Agostini, A.; Ballotta, D.; Righi, S.; Moretti, M.; Bertani, A.; Scarcelli, A.; Sartini, A.; Ercolani, M.; Nichelli, P.; Campieri, M.; et al. Stress and brain functional changes in patients with Crohn’s disease: A functional magnetic resonance imaging study. Neurogastroenterol. Motil. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Frolkis, A.; Dieleman, L.A.; Barkema, H.W.; Panaccione, R.; Ghosh, S.; Fedorak, R.N.; Madsen, K.; Kaplan, G.G.; Alberta IBD Consortium. Environment and the inflammatory bowel diseases. Can. J. Gastroenterol. 2013, 27, e18–e24. [Google Scholar] [CrossRef]
- Viggiano, D.; Ianiro, G.; Vanella, G.; Bibbò, S.; Bruno, G.; Simeone, G.; Mele, G. Gut barrier in health and disease: Focus on childhood. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1077–1085. [Google Scholar]
- Lopetuso, L.R.; Scaldaferri, F.; Bruno, G.; Petito, V.; Franceschi, F.; Gasbarrini, A. The therapeutic management of gut barrier leaking: The emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1068–1076. [Google Scholar]
- Carrière, J.; Darfeuille-Michaud, A.; Nguyen, H.T. Infectious etiopathogenesis of Crohn’s disease. World J. Gastroenterol 2014, 20, 12102–12117. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel diseases: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Ødum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef] [Green Version]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nat. Cell Boil. 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLOS ONE 2012, 7, e34938. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Abraham, B.P.; Quigley, E.M.M. Probiotics in inflammatory bowel disease. Gastroenterol. Clin. North Am. 2017, 46, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shao, W.; Zhang, C.; Xu, C.; Wang, Q.; Liu, H.; Sun, H.; Jiang, Z.; Gu, A. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 2017, 226, 268–276. [Google Scholar] [CrossRef]
- Mao, Q.; Manservisi, F.; Panzacchi, S.; Mandrioli, D.; Menghetti, I.; Vornoli, A.; Bua, L.; Falcioni, L.; Lesseur, C.; Chen, J.; et al. The Ramazzini Institute 13-week pilot study on glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: Effects on the microbiome. Environ. Health. 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Iszatt, N.; Janssen, S.; Lenters, V.; Dahl, C.; Stigum, H.; Knight, R.; Mandal, S.; Peddada, S.; González, A.; Midtvedt, T.; et al. Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
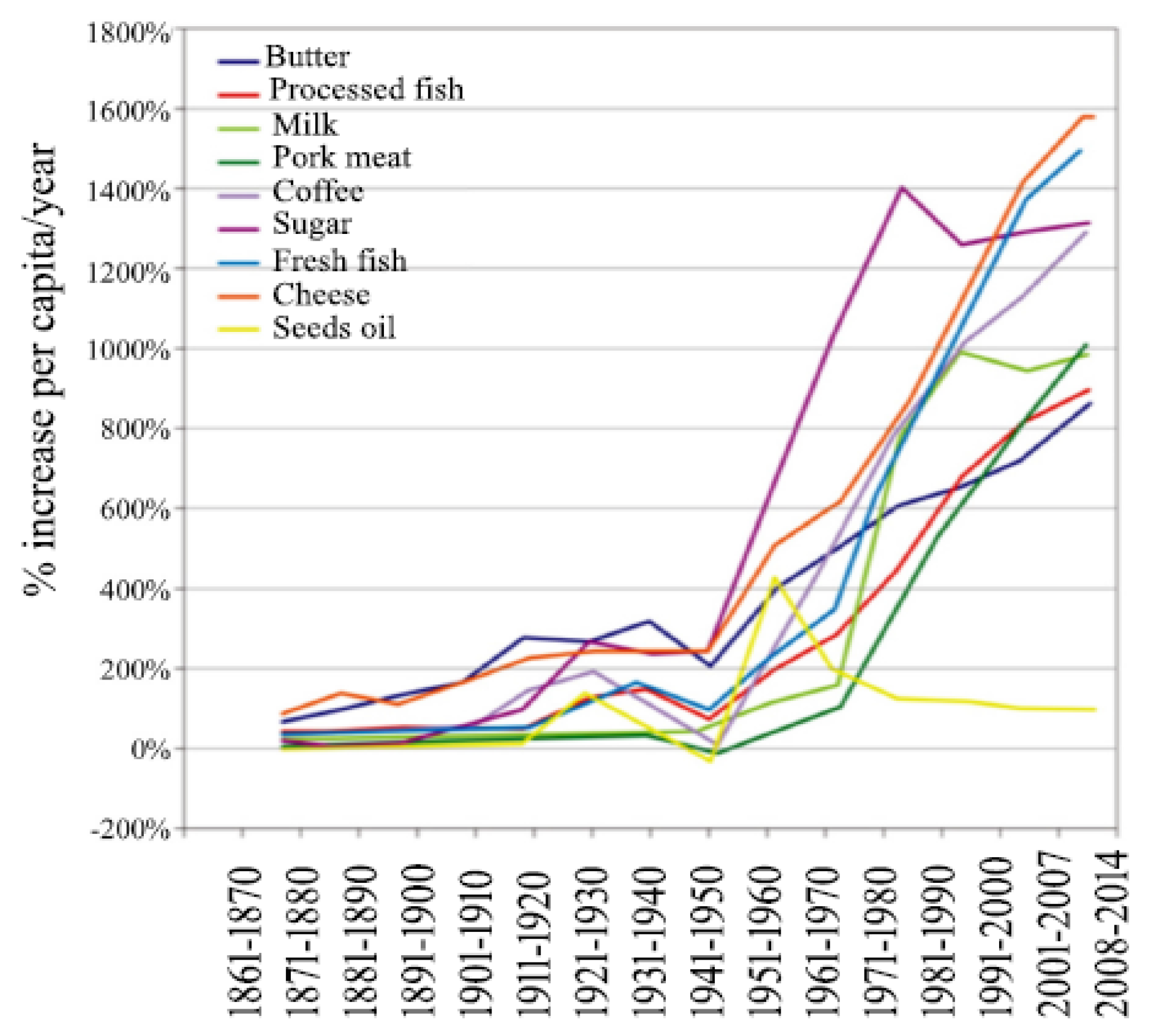
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Zeder, M.A.; Hesse, B. The initial domestication of goats (Capra hircus) in the Zagros mountains 10,000 years ago. Science 2000, 287, 2254–2257. [Google Scholar] [CrossRef] [PubMed]
- Copley, M.S.; Berstan, R.; Dudd, S.N.; Docherty, G.; Mukherjee, A.J.; Straker, V.; Payne, S.; Evershed, R.P. Direct chemical evidence for widespread dairying in prehistoric Britain. Proc. Natl. Acad. Sci. USA 2003, 100, 1524–1529. [Google Scholar] [CrossRef] [Green Version]
- Cleave, T.L. The Saccharine Disease; Stonebridge Press: Bristol, UK, 1974; pp. 6–27. [Google Scholar]
- Storck, J.; Teague, W.D. Flour for Man’s Bread, a History of Milling; University of Minnesota Press: Minneapolis, MS, USA, 1952. [Google Scholar]
- Galloway, P.J.; Thomson, G.A.; Fisher, B.M.; Semple, C.G. Insulin-induced hypoglycemia induces a rise in C-reactive protein. Diabetes Care. 2000, 23, 861–862. [Google Scholar] [CrossRef] [Green Version]
- Food Availability (Per Capita) Data System (Table: Sugar and sweeteners). Available online: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed on 6 May 2019).
- Cordain, L.; Watkins, B.A.; Florant, G.L.; Kelher, M.; Rogers, L.L.; Li, Y. Fatty acid analysis of wild ruminant tissues: Evolutionary implications for reducing diet-related chronic disease. Eur. J. Clin. Nutr. 2002, 56, 181–191. [Google Scholar] [CrossRef]
- Cordain, L. The nutritional characteristics of a contemporary diet based upon Paleolithic food groups. JANA 2002, 5, 15–24. [Google Scholar]
- Stahl, W.R. Organ weights in primates and other mammals. Science 1965, 150, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Rule, D.C.; Broughton, K.S.; Shellito, S.M.; Maiorano, G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken. J. Anim. Sci. 2002, 80, 1202–1211. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.J. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J. Gastroenterol 2016, 28, 8905–8909. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.C., Jr.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging—The pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Gotshall, R.; Eaton, S.B.; Eaton, S.B., 3rd. Physical activity, energy expenditure and fitness: An evolutionary perspective. Int. J. Sports Med. 1998, 19, 328–335. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public. Health. Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Kim, H.; Hu, E.A.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). Public Health Nutr. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Rai, S.K.; Fung, T.T.; Lu, N.; Keller, S.F.; Curhan, G.C.; Choi, H.K. The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: Prospective cohort study. BMJ 2017, 357, j1794. [Google Scholar] [CrossRef]
- Hariharan, D.; Vellanki, K.; Kramer, H. The Western diet and chronic kidney disease. Curr. Hypertens. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Melaku, Y.A.; Renzaho, A.; Gill, T.K.; Taylor, A.W.; Dal Grande, E.; De Courten, B.; Baye, E.; Gonzalez-Chica, D.; Hyppӧnen, E.; Shi, Z.; et al. Burden and trend of diet-related non-communicable diseases in Australia and comparison with 34 OECD countries, 1990–2015: Findings from the Global Burden of Disease Study 2015. Eur. J. Nutr. 2018, 58, 1299–1313. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. Cell Biol. 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.K.; Lindseth, I.A. The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, E365. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K. Dietary practices and inflammatory bowel disease. Indian. J. Gastroenterol. 2018, 37, 284–292. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. IBD: In food we trust. J. Crohns Colitis 2016, 10, 1351–1361. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and gut microbiota: A preliminary investigation in children living in rural and urban burkina faso and italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Kabi, A.; Nickerson, K.P.; McDonald, C. Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2015, 21, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Hou, J.K.; Lee, D.; Lewis, J. Diet and inflammatory bowel disease: Review of patient-targeted recommendations. Clin. Gastroenterol Hepatol. 2014, 12, 1592–1600. [Google Scholar] [CrossRef]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Kellermayer, R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease. J. Crohns Colitis 2019. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.-M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tomé, D. Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am. J. Pathol. 2017, 187, 476–486. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Kim, E. Cysteine-derived hydrogen sulfide and gut health: A matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 68–75. [Google Scholar] [CrossRef]
- Shoda, R.; Matsueda, K.; Yamato, S.; Umeda, N. Epidemiologic analysis of Crohn disease in Japan: Increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am. J. Clin. Nutr. 1996, 63, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, D.; Fan, L. Effect of personalized nutrition guidance on the birth rate of fetal macrosomia in Chinese population: A meta-analysis of nine randomized controlled trials. Cell Biochem. Biophys. 2015, 72, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Opstelten, J.L.; De Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2018, S0261–5614, 31202–31203. [Google Scholar] [CrossRef] [PubMed]
- Tasson, L.; Canova, C.; Vettorato, M.G.; Savarino, E.; Zanotti, R. Influence of diet on the course of inflammatory bowel disease. Dig. Dis. Sci. 2017, 62, 2087–2094. [Google Scholar] [CrossRef]
- Wang, Z.W.; Ji, F.; Teng, W.J.; Yuan, X.G.; Ye, X.M. Risk factors and gene polymorphisms of inflammatory bowel disease in population of Zhejiang, China. World J. Gastroenterol. 2011, 17, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Sepulveda, R.; Hing, T.; Shah, N.D.; Choe, M.; Limsui, D.; Shah, S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bianca, D.; Ficco, M.; Prandi, B.; Amaretti, A.; Anfelli, I.; Leonardi, A.; Raimondi, S.; Pecchioni, N.; De Vita, P.; Faccini, A.; et al. Comparison of gluten peptides and potential prebiotic carbohydrates in old and modern Triticum turgidum ssp. genotypes. Food Res. Int. 2018, 120, 568–576. [Google Scholar]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2018, 98, 2201–2209. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of inflammatory bowel diseases. Acta Biomed. 2018, 89, 60–75. [Google Scholar] [PubMed]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018, 154, 1037–1046. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, S.-K.; Hong, S.-J. Food elimination diet and nutritional deficiency in patients with inflammatory bowel disease. Clin. Nutr. Res. 2018, 7, 48–55. [Google Scholar] [CrossRef]
- Yılmaz, İ.; Dolar, M.E.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Shadnoush, M.; Hosseini, R.S.; Khalilnezhad, A.; Navai, L.; Goudarzi, H.; Vaezjalali, M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: A double-blind, placebo-controlled clinical trial. Korean J. Gastroenterol. 2015, 65, 215. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef]
- Andersen, V.; Chan, S.; Luben, R.; Khaw, K.T.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Grip, O.; Bergmann, M.M.; Boeing, H.; et al. Fiber intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J. Crohns Colitis 2018, 12, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275.e2. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Lee, T.H.; Hoover, R.L.; Williams, J.D.; Sperling, R.I.; Ravalese, J., 3rd; Spur, B.W.; Robinson, D.R.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. New Engl. J. Med. 1985, 312, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: A comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Wiese, D.M.; Horst, S.N.; Brown, C.T.; Allaman, M.M.; Hodges, M.E.; Slaughter, J.C.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T.; et al. Serum fatty acids are correlated with inflammatory cytokines in ulcerative colitis. PLOS ONE 2016, 11, e0156387. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Adams, D.W.; Motley, A.K.; Peyton, S.C.; Ferguson, S.L.; Horst, S.N.; Williams, C.S.; Beaulieu, D.B.; Schwartz, D.A.; et al. Serum polyunsaturated fatty acids correlate with serum cytokines and clinical disease activity in Crohn’s disease. Sci. Rep. 2019, 9, 2882. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The role of dietary nutrients in inflammatory bowel disease. Front. Immunol. 2019, 9, 3183. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.B.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-sectional analysis of overall dietary intake and Mediterranean dietary pattern in patients with Crohn’s disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef] [PubMed]
- Malavia, D.; Crawford, A.; Wilson, D. Nutritional immunity and fungal pathogenesis: The struggle for micronutrients at the host-pathogen interface. Adv. Microb. Physiol. 2017, 70, 85–103. [Google Scholar]
- Soares-Mota, M.; Silva, T.A.; Gomes, L.M.; Pinto, M.A.; Mc Mendonça, L.; Farias, M.L.F.; Nunes, T.; Ramalho, A.; Zaltman, C. High prevalence of vitamin A deficiency in Crohn’s disease patients according to serum retinol levels and the relative dose-response test. World J. Gastroenterol. 2015, 21, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Ardesia, M.; Ferlazzo, G.; Fries, W. Vitamin D and inflammatory bowel disease. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate–induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Kim, Y.S.; Lee, B.K.; Choi, J.H.; Woo, Y.M.; Kim, J.Y.; Moon, J.S. Vitamin D deficiency is associated with disease activity in patients with Crohn’s disease. Intest. Res. 2018, 17, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dadaei, T.; Safapoor, M.H.; Asadzadeh Aghdaei, H.A.; Balaii, H.; Pourhoseingholi, M.A.; Naderi, N.; Zojaji, H.; Azimzadeh, P.; Mohammadi, P.; Zali, M.R. Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterol. Hepatol. Bed Bench 2015, 8, 49–55. [Google Scholar] [PubMed]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between folate and vitamin B12 levels and inflammatory bowel disease: A meta-analysis. Nutrients 2017, 9, E382. [Google Scholar] [CrossRef] [PubMed]
- Madanchi, M.; Fagagnini, S.; Fournier, N.; Biedermann, L.; Zeitz, J.; Battegay, E.; Zimmerli, L.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. The relevance of vitamin and iron deficiency in patients with inflammatory bowel diseases in patients of the swiss IBD cohort. Inflamm. Bowel Dis. 2018, 24, 1768–1779. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Sartor, R.B. The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. J. Gastroenterol. 2014, 49, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Kakodkar, S.; Mutlu, E.A. Diet as a therapeutic option for adult inflammatory bowel disease. Gastroenterol. Clin. North Am. 2017, 46, 745–767. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G71–G77. [Google Scholar] [CrossRef] [Green Version]
- Reimund, J.-M.; Hirth, C.; Koehl, C.; Baumann, R.; Duclos, B. Antioxidant and immune status in active Crohn’s disease. A possible relationship. Clin. Nutr. 2000, 19, 43–48. [Google Scholar] [CrossRef]
- Han, Y.M.; Yoon, H.; Lim, S.; Sung, M.-K.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. Risk factors for vitamin D, zinc, and selenium deficiencies in Korean patients with inflammatory bowel disease. Gut Liver 2017, 11, 363–369. [Google Scholar] [CrossRef]
- Costa, C.S.; Rauber, F.; Leffa, P.S.; Sangalli, C.N.; Campagnolo, P.D.B.; Vitolo, M.R. Ultra-processed food consumption and its effects on anthropometric and glucose profile: A longitudinal study during childhood. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 177–184. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Patrón, S.V.; Calderón de la Barca, A.M. Old Fashioned vs. Ultra-Processed-Based current diets: Possible Implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods 2017, 6, E100. [Google Scholar] [CrossRef]
- Rother, K.I.; Conway, E.M.; Sylvetsky, A.C. How non-nutritive sweeteners influence hormones and health. Trends. Endocrinol. Metab. 2018, 29, 455–467. [Google Scholar] [CrossRef]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef]
- Chassaing, B.; Van De Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Stote, K.S.; Henderson, T.; Paul, D.R.; Okuma, K.; Tagami, H.; Kanahori, S.; Gordon, D.T.; Rumpler, W.V.; Ukhanova, M.; et al. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J. Nutr. 2014, 144, 1023–1029. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; DiNallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.V.; Van Limbergen, J.; Cahill, L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr. 2017, 5, 96. [Google Scholar] [CrossRef]
- Irwin, S.V.; Fisher, P.; Graham, E.; Malek, A.; Robidoux, A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLOS ONE 2017, 12, e0186629. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Bode, C.; Bode, J.C. Effect of alcohol consumption on the gut. Best Pr. Clin. Gastroenterol. 2003, 17, 575–592. [Google Scholar] [CrossRef]
- Salaspuro, M. Bacteriocolonic pathway for ethanol oxidation: Characteristics and implications. Ann. Med. 1996, 28, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mantzouranis, G.; Fafliora, E.; Saridi, M.; Tatsioni, A.; Glanztounis, G.; Albani, E.; Katsanos, K.H.; Christodoulou, D.K. Alcohol and narcotics use in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 649–658. [Google Scholar] [CrossRef]
- Swanson, G.R.; Sedghi, S.; Farhadi, A.; Keshavarzian, A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 2010, 44, 223–228. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Hernandez, V.; Bernigau, W.; Boeing, H.; Chan, S.S.; Luben, R.; Khaw, K.T.; Van Schaik, F.; Oldenburg, B.; Bueno-De-Mesquita, B.; et al. No association of alcohol use and the risk of ulcerative colitis or Crohn’s disease: Data from a European Prospective cohort study (EPIC). Eur. J. Clin. Nutr. 2017, 71, 512–518. [Google Scholar] [CrossRef]
- Magee, E.A.; Edmond, L.M.; Tasker, S.M.; Kong, S.C.; Curno, R.; Cummings, J.H. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr. J. 2005, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2007, CD000542. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: A randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J. Crohns Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Nakane, K.; Tsuji, T.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Ito, M.; Komatsu, M.; Yamada, K.; et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm. J. 2018, 22, 17–167. [Google Scholar] [CrossRef]
- Rajendran, N.; Kumar, D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: A pilot study. Colorectal Dis. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Gunasekeera, V.; Mendall, M.A.; Chan, D.; Kumar, D. treatment of Crohn’s disease with an IgG4-guided exclusion diet: A randomized controlled trial. Dig. Dis. Sci. 2016, 61, 1148–1157. [Google Scholar] [CrossRef]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The specific carbohydrate diet for inflammatory bowel disease: A case series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef] [PubMed]


| CNS Activity | Metabolic Regulations | Immune Homeostasis |
|---|---|---|
| GABA production in CNS Modulation of ENS and CNS function Serotonin biosynthesis | Energy source Intestinal gluconeogenesis Antilipolytic activity (WAT) Lepitin production (WAT) Release of major appetite and glucose regulatory peptides (PYY, GPL1) (L-cell) | Enhancement of IL-10 (DC) IL-18 biosynthesis (IEC) Treg polarization Reduction of TNF, IL-6, IFN (DC) IEC barrier fortification IgA promotion |
| Food Group | Include | Exclude |
|---|---|---|
| Fruit | Bananas, strawberry, raspberry, blueberry, orange, mandarin, clementine, cantaloupe, grapes, melons, lemon, lime, kiwi, passion fruit | Apple, applesauce, apricots, blackberries, cherries, nectarines, pears, peach, plum, prune, watermelon, grapefruit, dried fruit |
| Vegetables | Carrots, celery, corn, alfalfa, bean sprouts, bell pepper, broccoli (<1/2 cup), bok choy, cucumber, eggplant, green bean, kale, lettuce, potato, spinach, spring onion (green top), squash, tomato, turnip, zucchini | Brussels sprouts, asparagus, avocado, beetroot, cauliflower, cabbage, garlic, leek, mushroom, onion, pea shallot, snow pea, sweet corn, sweet potato |
| Grains | Rice, oats | Wheat, rye |
| Legumes | Certain legumes (soya) | Many legumes (chickpeas, lentils, beans) |
| Dairy | Lactose-free yoghurt and milk; almond, coconut, rice or soy “milk”, hard cheese, low-lactose cheese | Cow, goat, sheep milk, buttermilk, soymilk, soft cheese cream and ice cream |
| Beverage | Fruit juice and vegetable juices from permitted foods, wine | Soft drinks, sports drinks, fruit juice and vegetable juices from unpermitted foods, alcohol |
| Other | Maple syrup | Honey and sweeteners |
| Food Group | Include | Exclude |
|---|---|---|
| Fruit | All fresh fruit | Canned fruit (because of possible added sugars and starches) |
| Vegetables | Fresh vegetables | Canned or frozen vegetables (because of possible added sugars and starches), potatoes and yams |
| Grains | None | All cereal grain |
| Legumes | Certain legumes (lentils, split pea) | Certain legumes (chickpeas, soybeans) and soybean “milk” |
| Dairy | Lactose-free dairy (milk, cheese and yogurt) | Milk and all dairy products |
| Beverage | Wine | Instant tea, coffee and beer |
| Other | Honey, | Corn syrup, chocolate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. https://doi.org/10.3390/nu11051033
Rizzello F, Spisni E, Giovanardi E, Imbesi V, Salice M, Alvisi P, Valerii MC, Gionchetti P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients. 2019; 11(5):1033. https://doi.org/10.3390/nu11051033
Chicago/Turabian StyleRizzello, Fernando, Enzo Spisni, Elisabetta Giovanardi, Veronica Imbesi, Marco Salice, Patrizia Alvisi, Maria Chiara Valerii, and Paolo Gionchetti. 2019. "Implications of the Westernized Diet in the Onset and Progression of IBD" Nutrients 11, no. 5: 1033. https://doi.org/10.3390/nu11051033
APA StyleRizzello, F., Spisni, E., Giovanardi, E., Imbesi, V., Salice, M., Alvisi, P., Valerii, M. C., & Gionchetti, P. (2019). Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients, 11(5), 1033. https://doi.org/10.3390/nu11051033






