Serum Retinol but Not 25(OH)D Status Is Associated With Serum Hepcidin Levels in Older Mexican Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochemical Analysis
2.2. Definition of Variables
2.3. Statistics
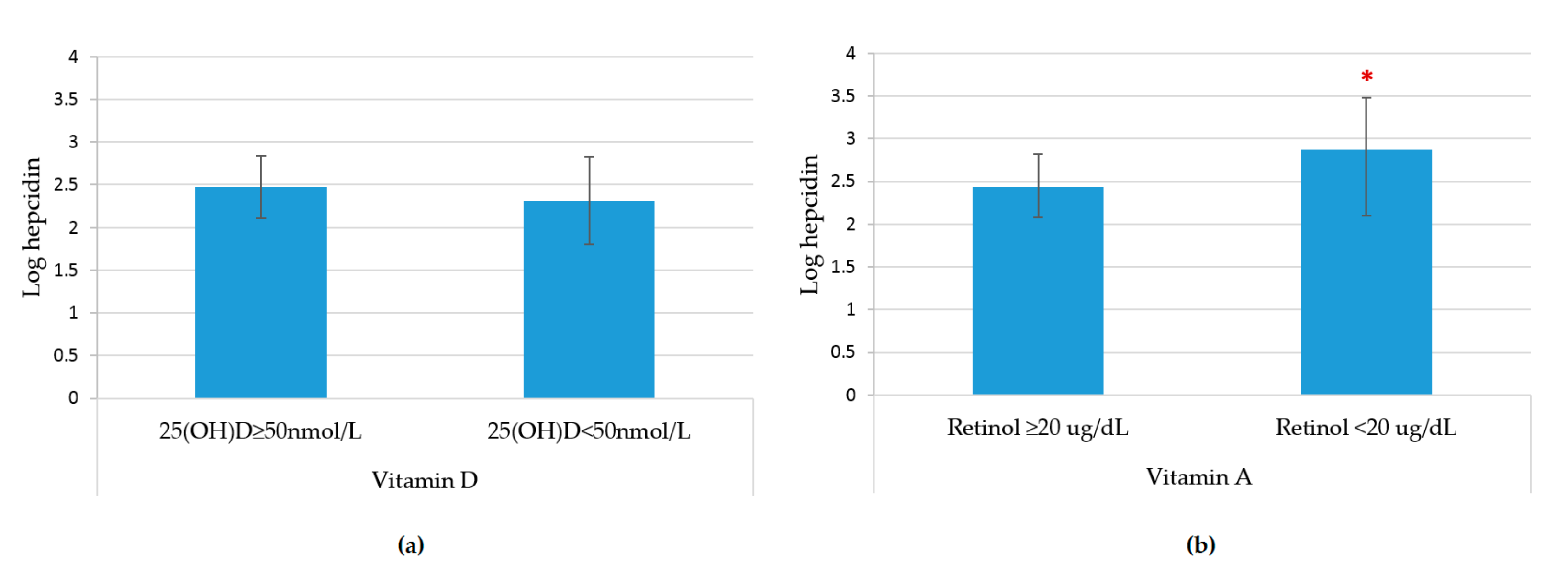
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin. Hematol. 2015, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Langer, A.L.; Ginzburg, Y.Z. Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment in anemia of chronic inflammation. Hemodial. Int. 2017, 21, 37–46. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune system dysfunction in the elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136–137, 138–147. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Cumming, R.G.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Waite, L.M.; Handelsman, D.J.; Seibel, M.J. Cross-sectional and longitudinal associations between the active vitamin D metabolite (1,25 dihydroxyvitamin D) and haemoglobin levels in older Australian men: The Concord Health and Ageing in Men Project. Age (Omaha) 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Pande, R.; Berliner, N.; Vanasse, G.J. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: Association with anemia of inflammation. Blood 2011, 117, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-dose vitamin D3 reduces circulating hepcidin concentrations: A pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin. Nutr. 2017, 36, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Citelli, M.; Bittencourt, L.L.; Da Silva, S.V.; Pierucci, A.P.T.; Pedrosa, C. Vitamin a modulates the expression of genes involved in iron bioavailability. Biol. Trace Elem. Res. 2012, 149, 64–70. [Google Scholar] [CrossRef]
- Arruda, S.F.; Siqueira, E.M.d.A.; de Valência, F.F. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition 2009, 25, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.S.B.; Campos Hankins, N.A.; Arruda, S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Biebinger, R.; Rohner, F.; Dib, A.; Zeder, C.; Hurrell, R.F.; Chaouki, N. Vitamin A supplementation in children with poor vitamin A and iron status increases erythropoietin and hemoglobin concentrations without changing total body iron. Am. J. Clin. Nutr. 2006, 84, 580–586. [Google Scholar] [CrossRef]
- Carrillo-Vega, M.F.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Vitamin D deficiency in older adults and its associated factors: A cross-sectional analysis of the Mexican Health and Aging Study. Arch. Osteoporos. 2017, 12, 8. [Google Scholar] [CrossRef]
- Min, K.-B.; Min, J.-Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1197–1203. [Google Scholar] [CrossRef]
- De la Cruz-Góngora, V. Causas de Anemia en Adultos Mayores: Papel de la Hepcidina, Vitamina A y Vitamina D. Ph.D. Thesis, Escuela de Salud Pública de México, Cuernavaca, Morelos, Mexico, 27 August 2018. [Google Scholar]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef]
- WHO. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. Available online: https://www.who.int/vmnis/indicators/retinol.pdf (accessed on 15 June 2018).
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://www.who.int/vmnis/indicators/haemoglobin/en/ (accessed on 25 May 2018).
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined indicator of vitamin B12 status: Modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- KDIGO; Society International Nephrology. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Den Elzen, W.P.J.; de Craen, A.J.M.; Wiegerinck, E.T.; Westendorp, R.G.J.; Swinkels, D.W.; Gussekloo, J. Plasma hepcidin levels and anemia in old age. The Leiden 85-plus study. Haematologica 2013, 98, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A. Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Namaste, S.M.; Aaron, G.J.; Varadhan, R.; Peerson, J.M.; Suchdev, P.S. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 333S–347S. [Google Scholar] [CrossRef]
- Colin Cameron, A.; Gelbach, J.B.; Miller, D.L. Bootstrap-based improvements for inference with clustered errors. Rev. Econ. Stat. 2008, 90, 414–427. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McLaren, G.D. Iron absorption and metabolism. Curr. Opin. Gastroenterol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional factors mediating retinoic acid signals in the control of energy metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.S.B.; Siqueira, E.M.A.; Trindade, L.S.; Arruda, S.F. Vitamin A deficiency modulates iron metabolism via ineffective erythropoiesis. J. Nutr. Biochem. 2014, 25, 1035–1044. [Google Scholar] [CrossRef]
- Mendes, J.F.R.; De Almeida Siqueira, E.M.; De Brito e Silva, J.G.M.; Arruda, S.F. Vitamin A deficiency modulates iron metabolism independent of hemojuvelin (Hfe2) and bone morphogenetic protein 6 (Bmp6) transcript levels. Genes Nutr. 2016, 11, 1. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17, 39–43. [Google Scholar] [CrossRef]
- Pesce, M.; Felaco, P.; Franceschelli, S.; Speranza, L.; Grilli, A.; De Lutiis, M.A.; Ferrone, A.; Sirolli, V.; Bonomini, M.; Felaco, M.; et al. Effect of erythropoietin on primed leucocyte expression profile. Open Biol. 2014, 4, 140026. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Alam, A.; Zimmermann, J.; Rauh, K.; Koljaja-Batzner, A.; Raff, U.; Wanner, C.; Schramm, L. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1573–1579. [Google Scholar] [CrossRef]
- Garimella, P.S.; Katz, R.; Patel, K.V.; Kritchevsky, S.B.; Parikh, C.R.; Ix, J.H.; Fried, L.F.; Newman, A.B.; Shlipak, M.G.; Harris, T.B.; et al. Association of Serum Erythropoietin with Cardiovascular Events, Kidney Function Decline, and Mortality: The Health Aging and Body Composition Study. Circ. Hear. Fail. 2016, 9, e002124. [Google Scholar] [CrossRef]
- Panwar, B.; McCann, D.; Olbina, G.; Westerman, M.; Gutiérrez, O.M. Effect of calcitriol on serum hepcidin in individuals with chronic kidney disease: A randomized controlled trial. BMC Nephrol. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Syed, S.; Michalski, E.S.; Tangpricha, V.; Chesdachai, S.; Kumar, A.; Prince, J.; Ziegler, T.R.; Suchdev, P.S.; Kugathasan, S. Vitamin D Status Is Associated with Hepcidin and Hemoglobin Concentrations in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1650–1658. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Heal. 2013, 2, 8. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Contreras-Manzano, A.; Villalpando, S.; Robledo-Pérez, R. Vitamin D status by sociodemographic factors and body mass index in Mexican women at reproductive age. Salud Publica Mex. 2017, 59, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.B.; Becker, T.; Kuhn, J.; Gummert, J.F.; Zittermann, A. Independent association of circulating vitamin D metabolites with anemia risk in patients scheduled for cardiac surgery. PLoS ONE 2015, 10, e0124751. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.P.; Rivera-Dommarco, J.Á; Shamah-Levy, T.; Villalpando-Hernández, S.; Franco, A.; Cuevas-Nasu, L.; Romero-Martínez, M.; Méndez Gómez-Humarán, I.; Hernández-Ávila, M. Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales. Available online: https://ensanut.insp.mx/informes/ENSANUT2012ResultadosNacionales.pdf (accessed on 8 June 2018).
- Vanasse, G.J.; Berliner, N. Anemia in elderly patients: An emerging problem for the 21st century. Hematology Am. Soc. Hematol. Educ. Program 2010, 2010, 271–275. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Macías, N.; Martínez-Aguilar, M.M.; Hidalgo-Bravo, A.; Flores, M.; Quezada-Sánchez, A.D.; Denova-Gutiérrez, E.; Cid, M.; Martínez-Hernández, A.; Orozco, L.; et al. Association between vitamin D deficiency and single nucleotide polymorphisms in the vitamin D receptor and GC genes and analysis of their distribution in Mexican postmenopausal women. Nutrients 2018, 10, 1175. [Google Scholar] [CrossRef]

| Characteristic | Sample (n) | Frequency (%) |
|---|---|---|
| Sex (women) | 471 | 60.2 |
| Age group (years) | ||
| 60–69 | 388 | 49.6 |
| 70–79 | 269 | 34.4 |
| 80+ | 126 | 16.1 |
| Speak an indigenous language (yes) | 264 | 33.7 |
| Tertile of SES (asset index) | 777 | |
| Tertile 1 | 261 | 33.6 |
| Tertile 2 | 269 | 34.6 |
| Tertile 3 | 247 | 31.8 |
| Body Mass Index | 770 | |
| Normal | 176 | 22.9 |
| Overweight | 291 | 37.8 |
| Obesity | 303 | 39.4 |
| CRP (>5 mg/L) | 264 | 33.7 |
| AGP (>1 g/dL) | 60 | 7.7 |
| IL6 (>10 pg/mL) | 85 | 10.9 |
| Status of inflammation | 783 | |
| Reference | 501 | 63.9 |
| Incubation | 222 | 28.3 |
| Early convalescence | 42 | 5.3 |
| Late convalescence | 18 | 2.3 |
| Functional disability | 783 | |
| ADL | 231 | 29.5 |
| IADL | 327 | 41.8 |
| Frailty | 783 | |
| Not frail | 314 | 40.1 |
| Pre-frail | 365 | 46.6 |
| Frail | 104 | 13.3 |
| Sarcopenia | 124 | 16.3 |
| Medical condition | 783 | |
| T2 Diabetes | 238 | 30.4 |
| Hypertension | 391 | 49.9 |
| Dyslipidemia | 273 | 34.8 |
| Arthritis | 95 | 12.1 |
| Cirrhosis | 157 | 20.1 |
| Cancer | 31 | 4 |
| Chronic kidney disease 1 | 187 | 23.8 |
| Use of medication | 574 | 73.3 |
| NSAID consumption | 120 | 15.3 |
| SAID consumption | 20 | 2.6 |
| Current Smoking | 195 | 24.9 |
| Anemia | 281 | 35.9 |
| Anemia causes | 783 | |
| No anemia | 502 | 64.11 |
| Renal | 81 | 10.34 |
| Nutritional | 22 | 2.81 |
| Inflammation | 41 | 5.24 |
| Multiple causes | 9 | 1.15 |
| Unexplained | 128 | 16.3 |
| Serum micronutrient deficiency 2 | ||
| B12 deficiency | 72 | 9.2 |
| Iron deficiency | 40 | 5.1 |
| Vitamin A Deficiency | 27 | 3.4 |
| Adjusted Vitamin A Deficiency | 27 | 2.4 |
| Vitamin D Deficiency | 74 | 9.5 |
| Adjusted Vitamin D Deficiency | 74 | 9.4 |
| Means of serum biochemical parameters3 | ||
| Hemoglobin (g/dL) | 783 | 12.8 ± 1.7 |
| CRP (mg/L) | 783 | 3.3 ± 3.3 |
| AGP (g/dL) | 783 | 0.5 ± 1.5 |
| IL-6 (pg/mL) | 783 | 2.7 ± 3.7 |
| EPO (mUI/mL) | 783 | 11 ± 1.6 |
| Creatinine (mg/dL) | 783 | 0.9 ± 0.6 |
| Estimated filtration glomerular rate by CKD-EPI Creatinine (mL/min/1.73 m2) | 783 | 78.9 ± 17.6 |
| Hepcidin (ng/mL) | 783 | 12.2 ± 3 |
| Vitamin D | Vitamin A | |||||
|---|---|---|---|---|---|---|
| 25(OH)D ≥50 nmol/L | 25(OH)D <50 nmol/L | p Value * | Retinol ≥20 μg/dL | Retinol <20 μg/dL | p Value * | |
| n = 709 % | n = 74 % | n = 756 % | n = 27 % | |||
| Sex (women) | 58.1 | 79.7 | <0.001 | 60.6 | 48.1 | 0.231 |
| Indigenous | 33.4 | 36.5 | 0.607 | 32.5 | 66.7 | 0.001 |
| Age group (years) | ||||||
| 60–69 | 51.2 | 33.8 | 50 | 37 | ||
| 70–79 | 33.4 | 42.2 | 34.3 | 37 | ||
| 80 and older | 15.4 | 23 | 0.012 | 15.7 | 25.9 | 0.253 |
| Tertile of SES (asset index) | ||||||
| Tertile 1 | 33.4 | 35.1 | 32.5 | 63 | ||
| Tertil 2 | 35.0 | 31.1 | 35.1 | 22.2 | ||
| Tertil 3 | 31.6 | 33.8 | 0.792 | 32.4 | 14.8 | 0.007 |
| Anemia causes | ||||||
| No anemia | 65.4 | 51.4 | 65.2 | 33.3 | ||
| Renal | 9.9 | 14.9 | 10.1 | 18.5 | ||
| Nutritional | 5.2 | 5.4 | 2.8 | 3.7 | ||
| Inflammation | 5.2 | 5.4 | 4.4 | 29.6 | ||
| Multiple causes | 0.7 | 5.4 | 1.2 | 0 | ||
| Unexplained | 16.2 | 17.6 | 0.008 | 16.4 | 14.8 | <0.001 |
| Vitamin D status | - | - | - | |||
| 25(OH)D ≥75 nmol/L | - | - | - | 48.4 | 74.1 | |
| 25(OH)D 50–74 nmol/L | - | - | - | 42.1 | 18.5 | |
| 25(OH)D <50 nmol/L | - | - | - | 9.5 | 7.4 | 0.025 |
| Retinol <20 ug/dL | 3.5 | 2.7 | 0.99 | - | - | - |
| Vitamin B12 deficiency | 10.1 | 16.2 | 0.119 | 10.4 | 18.5 | 0.202 |
| Iron deficiency | 4.8 | 8.1 | 0.218 | 5 | 7.4 | 0.643 |
| Low s-ferritin (<15 ng/mL) | 1.4 | 2.7 | 0.316 | 1.6 | 0 | 0.99 |
| Low serum iron (<60 ug/dL) | 8.5 | 9.5 | 0.826 | 7.5 | 37 | <0.001 |
| High s-ferritin (≥350 ng/mL) | 7.5 | 5.4 | 0.643 | 6.7 | 22.2 | 0.010 |
| CRP (>5 mg/L) | 34 | 31.1 | 0.699 | 32.5 | 66.7 | 0.001 |
| AGP (>1 g/L) | 7.5 | 9.5 | 0.494 | 7 | 25.9 | 0.003 |
| IL6 (>10 pg/mL) | 10.3 | 16.2 | 0.119 | 9.3 | 55.6 | <0.001 |
| Status of inflammation | ||||||
| Reference | 63.6 | 67.6 | 65.1 | 33.3 | ||
| Incubation | 28.9 | 23 | 27.9 | 40.7 | ||
| Early convalescence | 5.1 | 8.1 | 4.6 | 25.9 | ||
| Late convalescence | 2.4 | 1.4 | 0.488 | 2.4 | 0 | <0.001 |
| Body Mass Index | ||||||
| Normal | 22.8 | 23.2 | 21.9 | 50 | ||
| Overweight/Obese | 77.2 | 76.8 | 0.99 | 78.1 | 50 | 0.003 |
| Sarcopenia | 15 | 29.4 | 0.005 | 15.5 | 38.5 | 0.005 |
| Comorbidities previously diagnosed by a physician | ||||||
| Type 2 Diabetes | 29.2 | 41.9 | 0.033 | 30.4 | 29.6 | 0.99 |
| Hypertension | 48.9 | 59.5 | 0.089 | 50.1 | 44.4 | 0.696 |
| Dyslipidemia | 34.3 | 40.5 | 0.306 | 35.6 | 14.8 | 0.025 |
| Cancer | 3.8 | 5.4 | 0.524 | 4.1 | 0 | 0.620 |
| Cirrhosis | 2.1 | 4.1 | 0.238 | 1.9 | 14.8 | 0.002 |
| Chronic kidney disease 1 | 22.9 | 32.4 | 0.085 | 23.7 | 29.6 | 0.492 |
| Arthritis | 20 | 20.3 | 0.99 | 20.5 | 7.4 | 0.139 |
| Functional Disability | ||||||
| ALD | 27.4 | 50 | <0.001 | 29.2 | 37 | 0.394 |
| IALD | 39.6 | 62.2 | <0.001 | 41.3 | 55.6 | 0.165 |
| Frailty condition | ||||||
| No Frail | 42.2 | 20.3 | 40.5 | 29.6 | ||
| Pre-frail | 47 | 43.2 | 46.8 | 40.7 | ||
| Frail | 10.9 | 36.5 | <0.001 | 12.7 | 29.6 | 0.058 |
| Current smoking | 24.9 | 24.3 | 0.99 | 24.8 | 25.9 | 0.825 |
| Use of medication | 72.2 | 83.8 | 0.038 | 74.1 | 51.9 | 0.015 |
| NSAID | 14.5 | 22.9 | 0.062 | 15.6 | 7.4 | 0.411 |
| SAID | 2.4 | 4 | 0.425 | 2.5 | 3.7 | 0.509 |
| Means of serum biochemical parameters2 | ||||||
| Hemoglobin (g/dL) | 12.8 ± 1.7 | 12.1 ± 1.7 | 0.001 | 12.8 ± 1.7 | 12 ± 1.7 | 0.016 |
| CRP (mg/L) | 3.3 ± 3.3 | 3.3 ± 3 | 0.855 | 3.3 ± 3 | 10 ± 5.5 | <0.001 |
| AGP (g/dL) | 0.5 ± 1.5 | 0.6 ± 1.5 | 0.269 | 0.5 ± 1.5 | 0.5 ± 2.2 | 0.41 |
| IL-6 (pg/mL) | 2.5 ± 3.7 | 3.7 ± 3 | 0.016 | 2.5 ± 3.3 | 13.5 ± 3.3 | <0.001 |
| EPO (mUI/mL) | 11 ± 1.6 | 11 ± 1.6 | 0.269 | 10 ± 1.6 | 14.9 ± 2 | <0.001 |
| Creatinine (mg/dL) | 0.9 ± 0.5 | 1.1 ± 1.4 | 0.003 | 0.9 ± 0.7 | 0.9 ± 0.3 | 0.795 |
| Estimated filtration glomerular rate by CKD-EPI Creatinine (mL/min/1.73 m2) | 79.5 ± 16.9 | 72.7 ± 23.1 | 0.002 | 78.9 ± 17.5 | 77.4 ± 21.8 | 0.668 |
| Model 1, n = 777 | Model 2, n = 765 | Model 3, n = 765 | ||||
|---|---|---|---|---|---|---|
| Vitamin A | ||||||
| Outcome: Log of hepcidin | β | 95CI% | β | 95CI% | β | 95CI% |
| Retinol (μg/dL) | −0.003 | (−0.004, −0.003) | −0.004 | (−0.004, −0.003) | −0.003 | (−0.004, −0.002) |
| Log retinol | −0.14 | (−0.19, −0.1) | −0.15 | (−0.2, −0.09) | −0.1 | (−0.13, −0.06) |
| Decrement (10 u) | 0.03 | (0.03, 0.04) | 0.04 | (0.03, 0.04) | 0.03 | (0.02, 0.04) |
| VA deficiency | 0.43 | (0.15, 0.7) | 0.35 | (0.09, 0.62) | 0.24 | (0.08, 0.39) |
| Outcome: Tertile of hepcidin | OR | 95CI% | OR | 95CI% | OR | 95CI% |
| Retinol (μg/dL) | 0.994 | (0.993, 0.996) | 0.99 | (0.99, 0.995) | 0.99 | (0.993, 0.997) |
| Log retinol | 0.77 | (0.76, 0.78) | 0.77 | (0.73, 0.81) | 0.83 | (0.82, 0.84) |
| Decrement (10 u) | 1.06 | (1.05, 1.07) | 1.07 | (1.05, 1.08) | 1.05 | (1.03, 1.08) |
| VA deficiency | 2.29 | (1.36, 3.88) | 2.15 | (1.24, 3.74) | 1.82 | (1.23, 2.69) |
| Vitamin D | ||||||
| Outcome: Log of hepcidin | β | 95CI% | β | 95CI% | β | 95CI% |
| 25(OH)D (ng/mL) | 0.004 | (−0.002, 0.01) | 0.004 | (−0.001, 0.009) | 0.004 | (−0.002, 0.01) |
| Log vitamin D | 0.17 | (−0.04, 0.38) | 0.16 | (−0.02, 0.34) | 0.16 | (−0.03, 0.35) |
| Decrement (10 u) | −0.04 | (−0.11, 0.02) | −0.04 | (−0.09, 0.01) | −0.04 | (−0.1, 0.02) |
| VD deficiency | −0.14 | (−0.33, 0.04) | −0.18 | (−0.43, 0.06) | −0.19 | (−0.4, 0.02) |
| Outcome: Tertile of hepcidin | OR | 95CI% | OR | 95CI% | OR | 95CI% |
| 25(OH)D (ng/mL) | 1.01 | (0.99, 1.02) | 1.003 | (0.99, 1.01) | 1.003 | (0.99, 1.02) |
| Log vitamin D | 1.25 | (0.78, 1.99) | 1.17 | (0.78, 1.75) | 1.17 | (0.75, 1.83) |
| Decrement (10 u) | 0.95 | (0.83, 1.09) | 0.97 | (0.87, 1.09) | 0.97 | (0.85, 1.11) |
| VD deficiency | 0.79 | (0.53, 1.17) | 0.74 | (0.42, 1.28) | 0.73 | (0.44, 1.23) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Cruz-Góngora, V.; Salinas-Rodríguez, A.; Villalpando, S.; Flores-Aldana, M. Serum Retinol but Not 25(OH)D Status Is Associated With Serum Hepcidin Levels in Older Mexican Adults. Nutrients 2019, 11, 988. https://doi.org/10.3390/nu11050988
De la Cruz-Góngora V, Salinas-Rodríguez A, Villalpando S, Flores-Aldana M. Serum Retinol but Not 25(OH)D Status Is Associated With Serum Hepcidin Levels in Older Mexican Adults. Nutrients. 2019; 11(5):988. https://doi.org/10.3390/nu11050988
Chicago/Turabian StyleDe la Cruz-Góngora, Vanessa, Aarón Salinas-Rodríguez, Salvador Villalpando, and Mario Flores-Aldana. 2019. "Serum Retinol but Not 25(OH)D Status Is Associated With Serum Hepcidin Levels in Older Mexican Adults" Nutrients 11, no. 5: 988. https://doi.org/10.3390/nu11050988





