Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. RNAi-Mediated Knock-Down of ZIP8 and ZIP14
2.3. Antibodies
2.4. Real-Time PCR
2.5. Immunoblot Analysis
2.6. Cellular Fractionation
2.7. Cell Surface Biotinylation
2.8. ICP-MS Analysis of Metals
2.9. Preparation of Radiolabeled Metal Solutions
2.10. Metal Uptake
2.11. Statistical Analysis
3. Results
3.1. A549 Cells Express Higher Levels of ZIP8 than ZIP14
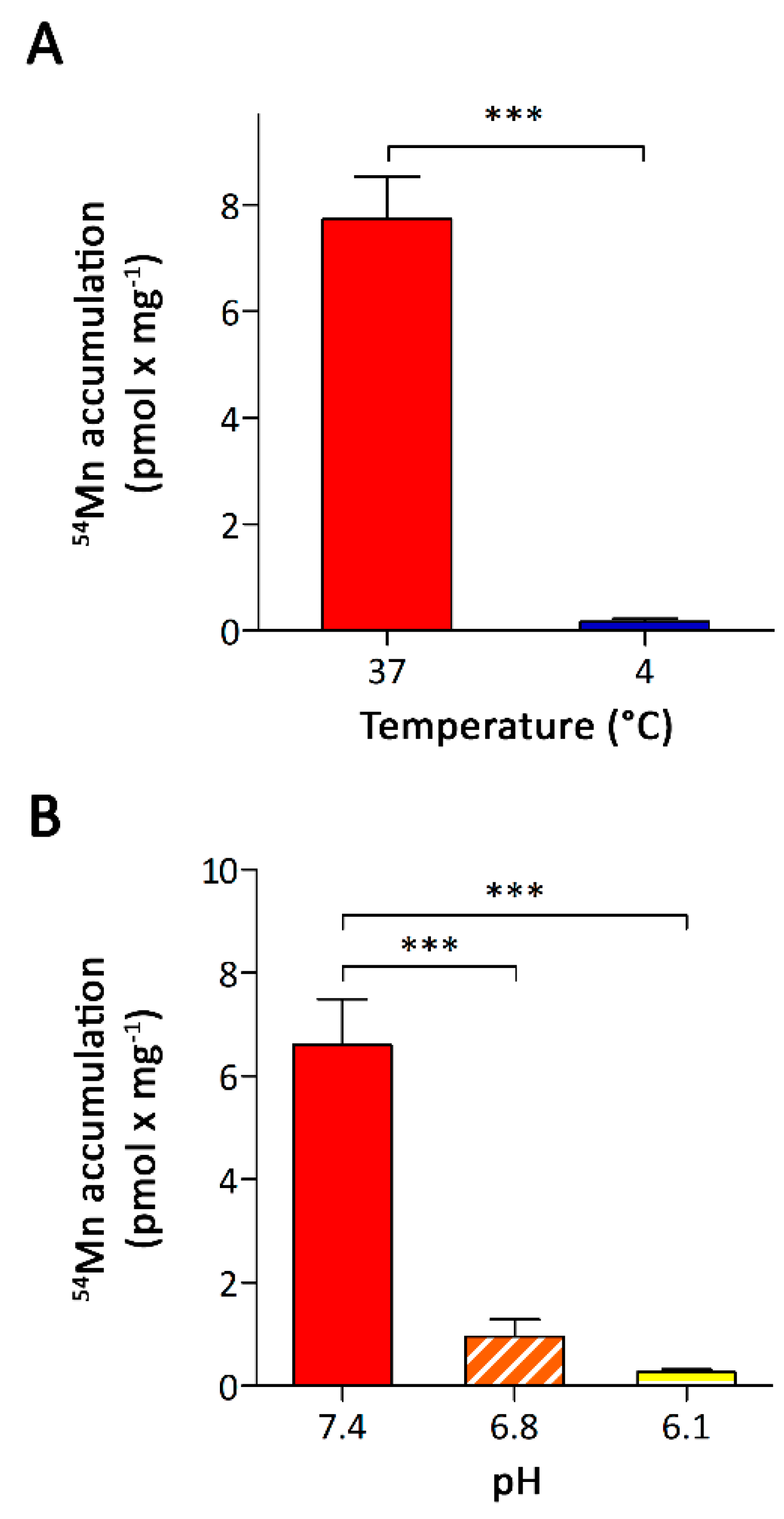
3.2. Characterization of Mn Accumulation by A549 Cells
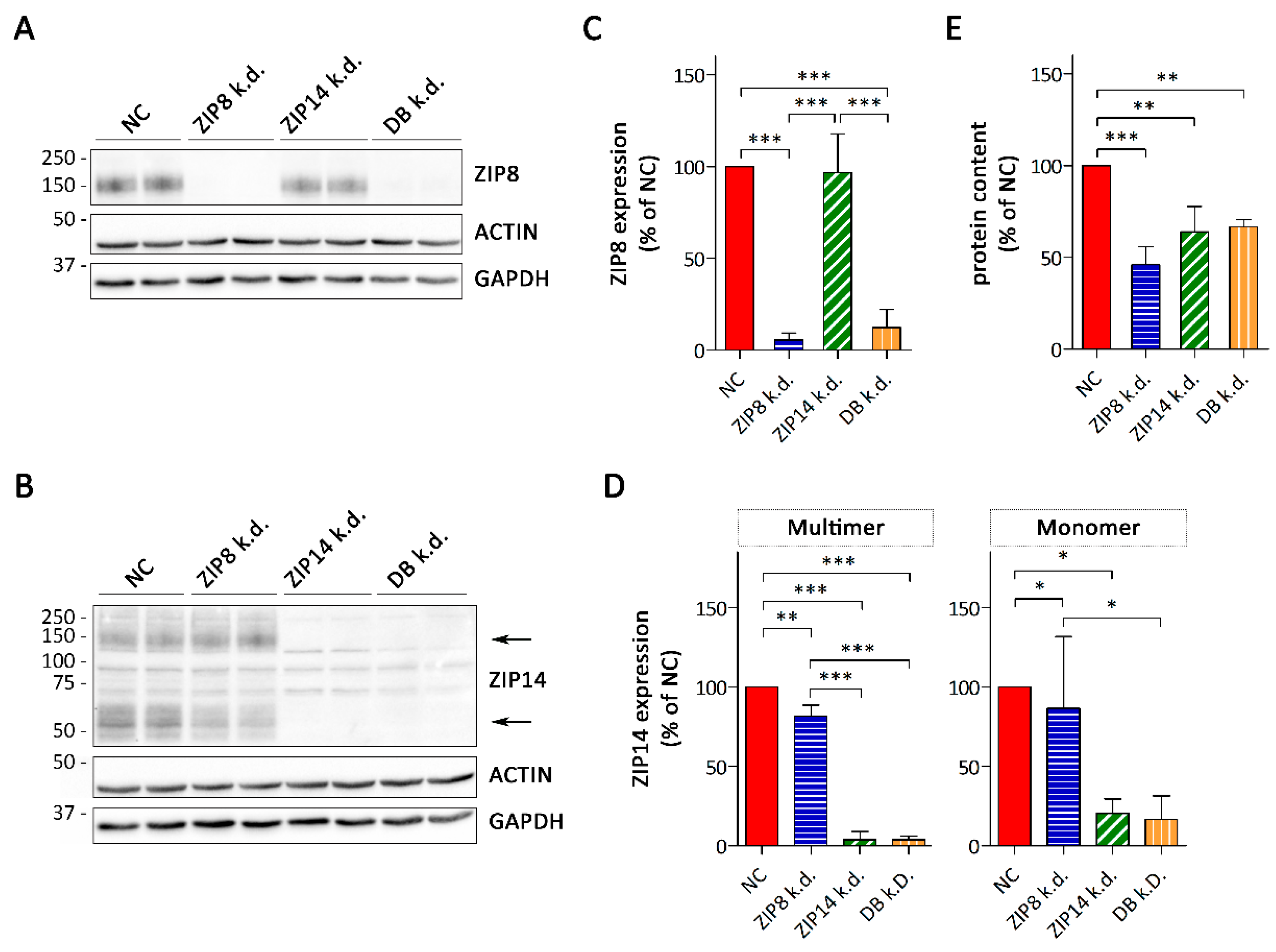
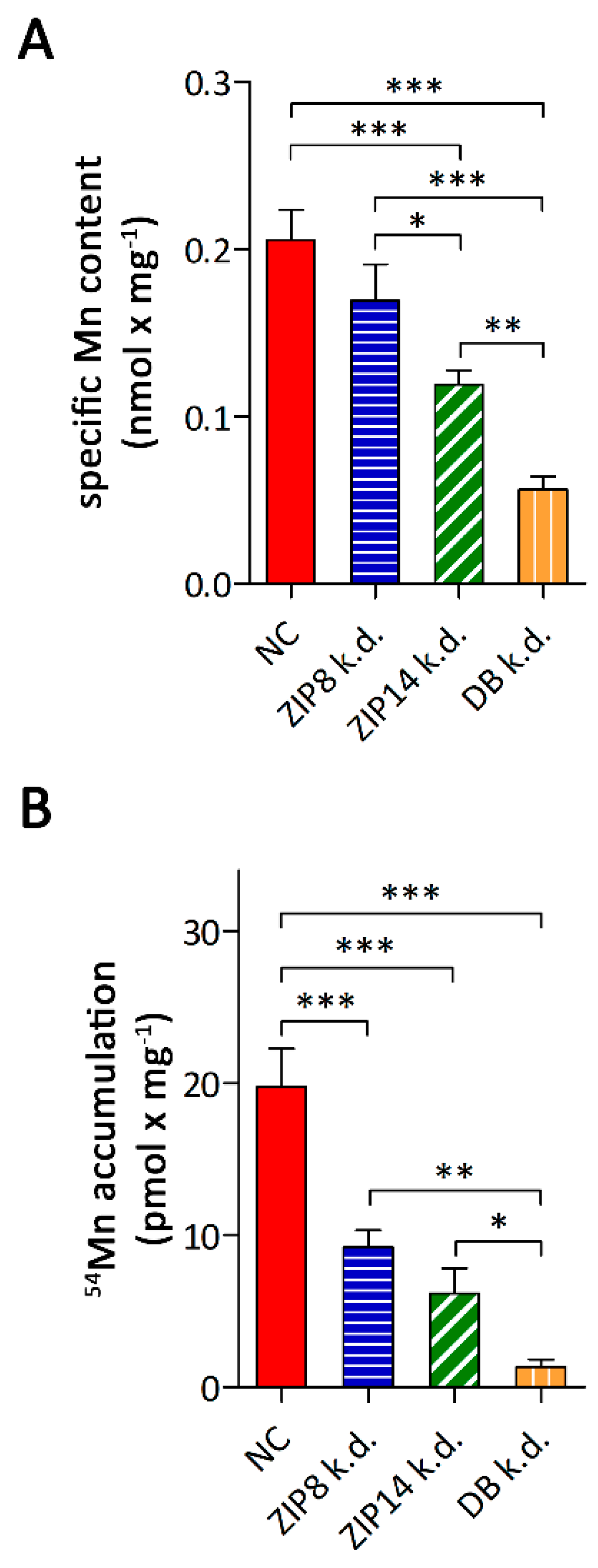
3.3. Consequences of siRNA-Mediated Knockdown of ZIP8 and ZIP14
3.4. Sucellular Localization of ZIP8 and ZIP14 in A549 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Wedler, F.C. Biochemical and nutritional role of manganese: An overview. Manganese Health Dis. 1994, 1–37. [Google Scholar]
- Peres, T.V.; Schettinger, M.R.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. “Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies”. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef]
- Kwakye, G.F.; Paoliello, M.M.; Mukhopadhyay, S.; Bowman, A.B.; Aschner, M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int. J. Environ. Res. Public Health 2015, 12, 7519–7540. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Johnson, P.E.; Johnson, L.K. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr. 1994, 60, 949–955. [Google Scholar] [CrossRef]
- Weigand, E.; Kirchgessner, M.; Helbig, U. True absorption and endogenous fecal excretion of manganese in relation to its dietary supply in growing rats. Biol. Trace Elem. Res. 1986, 10, 265–279. [Google Scholar] [CrossRef]
- Britton, A.A.; Cotzias, G.C. Dependence of manganese turnover on intake. Am. J. Physiol. 1966, 211, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.D.; Wolf, T.L.; Greger, J.L. Varying levels of manganese and iron affect absorption and gut endogenous losses of manganese by rats. J. Nutr. 1992, 122, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Dorman, D.C.; Struve, M.F.; James, R.A.; McManus, B.E.; Marshall, M.W.; Wong, B.A. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol. Sci. 2001, 60, 242–251. [Google Scholar] [CrossRef]
- Vieregge, P.; Heinzow, B.; Korf, G.; Teichert, H.M.; Schleifenbaum, P.; Mosinger, H.U. Long term exposure to manganese in rural well water has no neurological effects. Can. J. Neurol. Sci. 1995, 22, 286–289. [Google Scholar] [CrossRef]
- Brenneman, K.A.; Wong, B.A.; Buccellato, M.A.; Costa, E.R.; Gross, E.A.; Dorman, D.C. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: Toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol. Appl. Pharmacol. 2000, 169, 238–248. [Google Scholar] [CrossRef]
- Dorman, D.C.; Brenneman, K.A.; McElveen, A.M.; Lynch, S.E.; Roberts, K.C.; Wong, B.A. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. J. Toxicol. Environ. Health A 2002, 65, 1493–1511. [Google Scholar] [CrossRef]
- Schmitz, G.; Muller, G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991, 32, 1539–1570. [Google Scholar]
- Dobbs, L.G.; Johnson, M.D. Alveolar epithelial transport in the adult lung. Respir. Physiol. Neurobiol. 2007, 159, 283–300. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Richter, K.; Fronius, M. Ion transport by pulmonary epithelia. J. Biomed. Biotechnol. 2011, 2011, 174306. [Google Scholar] [CrossRef]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.; Sin, D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef]
- Heilig, E.A.; Thompson, K.J.; Molina, R.M.; Ivanov, A.R.; Brain, J.D.; Wessling-Resnick, M. Manganese and iron transport across pulmonary epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L1247–L1259. [Google Scholar] [CrossRef]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Kury, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Hogrebe, M.; Gruneberg, M.; DuChesne, I.; von der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef]
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; Mackenzie, B. ZIP14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, C862–C871. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef]
- Riley, L.G.; Cowley, M.J.; Gayevskiy, V.; Roscioli, T.; Thorburn, D.R.; Prelog, K.; Bahlo, M.; Sue, C.M.; Balasubramaniam, S.; Christodoulou, J. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J. Inherit. Metab. Dis. 2017, 40, 261–269. [Google Scholar] [CrossRef]
- Rodan, L.H.; Hauptman, M.; D’Gama, A.M.; Qualls, A.E.; Cao, S.; Tuschl, K.; Al-Jasmi, F.; Hertecant, J.; Hayflick, S.J.; Wessling-Resnick, M.; et al. Novel founder intronic variant in SLC39A14 in two families causing Manganism and potential treatment strategies. Mol. Genet. Metab. 2018, 124, 161–167. [Google Scholar] [CrossRef]
- Zeglam, A.; Abugrara, A.; Kabuka, M. Autosomal-recessive iron deficiency anemia, dystonia and hypermanganesemia caused by new variant mutation of the manganese transporter gene SLC39A14. Acta. Neurol. Belg. 2018. [Google Scholar] [CrossRef]
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet. J. Rare. Dis. 2018, 13, 28. [Google Scholar] [CrossRef]
- Juneja, M.; Shamim, U.; Joshi, A.; Mathur, A.; Uppili, B.; Sairam, S.; Ambawat, S.; Dixit, R.; Faruq, M. A novel mutation in SLC39A14 causing hypermanganesemia associated with infantile onset dystonia. J. Gene Med. 2018, 20, e3012. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [Green Version]
- Scheiber, I.F.; Wu, Y.; Morgan, S.E.; Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Liu, C.; Hutchens, S.; Jursa, T.; Shawlot, W.; Polishchuk, E.V.; Polishchuk, R.S.; Dray, B.K.; Gore, A.C.; Aschner, M.; Smith, D.R.; et al. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J. Biol. Chem. 2017, 292, 16605–16615. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Vann, D.R.; Doulias, P.T.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J.; et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Invest. 2017, 127, 2407–2417. [Google Scholar] [CrossRef] [Green Version]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef]
- Mei, Z.; Yan, P.; Wang, Y.; Liu, S.; He, F. Knockdown of zinc transporter ZIP8 expression inhibits neuroblastoma progression and metastasis in vitro. Mol. Med. Rep. 2018, 18, 477–485. [Google Scholar] [CrossRef]
- Gordon, S.J.V.; Fenker, D.E.; Vest, K.E.; Padilla-Benavides, T. Manganese influx and expression of ZIP8 is essential in primary myoblasts and contributes to activation of SOD2. Metallomics 2019. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Sitren, H.S.; Cousins, R.J. The zinc transporter ZIP14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology 2012, 142, 1536–1546.e5. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Ding, D.; Salvi, R.; Roth, J.A. Cellular localization and developmental changes of ZIP8, ZIP14 and transferrin receptor 1 in the inner ear of rats. Biometals 2014, 27, 731–744. [Google Scholar] [CrossRef]
- Zang, Z.S.; Xu, Y.M.; Lau, A.T.Y. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol. Res. (Camb) 2016, 5, 987–1002. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.J.; Hein, J.; Baez, A.; Sosa, J.C.; Wessling-Resnick, M. Manganese transport and toxicity in polarized WIF-B hepatocytes. Am. J. Physiol. Gastrointest Liver Physiol. 2018, 315, G351–G363. [Google Scholar] [CrossRef]
- Besecker, B.; Bao, S.; Bohacova, B.; Papp, A.; Sadee, W.; Knoell, D.L. The human zinc transporter SLC39A8 (ZIP8) is critical in zinc-mediated cytoprotection in lung epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L1127–L1136. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Liuzzi, J.P.; McClellan, S.; Cousins, R.J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J. Leukoc. Biol. 2009, 86, 337–348. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, A.S.; Wortham, A.M.; Jue, S.; Knutson, M.D.; Enns, C.A. The Tumor Suppressor, P53, Decreases the Metal Transporter, ZIP14. Nutrients 2017, 9, 1335. [Google Scholar] [CrossRef]
- Levy, M.; Elkoshi, N.; Barber-Zucker, S.; Hoch, E.; Zarivach, R.; Hershfinkel, M.; Sekler, I. Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn(2+) is driven by an active Ca(2+)-coupled exchange. J. Biol. Chem. 2019, 294, 5879–5889. [Google Scholar] [CrossRef]
- Choi, E.K.; Nguyen, T.T.; Gupta, N.; Iwase, S.; Seo, Y.A. Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Sci. Rep. 2018, 8, 3163. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiber, I.F.; Alarcon, N.O.; Zhao, N. Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14. Nutrients 2019, 11, 1473. https://doi.org/10.3390/nu11071473
Scheiber IF, Alarcon NO, Zhao N. Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14. Nutrients. 2019; 11(7):1473. https://doi.org/10.3390/nu11071473
Chicago/Turabian StyleScheiber, Ivo F., Neftali Ortega Alarcon, and Ningning Zhao. 2019. "Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14" Nutrients 11, no. 7: 1473. https://doi.org/10.3390/nu11071473
APA StyleScheiber, I. F., Alarcon, N. O., & Zhao, N. (2019). Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14. Nutrients, 11(7), 1473. https://doi.org/10.3390/nu11071473




