Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies
Abstract
:1. Introduction
1.1. Kidney Function in Health and Disease
1.2. Resveratrol
2. Resveratrol’s Effects on Kidney Disease
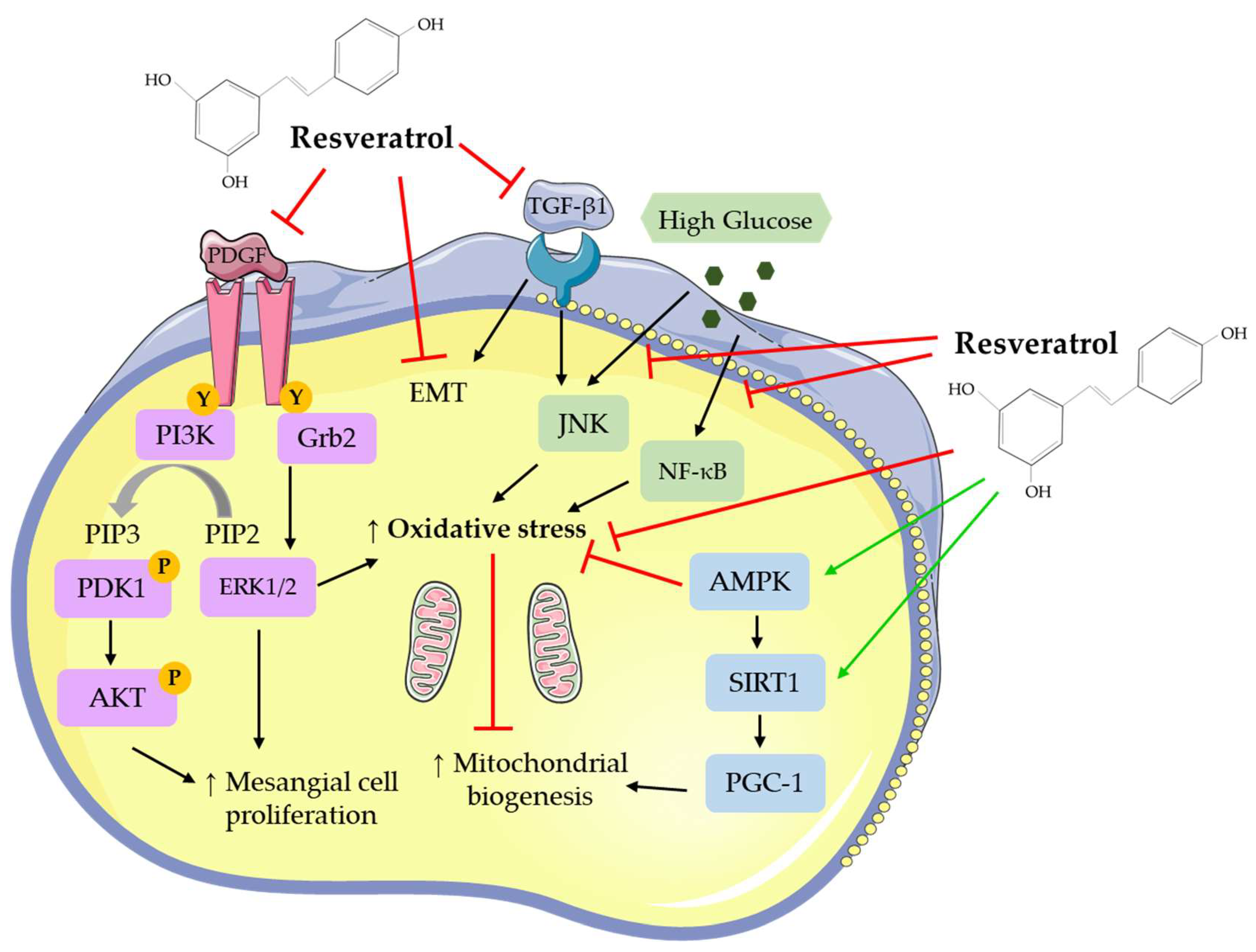
2.1. In Vitro Studies: Effects of Resveratrol on Mesangial Cells
2.2. In Vitro Studies: Effects of Resveratrol on Renal Epithelial Cells
2.3. In Vitro Studies: Effects of Resveratrol on Cells of the Renal Corpuscle
2.4. In Vitro Studies: Effects of Resveratrol on Embryonic Kidney Cells
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| HEK293 cells | 20 μM; 24 h | ↑Egr-1 protein ↑Egr-1 reporter mRNA ↑Ph-ERK1/2 protein ↓MKP-1 activity ↑Elk-1 transcriptional activation potential | [90] |
| HEK293 cells | 25 μM; 24–48 h | ↓OTA-induced
↑GSH levels | [92] |
| HEK293 cells | 2.5, 5, and 10 μM; 12–48 h | ↓High glucose-induced Aging β-galactosidase mRNA TXNIP mRNA ↑SIRT1 mRNA ↑Trx mRNA | [93] |
2.5. In Vitro Studies: Effects of Resveratrol on Kidney Fibroblasts
2.6. In Vitro Studies: Effects of Resveratrol on Renal Cancer Cells
2.7. In Vivo Animal Studies: Effects of Resveratrol on Diabetic Nephropathy
2.8. In Vivo Animal Studies: Effects of Resveratrol on Renal Fibrosis
3. Effects of Resveratrol on Human Kidneys
| Patients | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Nondialyzed CKD patients | 500 mg/day; 4 weeks | No significant effects | [112] |
| PD patients | 150 and 450 mg/day; 12 weeks | ↓UF volume and rate ↓PDE VEGF, Flk-1 and Ang-2 ↑PDE Tie-2 and Tsp-1 | [113] |
| T2DM patients | 10 mg/day; 4 weeks | ↑Kidney filtration ↑Insulin sensitivity ↓Glucose levels ↓Lipid levels ↓Serum creatinine | [116] |
| T2DM patients | 250 mg/day; 4 months | ↑Kidney function ↓Cholesterol levels ↓Triglyceride levels ↓Serum creatinine ↓Total protein excretion ↓Urea nitrogen levels | [117] |
4. Effects of RSV at the Cellular/Molecular Level
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Vart, P.; Grams, M.E. Measuring and assessing kidney function. Semin. Nephrol. 2016, 36, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.R.; Quaggin, S.E.; Hoenig, M.P.; Dworkin, L.D. The glomerulus: The sphere of influence. Clin. J. Am. Soc. Nephrol. 2014, 9, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Mitrakou, A. Kidney: Its impact on glucose homeostasis and hormonal regulation. Diabetes Res. Clin. Pr. 2011, 93, S66–S72. [Google Scholar] [CrossRef]
- Dalal, R.; Sehdev, J.S. Physiology, renal, blood flow and filtration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Kurtz, S.B. Renal Function: Mechanisms Preserving Fluid and Solute Balance in Health. Mayo Clin. Proc. 1984, 59, 210. [Google Scholar] [CrossRef]
- Hansell, P.; Welch, W.J.; Blantz, R.C.; Palm, F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin. Exp. Pharmacol. Physiol. 2013, 40, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Robson, L. The kidney—An organ of critical importance in physiology. J. Physiol. 2014, 592, 3953–3954. [Google Scholar] [CrossRef] [PubMed]
- Schlondorff, D. The glomerular mesangial cell: An expanding role for a specialized pericyte. FASEB J. 1987, 1, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Pavenstädt, H. Roles of the podocyte in glomerular function. Am. J. Physiol. Physiol. 2000, 278, 173. [Google Scholar] [CrossRef]
- Strutz, F.; Zeisberg, M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2992–2998. [Google Scholar] [CrossRef]
- Scott, R.P.; Quaggin, S.E. The cell biology of renal filtration. J. Cell Boil. 2015, 209, 199–210. [Google Scholar] [CrossRef]
- Smith, P.L.; Buffington, D.A.; Humes, H.D. Kidney epithelial cells. In Methods in Enzymology; Klimanskaya, I., Lanza, R., Eds.; Adult Stem Cells; Academic Press: London, UK, 2006; Volume 419, pp. 194–207. [Google Scholar]
- Subramanya, A.R.; Ellison, D.H. Distal convoluted tubule. CJASN 2014, 9, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.P.; Basit, H.; Knohl, S.J. Physiology, glomerular filtration rate (GFR). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Musso, C.G.; Álvarez-Gregori, J.; Jáuregui, J.; Macías-Núñez, J.F. Glomerular filtration rate equations: A comprehensive review. Int. Urol. Nephrol. 2016, 48, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Issar, T.; Krishnan, A.V.; A Pussell, B. Neurological complications in chronic kidney disease. JRSM Cardiovasc. Dis. 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Goldsmith, D.; De Jongh, R. Vitamin D and osteoporosis in chronic kidney disease. J. Nephrol. 2017, 30, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Iorember, F.M. Malnutrition in chronic kidney disease. Front Pediatr. 2018, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Hemmelgarn, B.; Culleton, B.; Tobe, S.; McFarlane, P.; Ruzicka, M.; Burns, K.; Manns, B.; White, C.; Madore, F.; et al. Guidelines for the management of chronic kidney disease. Can. Med Assoc. J. 2008, 179, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, P.; Vasa, P.; Brenner, D.; Iglar, K.; McFarlane, P.; Morrison, H.; Badawi, A. Prevalence estimates of chronic kidney disease in Canada: Results of a nationally representative survey. Can. Med Assoc. J. 2013, 185, E417–E423. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Heal. Organ. 2018, 96, 414–422. [Google Scholar] [CrossRef]
- Gajjala, P.R.; Sanati, M.; Jankowski, J. Cellular and molecular mechanisms of chronic kidney disease with diabetes mellitus and cardiovascular diseases as its comorbidities. Front. Immunol. 2015, 6, 6. [Google Scholar] [CrossRef]
- Gewin, L.; Zent, R.; Pozzi, A. Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int. 2017, 91, 552–560. [Google Scholar] [CrossRef]
- Zoja, C.; Abbate, M.; Remuzzi, G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol. Dial. Transplant. 2015, 30, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Bauer, C.; Abramowitz, M.K.; Melamed, M.L.; Hostetter, T.H. Treatment of chronic kidney disease. Kidney Int. 2012, 81, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, S.D.; Blakeman, T.; Blakeman, T. Chronic kidney disease: Identification and management in primary care. Pragmatic Obs. Res. 2016, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lee-Law, P.Y.; Van De Laarschot, L.F.; Banales, J.M.; Drenth, J.P. Genetics of polycystic liver diseases. Curr. Opin. Gastroenterol. 2019, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef]
- Shahbazian, H.; Rezaii, I. Diabetic kidney disease; review of the current knowledge. J. Renal. Inj. Prev. 2013, 2, 73–80. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Nabi, S.; Kessler, E.R.; Bernard, B.; Flaig, T.W.; Lam, E.T. Renal cell carcinoma: A review of biology and pathophysiology. F1000Res. 2018, 7, 307. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of renal cell carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Barata, P.C.; Rini, B.I. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Prevention of metabolic diseases: Fruits (including fruit sugars) vs. vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.-U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrition 2018, 11, 53. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Sautter, C.K.; DeNardin, S.; Alves, A.O.; Mallmann, C.A.; Penna, N.G.; Hecktheuer, L.H. Determinação de resveratrol em sucos de uva no Brasil. Food Sci. Technol. 2005, 25, 437–442. [Google Scholar] [CrossRef]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 888, 1–7. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.A.; Bartolo, M.; Maciąg, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Fan, X.; Tan, H.; Zeng, H.; Wang, Y.; Chen, P.; Huang, M.; Bi, H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1–p53 signaling pathways. Toxicol. Lett. 2015, 236, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Peiyuan, H.; Zhiping, H.; Chengjun, S.; Chunqing, W.; Bingqing, L.; Imam, M.U. Resveratrol ameliorates experimental alcoholic liver disease by modulating oxidative stress. Evid. Based Complement. Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L.; Laguillier-Morizot, C.; Laguillier-Morizot, C.; Nivet-Antoine, V.; Laguillier-Morizot, C. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- El-Mohsen, M.A.; Bayele, H.; Kuhnle, G.; Gibson, G.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; E Spencer, J.P. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br. J. Nutr. 2006, 96, 62–70. [Google Scholar] [CrossRef]
- Juan, M.E.; Maijó, M.; Planas, J.M. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Espín, J.C.; Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T.; et al. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [Green Version]
- Burkon, A.; Somoza, V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides—Two novel resveratrol metabolites in human plasma. Mol. Nutr. Food Res. 2008, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Kitching, A.R.; Hutton, H.L. The players: Cells involved in glomerular disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 1664–1674. [Google Scholar] [CrossRef]
- Uchida, Y.; Yamazaki, H.; Watanabe, S.; Hayakawa, K.; Meng, Y.; Hiramatsu, N.; Kasai, A.; Yamauchi, K.; Yao, J.; Kitamura, M. Enhancement of NF-kappaB activity by resveratrol in cytokine-exposed mesangial cells. Clin. Exp. Immunol. 2005, 142, 76–83. [Google Scholar] [CrossRef]
- Chen, F.; Castranova, V.; Shi, X.; Demers, L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999, 45, 7–17. [Google Scholar] [PubMed]
- Morales, A.I.; Rodríguez-Barbero, A.; Vicente-Sánchez, C.; Mayoral, P.; Lopez-Novoa, J.M.; Pérez-Barriocanal, F. Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sci. 2006, 78, 2373–2377. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Ghosh-Choudhury, N.; Das, F.; Mahimainathan, L.; Kamat, A.; Kasinath, B.S.; Abboud, H.E.; Choudhury, G.G. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: Role of PTP1B. FASEB J. 2008, 22, 3469–3482. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nie, L.; Yin, Y.-G.; Tang, J.-L.; Zhou, J.-Y.; Li, D.-D.; Zhou, S.-W. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 2012, 259, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Boil. 2012, 44, 629–638. [Google Scholar] [CrossRef]
- Ji, H.; Wu, L.; Ma, X.; Ma, X.; Qin, G. The effect of resveratrol on the expression of AdipoR1 in kidneys of diabetic nephropathy. Mol. Boil. Rep. 2014, 41, 2151–2159. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Cui, W.; Yuan, H.; Sun, J.; Wu, M.; Guo, Q.; Kong, L.; Wu, H.; Miao, L. Resveratrol prevention of diabetic nephropathy is associated with the suppression of renal inflammation and mesangial cell proliferation: Possible roles of Akt/NF-κB pathway. Int. J. Endocrinol. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Qiao, Y.; Gao, K.; Wang, Y.; Wang, X.; Cui, B. Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-β1 pathway. Exp. Ther. Med. 2017, 13, 3223–3230. [Google Scholar] [CrossRef]
- Hui, Y.; Lu, M.; Han, Y.; Zhou, H.; Liu, W.; Li, L.; Jin, R. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Lee, M.-J.; Feliers, D.; Sataranatarajan, K.; Mariappan, M.M.; Li, M.; Barnes, J.L.; Choudhury, G.G.; Kasinath, B.S. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell. Signal. 2010, 22, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Han, M.K.; Lee, S.Y.; Ramkumar, K.M.; et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Renal Physiol. 2011, 301, F427–F435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.H.; Lee, H.-J.; Sohn, E.J.; Ko, H.-S.; Shim, B.S.; Ahn, K.S.; Kim, S.-H. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol. Rep. 2013, 65, 970–979. [Google Scholar] [CrossRef]
- Weixel, K.M.; Marciszyn, A.; Alzamora, R.; Li, H.; Fischer, O.; Edinger, R.S.; Hallows, K.R.; Johnson, J.P. Resveratrol inhibits the epithelial sodium channel via phopshoinositides and AMP-activated protein kinase in kidney collecting duct cells. PLoS ONE 2013, 8, e78019. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lu, H.; Wu, C.; Liang, Y.; Wang, S.; Lin, C.; Chen, B.; Xia, P. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem. Pharmacol. 2014, 92, 484–493. [Google Scholar] [CrossRef]
- He, T.; Guan, X.; Wang, S.; Xiao, T.; Yang, K.; Xu, X.; Wang, J.; Zhao, J. Resveratrol prevents high glucose-induced epithelial–mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Mol. Cell. Endocrinol. 2015, 402, 13–20. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, C.; Meng, T.; Zhang, W.; Zhou, Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Exp. Biol. Med. 2016, 241, 140–146. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, Y.Y.; Lai, Y.H.; Cheng, C.C.; Lin, T.C.; Su, Y.S.; Liu, C.H.; Lai, P.C. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Int. J. Mol. Med. 2016, 37, 83–91. [Google Scholar] [CrossRef]
- Gu, J.; Mei, S.; Xu, D.; Chen, M.; Chen, S.; Hu, H.; Mei, C.; Wu, M.; Jing, Y.; Yao, Q.; et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol. Dial. Transplant. 2016, 31, 1826–1834. [Google Scholar]
- Wang, N.; Mao, L.; Yang, L.; Zou, J.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 2017, 8, 36449–36461. [Google Scholar]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, M.; Zhou, Y.; Wang, C.; Yuan, Y.; Li, L.; Bresette, W.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine 2018, 57, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-C.; Zhu, X.-L.; Zhang, H.-Q.; Li, W.-D. Study of resveratrol suppressing TGF-beta1 induced transdifferentiation of podocytes. Chin. J. Integr. Tradit. West. Med. 2013, 33, 1677–1682. [Google Scholar]
- Zhang, T.; Chi, Y.; Kang, Y.; Lu, H.; Niu, H.; Liu, W.; Li, Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019, 234, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.S.; Dressler, G.R. The molecular basis of embryonic kidney development. Mech. Dev. 1997, 62, 105–120. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rössler, O.G.; Glatzel, D.; Thiel, G. Resveratrol upregulates Egr-1 expression and activity involving extracellular signal-regulated protein kinase and ternary complex factors. Exp. Cell Res. 2015, 332, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef]
- Raghubeer, S.; Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. The phytoalexin resveratrol ameliorates ochratoxin a toxicity in human embryonic kidney (HEK293) cells. J. Cell. Biochem. 2015, 116, 2947–2955. [Google Scholar] [CrossRef]
- Abharzanjani, F.; Afshar, M.; Hemmati, M.; Moossavi, M. Short-term high dose of quercetin and resveratrol alters aging markers in human kidney cells. Int. J. Prev. Med. 2017, 8, 64. [Google Scholar]
- He, T.; Xiong, J.; Nie, L.; Yu, Y.; Guan, X.; Xu, X.; Xiao, T.; Yang, K.; Liu, L.; Zhang, D.; et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J. Mol. Med. 2016, 94, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, R.; Zhu, L. Inhibitory effect of resveratrol on the expression of the VEGF gene and proliferation in renal cancer cells. Mol. Med. Rep. 2011, 4, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Baek, S.H.; Um, J.-Y.; Shim, B.S.; Ahn, K.S. Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPε and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrol. 2016, 17, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Vardyani, M.; Sheervalilou, R.; Mohammadi, M.; Somi, M.H. Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. Gen. Physiol. Biophys. 2012, 31, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Guo, L.; Li, B.-Y.; Zhen, J.-H.; Song, J.; Peng, T.; Yang, X.-D.; Hu, Z.; Gao, H.-Q. Resveratrol attenuates early diabetic nephropathy by down-regulating glutathione s-transferases mu in diabetic rats. J. Med. Food 2013, 16, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Ko, S.H.; Koh, S.H.; Shin, S.J.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Vardi, N.; Esrefoglu, M.; Ates, B.; Yologlu, S.; Taskapan, C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. [Google Scholar] [CrossRef]
- Yan, C.; Xu, W.; Huang, Y.; Li, M.; Shen, Y.; You, H.; Liang, X. HRD1-mediated IGF-1R ubiquitination contributes to renal protection of resveratrol in db/db mice. Mol. Endocrinol. 2016, 30, 600–613. [Google Scholar] [CrossRef]
- Park, H.S.; Lim, J.H.; Kim, M.Y.; Kim, Y.; Hong, Y.A.; Choi, S.R.; Chung, S.; Kim, H.W.; Choi, B.S.; Kim, Y.S.; et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J. Transl. Med. 2016, 14, 132. [Google Scholar]
- Ma, L.; Fu, R.; Duan, Z.; Lu, J.; Gao, J.; Tian, L.; Lv, Z.; Chen, Z.; Han, J.; Jia, L.; et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pr. 2016, 212, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H.; Kilarkaje, N. Trans-resveratrol mitigates type 1 diabetes-induced oxidative DNA damage and accumulation of advanced glycation end products in glomeruli and tubules of rat kidneys. Toxicol. Appl. Pharmacol. 2018, 339, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, L. Resveratrol provides benefits in mice with type II diabetes-induced chronic renal failure through AMPK signaling pathway. Exp. Ther. Med. 2018, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Ge, M.; Jing, J.; Chen, Y.; Jiang, H.; Yu, H.; Li, N.; Zhang, Z. Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutr. Res. Pr. 2014, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K.-S. SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell Longev. 2016, 2016, 7296092. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Tao, S.; Cao, D.; Xie, H.; Zeng, Q. Protection of resveratrol on acute kidney injury in septic rats. Hum. Exp. Toxicol. 2017, 36, 1015–1022. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of resveratrol supplementation in Nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: A randomized, double-blind, placebo-controlled, crossover clinical trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef]
- Lin, C.-T.; Sun, X.-Y.; Lin, A.-X. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: a prospective, randomized, double-blind study. Ren. Fail. 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.D.O.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]

| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Rat primary mesangial cells and LLCPK1 cells | 50–75 µM; 24 h | ↑NF-κB activation | [63] |
| Rat primary mesangial cells | 10 µM; 1 h | ↓Gentamicin-induced contraction | [65] |
| Rat mesangial cells | 10 µM; 1 h | ↓PDGF-induced cell proliferation ↓PDGFR Y-751 phosphorylation ↓PDGFR Y-761 phosphorylation ↓PDGF-induced PI3K, Akt, ERK1/2, c-Src activity ↑PTP1B activity | [66] |
| Rat primary mesangial cells | 10 μM; 6 h | ↓High glucose-induced
↑ Mitochondrial complex III activity ↑ ∆Ψm hyperpolarization ↑ SIRT1 activity | [67] |
| CRL-2573 and primary mesangial cells | 5–10 µM; 24 h | ↓ High glucose-induced
| [68] |
| HBYZ-1 cells | 20 μM; 72 h | ↑High glucose-induced AdipoR1 mRNA and protein ↑FOX01 activity ↓FOX01 phosphorylation | [69] |
| Rat mesangial cells | 25 μM; 48 h | ↓High glucose-induced
| [70] |
| CRL-2573 cells | 10 µM; 48 h | ↓High glucose-induced
| [71] |
| SV40 MES 13 cells | 10 µM; 46 h | ↓TGF-β1-induced ROS production ↑TGF-β1-induced
| [72] |
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Rodent glomerular epithelial cells | 30 μM and 50 μM; 72 h | ↓High glucose-induced
| [73] |
| Mouse proximal tubular epithelial cells | 100 μM; 30 min | ↓Cisplatin-induced
↑Bcl-xL, Bax, and Bak protein | [74] |
| Human renal epithelial cells | 0, 40 and 80 µM; 24 h | ↓Oxalate-induced
| [75] |
| mpkCCDC14 cells | 25–400 µM; 30 min to 24 h | ↓Sodium transport ↑GFP-AKT-PH redistribution ↑AMPKα protein | [76] |
| NRK-52E cells | 10 and 100 μM; 24 h | ↓TGF-β1-induced
| [77] |
| HK-2 cells | 5–20 µM; 4 h | ↓High glucose-induced
| [78] |
| HK-2 cells | 20 μM; 48 h | ↓EMT ↓β-catenin nuclear translocation ↑E-cadherin and SIRT1 mRNA and protein ↓MMP7, α-SMA, and COLIA1 mRNA and protein | [79] |
| HK-2 cells | 12.5 µM; 48 h | ↓Ioxitalamate-induced
↑ Caspase 3 activity | [80] |
| OX161 and UCL93 human renal epithelial cells; MDCK canine renal epithelial cells | 2–50 µM; 48 h | ↓Cyst number ↓MCP-1 protein and activity ↓TNF-α protein and activity ↓CFB protein and activity ↑SOD2 protein | [81] |
| HK-2 cells | 20 µM; 12 h | ↑Cell viability ↓Ph-NFκB protein ↓TNF-α, IL-1β, and IL-6 mRNA and protein ↓IRE1 activation | [82] |
| HK-2 cells | 25 µM; 72 h | ↓High glucose-induced oxidative stress ↓MDA and ROS activity ↑CAT and SIRT1 protein ↑SIRT1 activity ↓Acetyl-FOXO3a protein | [83] |
| TCMK-1 cells | 25 µM; 72 h | ↓Cadmium-induced apoptosis ↓mROS production ↑mSIRT3 protein and activity ↑PGC-1α and SOD2 mRNA | [84] |
| HK-2 cells | 5–20, 40 µM; 72 h | 5–20 µM RSV: ↓TGF-β-induced EMT ↓Cytotoxicity ↑SIRT1 and E-cadherin protein ↓α-SMA and fibronectin protein ↓Ph-Smad3 ↓SIRT1-Smad3/4 40 µM RSV: ↑Cytotoxicity ↑mtROS release ↑Bax, fibronectin, and α-SMA protein ↓Bcl-2 protein ↓ATP production ↓PGC-1α and TFAM protein | [85] |
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Mouse podocytes | 2–5 µM; 30 min | ↓Albumin permeability ↓Podocyte death ↑E-cadherin expression ↑P-cadherin, ZO-1, and NEPH1 protein ↓α-SMA protein | [86] |
| Immortalized podocytes | 10 μM; 48 h | ↓High glucose-induced
↑Mitochondrial membrane potential ↑SIRT1, PGC-1α, NRF1, TFAM mRNA and protein | [87] |
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| NRF-49F cells | 5, 10, and 20 µM; 1 h | ↓High glucose-induced
| [94] |
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| 786-0 cells | 0, 10, 20 and 40 µM; 24, 48 and 72 h | ↓Cell growth ↓VEGF mRNA and protein | [95] |
| Caki-1 and 786-0 cells | 0, 10, 30 and 50 µM; 6 h | ↑Apoptosis ↓Survival ↓Migration ↓STAT3 and STAT5 activation ↑PTPε and SHP-2 protein ↓JAK1, JAK2, and c-Src protein ↓Bcl-2, bcl-xL, survivin, IAP-1, and IAP-2 protein ↑Caspase-3 protein | [96] |
| ACHN and A498 cells | 50 μM; 12 h | ↓Cell growth ↓Cell-to-cell contact ↓Migration ↓Filopodia formation ↓N-cadherin, vimentin, snail, MMP-2, MMP-9, ph-Akt and ph-ERK1/2 protein ↑E-cadherin and TIMP-1 protein | [97] |
| Animal | Resveratrol Concentration/Duration | Serum Effects | Other Effects | Reference |
|---|---|---|---|---|
| db/db mice | 0.3% diet; 8 weeks | ↓Glucose levels ↓Insulin levels ↓Triglyceride levels ↓FFA levels | ↓Albuminuria ↓Mesangial expansion ↓Fibronectin accumulation ↓Macrophage infiltration ↑O2− scavenging ↑MnSOD activity ↓Mitochondrial biogenesis mRNA | [98] |
| Male Wistar rats | 5 mg/kg/day; 16 weeks | ↓Glucose levels ↓SOD activity ↓TBARS levels ↓TNF-α ↓IL-6 | ↓Apoptosis rate of kidney cells ↓NF-κB activity | [99] |
| Male Wistar rats | 20 mg/kg/day; 8 weeks | ↓Glucose levels ↓Creatinine levels | ↓Urinary protein excretion ↓Renal hypertrophy ↓Mesangial matrix expansion ↓Mesangial cell hyperplasia ↓GSTM expression | [100] |
| db/db mice | 20 mg/kg/day; 12 weeks | No measured effects | ↓Kidney albuminuria ↓Kidney NEFA and triacylglycerol ↓Mesangial area ↓Oxidative stress ↓Type IV collagen ↓TGF-β1 ↓F4/80 positive cells ↑Ph-AMPK ↑SIRT1 protein ↓PI3K-Akt protein and activity ↓Ph-FOXO3a ↓BAX protein ↑BCL-2 production ↓Renal and Urinary 8-OHdG | [101] |
| FVB mice | 10 mg/kg/day; 12 weeks | No measured effects | ↓Glomerular area ↓Extracellular matrix ↓Albumin levels ↓Ph-Akt protein ↓PAI-1 protein ↓ICAM-1 protein ↓PCNA mRNA | [70] |
| Sprague–Dawley rats | 200 mg/kg/day; 12 weeks | No measured effects | ↓Glomerular area ↓Mesangial cell expansion ↓Glomerular basement membrane thickness ↓Collagen IV ↓Fibronectin ↑AdipoR1 expression ↓MDA production | [69] |
| Male Wistar rats | 10 mg/kg/day; 30 days | ↓Glucose levels ↓Urea nitrogen levels | ↓Glomeruli sclerotic changes ↓Epithelial desquamation ↓Tissue swelling ↓Intracytoplasmic vacuolization ↓Brush border loss ↓Kidney TGF-β1 ↑SOD and CAT activities ↓MDA levels | [102] |
| db/db mice | 40 mg/kg/day; 12 weeks | ↓BUN levels ↓Creatinine levels | ↓Glomerulosclerosis ↓Tubulointerstitial fibrosis ↓Albuminuria ↑Kidney SOD, Mn-SOD, Catalase protein ↓Renal MDA ↓α-SMA protein ↓E-cadherin protein ↓TGF-β, pSmad3, ph-Akt, ph-ERK ↓IGF-1R expression ↑HRD1 expression | [103] |
| db/db mice | 20 mg/kg/day; 12 weeks | ↓Triacylglycerol levels ↓NEFA levels ↑Adiponectin levels | ↓Glomerular matrix expansion ↓Albuminuria ↑AdipoR1 and AdipoR2 ↑Ph-AMPK, SIRT1, total FoxO1, total FoxO3a ↑PGC-1α, ERR-1α, ph-ACC ↓SREBP-1c ↓Bax ↑Bcl-2 ↓8-OHdG levels ↓8-isoprostane levels | [104] |
| db/db mice | 40 mg/kg/day; 12 weeks | No measured effects | ↓Mesangial area ↓Albuminuria ↓Collagen deposition ↓FSP-1, α-SMA, and fibronectin protein ↓NOX4 protein ↑Ph-AMPK, ph-ACC | [94] |
| Sprague-Dawley rats | 5 mg/kg/day; 4 months | ↓Glucose levels ↓Cholesterol levels ↓Triglyceride levels ↓HbA1c levels ↓Creatinine levels ↓Urea nitrogen levels ↓Cycstatin C levels ↓TNF-a, IL-6, IL-1B, and IL-10 levels | ↓Albuminuria ↓Renal 8-OHdG ↑SIRT1 mRNA and protein ↑Atg5 and Atg7 mRNA | [105] |
| Male Wistar rats | 30 mg/kg/day; 16 weeks | ↓Creatinine levels | ↑Renal function ↓Kidney weight ↑Kidney SOD activity ↓Kidney MDA content ↑CAT protein ↓SIRT1 protein ↑SIRT1 activity ↓Acetylated-FOXO3a | [83] |
| Sprague-Dawley rats | 20 mg/kg/day; 4 weeks | ↓Glucose levels ↓Creatinine levels | ↓Kidney weight ↓Glomerular thickening ↓Interstitial fibrosis ↓Epithelial cellular vacuolar degeneration ↓Hyaline casts ↓Arteriolopathy ↓Ph-p38 and p38 protein ↓TGF-β1 protein ↓Fibronectin protein ↓Urinary albumin | [71] |
| Male Wistar rats | 5 mg/kg/day; 45 days | No measured effects | ↓Renal hypertrophy ↓Mesangial expansion ↓Fibrosis ↓Oxidative damage ↓Kidney AGE accumulation ↓DNA damage ↓4-HNE protein ↓Caspase-3 protein ↓Cleaved caspase-3 protein | [106] |
| C57BL/KsJ db/+ mice | 10 mg/kg/day; 8 weeks | ↓Glucose levels ↓Insulin levels ↓IL-1β, IL-17, IL-10 and TNF-α levels ↑IL-6 and VEGF levels | ↓Renal cell apoptosis ↓Apaf-1, caspase-3, caspase-8 and caspase-9 mRNA ↓Ph-AMPK ↓Total thiol level ↑GSH level | [107] |
| CD-1 mice | 30 mg/kg/day; 12 weeks | ↓Glucose levels ↓Cholesterol levels ↓Urea nitrogen levels | ↓Glomerular thickening ↓Mesangial area ↑Podocyte mitochondria ↓Renal cell apoptosis ↑Nephrin, SIRT1, PGC-1α, NRF1, TFAM protein ↓Kidney MDA content ↓Kidney Mn-SOD activity | [87] |
| Animal | Resveratrol Concentration/Duration | Serum Effects | Other Effects | Reference |
|---|---|---|---|---|
| Sprague–Dawley rats | 10 mg/kg/day; 21 days | ↓MDA levels | ↓Urine calcium oxalate crystals ↓Hyaluronan protein ↓Osteopontin protein ↑GPx protein ↑CAT protein ↑SOD protein | [75] |
| Male Wistar rats | 8 mg/kg/alternating days; 8 days | ↓Creatinine levels ↓Urea nitrogen levels | ↓Oxidative stress ↓Renal tubular epithelial cell necrosis ↓MDA, BUN, CRE, and ROS levels ↑SOD and GPx levels ↑Selenium content | [108] |
| C57BL/6J mice | 20 mg/kg/day; 14 days | No measured effects | ↓Extracellular matrix deposition ↓Tubulointerstitium damage ↓Oxidative stress ↓ICAM-1 mRNA ↓TNF-α mRNA ↓TGF-β mRNA ↓Acetyl-Smad3 ↓Fibronectin | [109] |
| UUO-Sprague-Dawley rats | 20 mg/kg/day; 7–14 days | ↓Creatinine levels | ↓Renal interstitial damage ↓Tubular dilation and atrophy ↓Collagen deposition ↓Inflammation cell infiltration ↓α-SMA and type III collagen mRNA and protein ↑E-cadherin protein and mRNA ↓TGF-β1 expression | [77] |
| I/R and UUO C57BL/6 mice | 20 mg/kg/day; 6 weeks | ↓Creatinine levels ↓BUN levels | ↑α-SMA protein ↑COL1A1 protein | [79] |
| Sprague–Dawley rats | 50 mg/kg; 8 h | ↓Creatinine levels ↓Urea nitrogen levels | ↓Apoptosis ↑SIRT1 activity and protein ↑SIRT3 activity and protein ↑SOD2 protein ↓Acetyl-SOD2 ↑GSH and ATP content ↑GSH/GSSG ratio ↑CAT activity ↓mPTP opening | [110] |
| Male cystic (Cy/+) rats | 200 mg/kg/day; 5 weeks | ↓BUN levels ↓Creatinine levels | ↓Cyst density ↓Macrophage infiltration ↓MCP-1 ↓TNF-α ↓CFB ↓Ph-p65, ph-S6K and p50 | [81] |
| Sprague–Dawley rats | 3 and 10 mg/kg/injection; 70 h | ↓BUN levels ↓Creatinine levels ↓Nitrogen levels | ↑Survival ↓Cystatin C ↓KIM-1 ↓TNF-α ↓IL-1B ↓IL-6 ↓Renal injury index | [111] |
| Kunming mice | 10 mg/kg/day; 1 week | ↓BUN levels ↓Creatinine levels | ↓Apoptosis ↓Caspase-3 activity ↓Bax protein ↓ERK1/2 protein | [84] |
| Male AKI rats | 30 mg/kg; 12 h | ↓Creatinine levels ↓Urea nitrogen levels ↓TNF-α, IL-1β, IL-6 levels | ↑Renal function ↓Tubular epithelial cell injury ↑Survival ↓p-65 positive cells ↓Renal TNF-α, IL-1β, IL-6 mRNA ↓IRE1 protein | [82] |
| 5/6 Nephrectomized Sprague–Dawley rats | 20 mg/kg/day; 4 weeks | No measured effects | ↓Mesangial cell proliferation ↓Glomeruli matrix expansion ↓TGF-β ↑ATP production ↓ROS production ↑Activities of complex I and III ↑ATP synthase B ↑COX I, Opa1, Mfn2 ↓Drp1 | [72] |
| C57BL/6 mice | 25 and 100 mg/kg/day; 2 weeks | ↓Creatinine levels | 25 mg/kg RSV: ↓Renal fibrosis ↓Tubular lesion score ↓Interstitial collagen deposition ↓α-SMA protein ↓Snail protein ↓Fibronectin protein ↑SIRT1 ↓Phospho-Smad3 100 mg/kg RSV: ↑Renal fibrosis ↑α-SMA and TFAM | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. https://doi.org/10.3390/nu11071624
Den Hartogh DJ, Tsiani E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients. 2019; 11(7):1624. https://doi.org/10.3390/nu11071624
Chicago/Turabian StyleDen Hartogh, Danja J., and Evangelia Tsiani. 2019. "Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies" Nutrients 11, no. 7: 1624. https://doi.org/10.3390/nu11071624
APA StyleDen Hartogh, D. J., & Tsiani, E. (2019). Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients, 11(7), 1624. https://doi.org/10.3390/nu11071624






