The Effects of High Peripubertal Caffeine Exposure on the Adrenal Gland in Immature Male and Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Experimental Design
2.3. Weighing the Adrenal Glands
2.4. Histological Analysis of the Adrenal Glands
2.5. Hormone Measurement
2.6. Statistical Analysis
3. Results
3.1. Body Weight Change
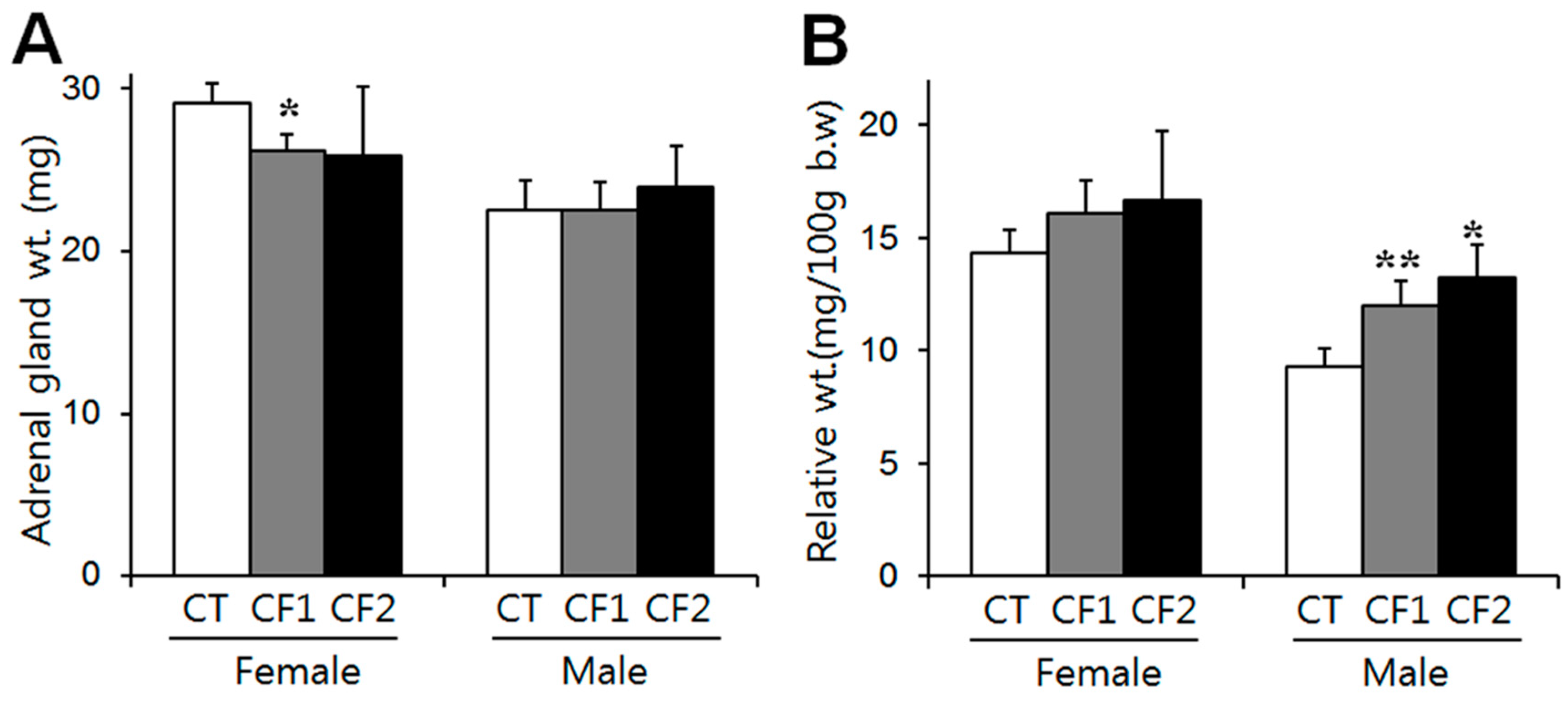
3.2. Adrenal Gland Weights
3.3. Histological Findings
3.4. Serum Corticosterone and ACTH Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated energy drinks—A growing problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Cotter, B.V.; Merchant, R.C.; Babu, K.M.; Baird, J.R.; Nirenberg, T.; Linakis, J.G. Behavioral and physiologic adverse effects in adolescent and young adult emergency department patients reporting use of energy drinks and caffeine. Clin. Toxicol. 2013, 51, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Aguila, Y.; Cuevas-Romero, E.; Castelán, F.; Martínez-Gómez, M.; Rodríguez-Antolín, J.; Nicolás-Toledo, L. Chronic stress and high sucrose intake cause distinctive morphometric effects in the adrenal glands of post-weaned rats. Biotech. Histochem. 2018, 93, 565–574. [Google Scholar] [CrossRef]
- Ribelin, W.E. The effects of drugs and chemicals upon the structure of the adrenal gland. Fundam. Appl. Toxicol. 1984, 4, 105–119. [Google Scholar] [CrossRef]
- O’Neill, C.E.; Newsom, R.J.; Stafford, J.; Scott, T.; Archuleta, S.; Levis, S.C.; Spencer, R.L.; Campeau, S.; Bachtell, R.K. Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology 2016, 67, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Wade, D.; Workman, R.; Woosley, R.L.; Oates, J.A. Tolerance to the humoral and hemodynamic effects of caffeine in man. J. Clin. Investig. 1981, 67, 1111–1117. [Google Scholar] [CrossRef]
- Wu, D.M.; He, Z.; Ma, L.P.; Wang, L.L.; Ping, J.; Wang, H. Increased DNA methylation of scavenger receptor class B type I contributes to inhibitory effects of prenatal caffeine ingestion on cholesterol uptake and steroidogenesis in fetal adrenals. Toxicol. Appl. Pharmacol. 2015, 285, 89–97. [Google Scholar] [CrossRef]
- He, Z.; Zhu, C.; Huang, H.; Liu, L.; Wang, L.; Chen, L.; Magdalou, J.; Wang, H. Prenatal caffeine exposure-induced adrenal developmental abnormality in male offspring rats and its possible intrauterine programming mechanisms. Toxicol. Res. 2016, 5, 388–398. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, B.; Liang, G.; Ping, J.; Kou, H.; Li, X.; Xiong, J.; Hu, D.; Chen, L.; Magdalou, J.; et al. Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS ONE 2012, 7, e44497. [Google Scholar] [CrossRef]
- Xing, Y.; Lerario, A.M.; Rainey, W.; Hammer, G.D. Development of adrenal cortex zonation. Endocrinol. Metab. Clin. N. Am. 2015, 44, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.D.; Sapolsky, R.M.; Meaney, M.J.; Vale, W.W.; Rivier, C.L. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology 1986, 119, 1816–1821. [Google Scholar] [CrossRef]
- Anderson, N.L.; Hughes, R.N. Increased emotional reactivity in rats following exposure to caffeine during adolescence. Neurotoxicol. Teratol. 2008, 30, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Winget, C.; Hollingshead, G.W.; Levine, S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology 1973, 12, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, Y.; Choi, H.; Yim, J.Y.; Roh, J. High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. Int. J. Endocrinol. 2015, 2015, 368475. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Choi, H.; Bae, J.; Choi, Y.Y.; Roh, J. Peri-pubertal high caffeine exposure increases ovarian estradiol production in immature rats. Reprod. Toxicol. 2017, 69, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vik, T.; Bakketeig, L.S.; Trygg, K.U.; Lund-Larsen, K.; Jacobsen, G. High caffeine consumption in the third trimester of pregnancy: Gender-specific effects on fetal growth. Paediatr. Perinat. Epidemiol. 2003, 17, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, S.R.; Skinner, M.K. Puberty in the rat. In Knobil and Neill’s Physiology of Reproduction; Elsevier: Amsterdam, The Netherlands, 2006; pp. 2061–2126. [Google Scholar]
- Huang, T.H.; Yang, R.S.; Hsieh, S.S.; Liu, S.H. Effects of caffeine and exercise on the development of bone: A densitometric and histomorphometric study in young Wistar rats. Bone 2002, 30, 293–299. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Faith, M.S.; Wang, J.; Brambilla, P.; Chiumello, G.; Heymsfield, S.B. Association of lean tissue and fat mass with bone mineral content in children and adolescents. Obes. Res. 2002, 10, 56–60. [Google Scholar] [CrossRef]
- Ishimoto, H.; Jaffe, R.B. Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocr. Rev. 2011, 32, 317–355. [Google Scholar] [PubMed]
- Mesiano, S.; Jaffe, R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997, 18, 378–403. [Google Scholar] [PubMed]
- Beach, E.F.; Turner, J.J. An enzymatic method for glucose determination in body fluids. Clin. Chem. 1958, 4, 462–475. [Google Scholar]
- Novello, L.; Speiser, P.W. Premature adrenarche. Pediatr. Ann. 2018, 47, e7–e11. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.S.; Rebuffat, P.; Malendowicz, L.K.; Mazzocchi, G.; Rocco, S.; Nussdorfer, G.G. Age-related changes in the morphology and function of the zona glomerulosa of the rat adrenal cortex. Tissue Cell 1992, 24, 835–842. [Google Scholar] [CrossRef]
- Bailey, S.A.; Zidell, R.H.; Perry, R.W. Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol. Pathol. 2004, 32, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, M.; Malendowicz, L.K. Sex differences in adrenocortical structure and function. XII. Stereologic studies of rat adrenal cortex in the course of maturation. Cell Tissue Res. 1983, 232, 457–469. [Google Scholar] [CrossRef]
- Rosol, T.J.; Yarrington, J.T.; Latendresse, J.; Capen, C.C. Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicol. Pathol. 2001, 29, 41–48. [Google Scholar] [CrossRef]
- McCormick, C.M.; Mathews, I.Z. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007, 86, 220–233. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, C.; Duan, F.; Liu, Y.; Zhang, J.; He, Z.; Huang, H.; He, C.; Wang, H. IGF1/MAPK/ERK signaling pathway-mediated programming alterations of adrenal cortex cell proliferation by prenatal caffeine exposure in male offspring rats. Toxicol. Appl. Pharmacol. 2018, 341, 64–76. [Google Scholar] [CrossRef]
- Leblanc, J.; Richard, D.; Racotta, I.S. Metabolic and hormone-related responses to caffeine in rats. Pharmacol. Res. 1995, 32, 129–133. [Google Scholar] [CrossRef]
- Patz, M.D.; Day, H.E.; Burow, A.; Campeau, S. Modulation of the hypothalamo-pituitary-adrenocortical axis by caffeine. Psychoneuroendocrinology 2006, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]




| Group | Female | Male | ||||
|---|---|---|---|---|---|---|
| CT | CF1 | CF2 | CT | CF1 | CF2 | |
| Cortical cells | 522 ± 29 | 505 ± 23 | 356 ± 79 ∗ | 936 ± 42 | 480 ± 31 ** | 585 ± 19 **’† |
| Dilated blood sinusoids (ZF) | 4 ± 0.3 | 5 ± 0.8 | 12 ± 2.1 **’‡ | 0.8 ± 1.0 | 1.3 ± 1.0 | 12 ± 1.3 **’‡ |
| Foamy swelling of cortical cell | 15 ± 7.5 | 25 ± 5.5 | 27 ± 5.3 ∗ | 35 ± 14.9 | 76 ± 7.9 ∗ | 62 ± 10.8 ∗ |
| Cell cords | 24 ± 3.5 | 15 ± 2.1 ∗ | 11 ± 3.7** | 56 ± 18.4 | 25 ± 1.9 | 43 ± 3.5 † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, K.-Y.; Roh, J. The Effects of High Peripubertal Caffeine Exposure on the Adrenal Gland in Immature Male and Female Rats. Nutrients 2019, 11, 951. https://doi.org/10.3390/nu11050951
Ryu K-Y, Roh J. The Effects of High Peripubertal Caffeine Exposure on the Adrenal Gland in Immature Male and Female Rats. Nutrients. 2019; 11(5):951. https://doi.org/10.3390/nu11050951
Chicago/Turabian StyleRyu, Ki-Young, and Jaesook Roh. 2019. "The Effects of High Peripubertal Caffeine Exposure on the Adrenal Gland in Immature Male and Female Rats" Nutrients 11, no. 5: 951. https://doi.org/10.3390/nu11050951
APA StyleRyu, K.-Y., & Roh, J. (2019). The Effects of High Peripubertal Caffeine Exposure on the Adrenal Gland in Immature Male and Female Rats. Nutrients, 11(5), 951. https://doi.org/10.3390/nu11050951





