A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Study Design
2.3. Dietary Advice
2.4. Questionnaires
2.4.1. Study Questionnaire
2.4.2. Food Diary
2.4.3. Rome IV Questionnaire
2.4.4. Irritable Bowel Syndrome-Symptom Severity Scale
2.4.5. Visual Analog Scale for Irritable Bowel Syndrome
2.4.6. Sweet Cravings
2.4.7. Statistical Analyses
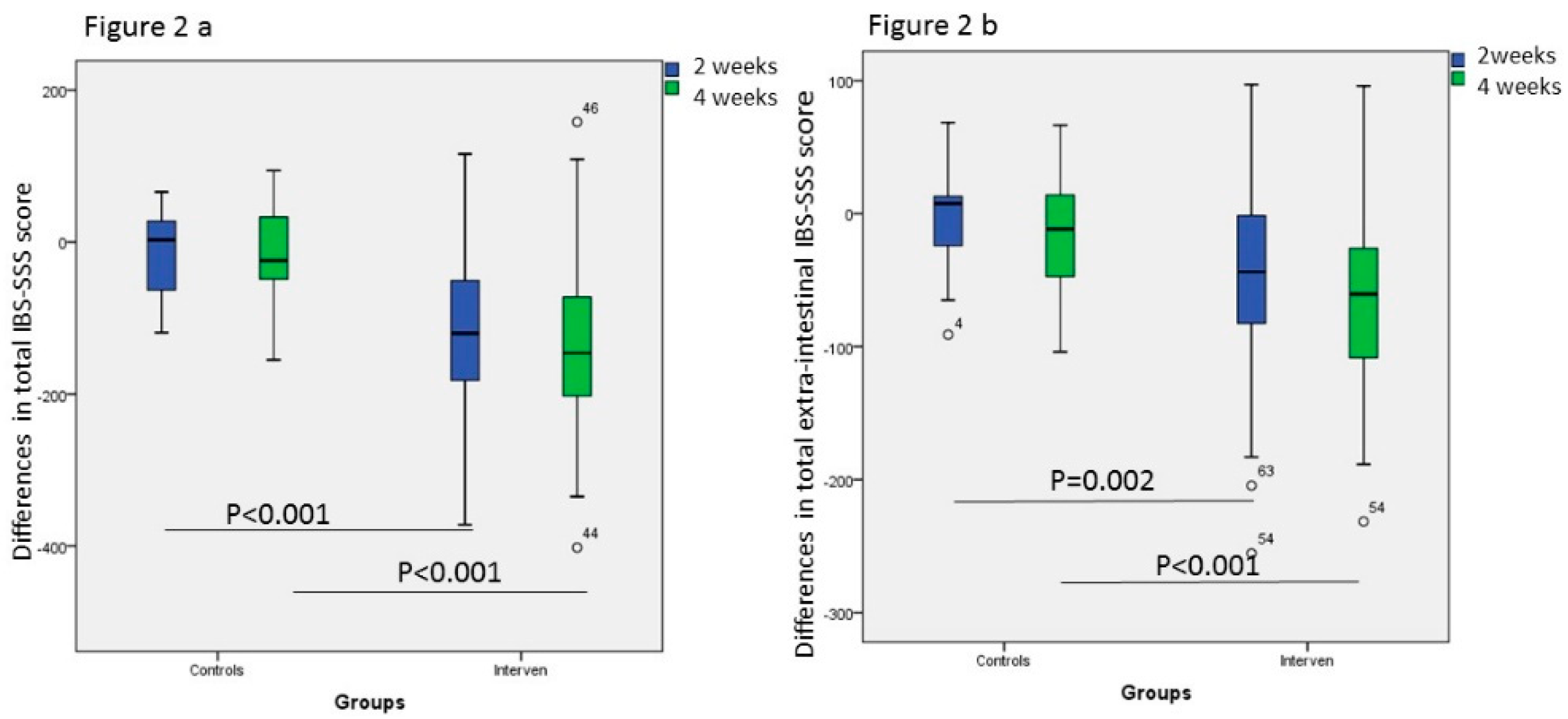
3. Results
3.1. Basal Characteristics
3.2. Disease Classification
3.3. Anthropometric Parameters and Sweet Cravings
3.4. Gastrointestinal and Extra-Intestinal Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 393–1407. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Manjer, J. Physical inactivity during leisure time and irregular meals are associated with functional gastrointestinal complaints in middle-aged and elder subjects. Scand. J. Gastroenterol. 2016, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khayyatzadeh, S.S.; Esmaillzadeh, A.; Saneei, P.; Keshteli, A.H.; Adibi, P. Dietary patterns and prevalence of irritable bowel syndrome in Iranian adults. Neurogastroenterol. Motil. 2016, 28, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Hercberg, S.; Benamouzig, R.; Julia, C. Western Dietary Pattern Is Associated with Irritable Bowel Syndrome in the French NutriNet Cohort. Nutrients 2017, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Zaribaf, F.; Keshteli, A.H.; Esmaillzadeh, A.; Saneei, P.; Feizi, A.; Daghaghzadeh, H.; Feinle-Bisset, C.; Adibi, P. Empirically derived dietary habits are associated with irritable bowel syndrome. Eur. J. Clin. Nutr. 2018, 72, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Khayyatzadeh, S.S.; Kazemi-Bajestani, S.M.; Mirmousavi, S.J.; Heshmati, M.; Khoshmohabbat, S.; A Ferns, G.; Ghayour-Mobarhan, M.; Kazemi-Bajestani, S.M.; Ghayour-Mobarhan, M. Dietary behaviors in relation to prevalence of irritable bowel syndrome in adolescent girls. J. Gastroenterol. Hepatol. 2018, 33, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.; Ohlsson, B. Irregular Dietary Habits with a High Intake of Cereals and Sweets Are Associated with More Severe Gastrointestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef]
- Frazier, T.H.; DiBaise, J.K.; McClain, C.J. Gut Microbiota, Intestinal Permeability, Obesity-Induced Inflammation, and Liver Injury. J. Parenter. Enter. Nutr. 2011, 35, 14–20. [Google Scholar] [CrossRef]
- El-Salhy, M. Irritable bowel syndrome: Diagnosis and pathogenesis. World J. Gastroenterol. 2012, 18, 5151–5163. [Google Scholar]
- Linton, S.J.; Shaw, W.S. Impact of Psychological Factors in the Experience of Pain. Phys. Ther. 2011, 91, 700–711. [Google Scholar] [CrossRef]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological disorders in gastrointestinal disease: Epiphenomenon, cause or consequence? Ann. Gastroenterol. 2014, 27, 224–230. [Google Scholar]
- Icenhour, A.; Witt, S.T.; Elsenbruch, S.; Lowén, M.; Engström, M.; Tillisch, K.; Mayer, E.A.; Walter, S. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin. 2017, 15, 449–457. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Lamb, K.; Gebhart, G.F. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am. J. Physiol. Liver Physiol. 2006, 291, G658–G665. [Google Scholar] [CrossRef]
- Pezzone, M.A.; Liang, R.; Fraser, M.O. A Model of Neural Cross-Talk and Irritation in the Pelvis: Implications for the Overlap of Chronic Pelvic Pain Disorders. Gastroenterology 2005, 128, 1953–1964. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. NICE Clinical Guideline 61. In Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care; National Institute for Health and Clinical Excellence: London, UK, 2008. [Google Scholar]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef]
- Ohlsson, B.; Darwiche, G.; Roth, B.; Bengtsson, M.; Hoglund, P. High Fiber Fat and Protein Contents Lead to Increased Satiety Reduced Sweet Cravings and Decreased Gastrointestinal Symptoms Independently of Anthropometric Hormonal and Metabolic Factors. J. Diabetes Metab. 2017, 8. [Google Scholar] [CrossRef]
- Sucrose Intolerance. Genetic Sucrase-Isomaltase Deficiency. Available online: https://www.sucroseintolerance.com/choosing-your-foods (accessed on 10 July 2018).
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Zheng, T.; Bonfiglio, F.; Bujanda, L.; Dlugosz, A.; Lindberg, G.; Schmidt, P.T.; Karling, P.; Ohlsson, B.; Simren, M.; et al. Increased Prevalence of Rare Sucrase-isomaltase Pathogenic Variants in Irritable Bowel Syndrome Patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1673–1676. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.; et al. Development and Validation of the Rome IV Diagnostic Questionnaire for Adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B. The brief Visual Analogue Scale for Irritable Bowel Syndrome questionnaire can be used to evaluate psychological well-being in patients with irritable bowel syndrome. Eur. J. Intern. Med. 2013, 24, e82–e83. [Google Scholar] [CrossRef]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef]
- Erlanson-Albertsson, C.; Lindqvist, A. Fructose affects enzymes involved in the synthesis and degradation of hypothalamic endocannabinoids. Regul. Pept. 2010, 161, 87–91. [Google Scholar] [CrossRef]
- Jewett, D.C.; Grace, M.K.; Levine, A.S. Chronic sucrose ingestion enhances mu-opioid discriminative stimulus effects. Brain Res. 2005, 1050, 48–52. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012, 11, 1–24. [Google Scholar]
- Hajnal, A.; De Jonghe, B.C.; Covasa, M. Dopamine D2 receptors contribute to increased avidity for sucrose in obese OLETF rats lacking CCK-1 receptors. Neuroscience 2007, 148, 584–592. [Google Scholar] [CrossRef]
- Ochoa, M.; Lallès, J.P.; Malbert, C.H.; Val-Laillet, D. Dietary sugars: Their detection by the gut-brain axis and their peripheral and central effects in health and diseases. Eur. J. Nutr. 2015, 54, 1–24. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.; Whelan, K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 256–266. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Treem, W.R. Clinical Aspects and Treatment of Congenital Sucrase-Isomaltase Deficiency. J. Pediatr. Gastroenterol. Nutr. 2012, 55, S7–S13. [Google Scholar] [CrossRef]
- Burns, G.; Carroll, G.; Mathe, A.; Horvat, J.; Foster, P.; Walker, M.M.; Talley, N.J.; Keely, S. Evidence for Local and Systemic Immune Activation in Functional Dyspepsia and the Irritable Bowel Syndrome: A Systematic Review. Am. J. Gastroenterol. 2018, 14. [Google Scholar] [CrossRef]
- Meneses, M.E.; Camargo, A.; Jimenez-Gomez, Y.; Paniagua, J.A.; Tinahones, F.J.; Roche, H.M.; Perez-Jimenez, F.; Malagón, M.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Postprandial inflammatory response in adipose tissue of patients with metabolic syndrome after the intake of different dietary models. Mol. Nutr. Food Res. 2011, 55, 1759–1770. [Google Scholar] [CrossRef]
- Nasser, Y.; Petes, C.; Simmers, C.; Basso, L.; Altier, C.; Gee, K.; Vanner, S.J. Activation of Peripheral Blood CD4+ T-Cells in IBS is not Associated with Gastrointestinal or Psychological Symptoms. Sci. Rep. 2019, 9, 3710. [Google Scholar] [CrossRef]
- Giamberardino, M.A. Recent and forgotten aspects of visceral pain. Eur. J. Pain 1999, 3, 77–92. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Stanghellini, V.; Barbara, G. Recent advances in understanding non-celiac gluten sensitivity. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Johannesson, E.; Ringström, G.; Abrahamsson, H.; Sadik, R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J. Gastroenterol. 2015, 21, 600–608. [Google Scholar] [CrossRef]
- Thompson, R.L.; Summerbell, C.D.; Hooper, L.; Higgins, J.P.; Little, P.S.; Talbot, D.; Ebrahim, S.; Little, P. Dietary advice given by a dietitian versus other health professional or self-help resources to reduce blood cholesterol. Cochrane Database Syst. Rev. 2003, 3, CD001366. [Google Scholar] [CrossRef]


| Control Group N = 25 | Differences within Group | Intervention Group N = 80 | Differences within Group | P-Value | |
|---|---|---|---|---|---|
| Times/4 days | Times/4 days | ||||
| Baseline Missing | 1 | 2 | |||
| Meat | 4 (1.25–8) | 4 (2–8) | 0.393 | ||
| Fish/seafood | 1 (0–1.75) | 1 (0–2) | 0.806 | ||
| Vegetable/legumes | 4 (2.25–4) | 4 (3–4) | 0.302 | ||
| Fruits/nuts | 2 (2–4) | 3 (1–4) | 0.652 | ||
| Dairy products | 4 (2–4) | 4 (2–8) | 0.604 | ||
| Cereals | 8 (4–8) | 8 (4–8) | 0.504 | ||
| Sweets/soft drinks | 4 (2–8) | 4 (2–8) | 0.920 | ||
| After 2 weeks Missing | 3 | 6 | |||
| Meat | 4 (2–7) | 0 (−0.5–0.5) | 4 (2–4) | 0 (−2.25–0) | 0.793 |
| Fish/seafood | 1 (0–1.25) | 0 (−1–1) | 1 (1–2) | 0 (−2.25–1) | 0.022 |
| Vegetable/legumes | 4 (2.5–5) | 0 (0–1) | 4 (4–8) | 0 (0–4) | 0.034 |
| Fruits/nuts | 2 (0.5–5) | 0 (−1.5–1.5) | 8 (4–8) | 4 (0–6) | <0.001 |
| Dairy products | 4 (3–4) | 0 (−1–0) | 4 (3–8) | 1 (0–4) | 0.036 |
| Cereals | 8 (4–8) | 0 (−0.5–0) | 4 (1–8) | −4 (−6.25–0) | 0.002 |
| Sweets/soft drinks | 4 (0.5–4) | 0 (−1–0) | 0 (0–2) | −3 (−4–1) | 0.001 |
| Control Group N = 25 | Intervention Group N = 80 | P-Value | |
|---|---|---|---|
| Rome IV criteria | |||
| Baseline | |||
| Unspecified FGID | 6 (24.0) | 11 (13.8) | 0.352 |
| IBS | 19 (76.0) | 67 (83.8) | |
| Mixed IBS | 8 | 29 | 0.270 * |
| IBS-D | 3 | 23 | |
| IBS-C | 7 | 13 | |
| Unspecified IBS | 1 | 2 | |
| Missing value | 2 (2.5) | ||
| 4 weeks | |||
| No FGID/IBS | 23 (28.8) | 0.001 | |
| Unspecified FGID | 7 (28.0) | 9 (11.2) | |
| IBS | 16 (64.0) | 39 (48.8) | |
| Mixed IBS | 5 | 10 | 0.815 * |
| IBS-D | 5 | 17 | |
| IBS-C | 5 | 11 | |
| Unspecified IBS | 1 | 1 | |
| Missing value | 2 (8.0) | 9 (11.2) | |
| IBS-SSS total score | |||
| Baseline | |||
| Moderate | 11 (44.0) | 37 (46.3) | 0.821 |
| Severe | 14 (56.0) | 41 (51.2) | |
| Missing value | 2 (2.5) | ||
| 4 weeks | |||
| <75 | 15 (18.8) | <0.001 | |
| Mild 75–174 | 2 (8.0) | 24 (30.0) | |
| Moderate 175–299 | 9 (36.0) | 24 (30.0) | |
| Severe ≥ 300 | 12 (48.0) | 9 (11.9) | |
| Missing value | 2 (8.0) | 8 (10.0) |
| Control Group N = 25 | P-Value | Intervention Group N = 80 | P-Value | |
|---|---|---|---|---|
| Weight (kg/m2) | ||||
| Baseline | 68 (57–75) (0) | 72 (64–85) (3) | ||
| 4 weeks | 68 (61–76) (2) | 0.158 | 71 (64–82) (9) | <0.001 |
| Systolic blood pressure (mm/Hg) | ||||
| Baseline | 127 (116–140) (1) | 125 (115–135) (7) | ||
| 4 weeks | 125 (120–148) (4) | 0.974 | 120 (115–130) (8) | 0.431 |
| Diastolic blood pressure (mm/Hg) | ||||
| Baseline | 80 (75–90) (1) | 80 (70–85) (7) | ||
| 4 weeks | 80 (75–85) (4) | 0.222 | 80 (70–84) (8) | 0.074 |
| Sweet cravings (mm) | ||||
| Baseline | 51 (34–70) (2) | 60 (32–79) (10) | ||
| 4 weeks | 57 (30–70) (3) | 0.671 | 21 (10–42) (11) | <0.001 |
| Control Group N = 25 | P-value | Intervention Group N = 80 | P-value | |
|---|---|---|---|---|
| IBS-SSS Total Score | ||||
| Baseline | 310 (247–351) | 306 (250–356) (2) | ||
| 2 weeks | 271 (238–325) (5) | 0.548 | 190 (118–282) (6) | <0.001 |
| 4 weeks | 300 (233–331) (2) | 0.248 | 156 (88–250) (8) | <0.001 |
| IBS-SSS Extra-Intestinal | ||||
| Baseline | 197 (106–257) | 184 (125–254) (3) | ||
| 2 weeks | 154 (121–241) (7) | 0.823 | 117 (77–215) (6) | <0.001 |
| 4 weeks | 169 (107–208) (3) | 0.231 | 98 (61–174) (6) | <0.001 |
| Psychological Well-Being | ||||
| Baseline | 47 (24–71) | 50 (24–69) (2) | ||
| 2 weeks | 49 (31–66) (5) | 0.658 | 41 (14–60) (6) | 0.002 |
| 4 weeks | 48 (32–60) (2) | 0.732 | 36 (13–53) (6) | <0.001 |
| Nausea | ||||
| Baseline | 29 (6–50) | 11 (1–34) (2) | ||
| 2 weeks | 20 (3–38) (5) | 0.820 | 4 (0–28) (6) | 0.011 |
| 4 weeks | 12 (2–56) (2) | 0.112 | 2 (0–24) (6) | 0.004 |
| Difficulty to Eat a Whole Portion | ||||
| Baseline | 9 (1–26) | 12 (1–30) (2) | ||
| 2 weeks | 8 (1–27) (5) | 0.941 | 4 (0–22) (6) | 0.003 |
| 4 weeks | 6 (1–20) (2) | 0.071 | 3 (0–17) (6) | <0.001 |
| Reflux | ||||
| Baseline | 17 (2–78) | 16 (3–54) (2) | ||
| 2 weeks | 14 (4–64) (5) | 0.806 | 5 (1–23) (6) | <0.001 |
| 4 weeks | 8 (1–50) (2) | 0.061 | 4 (0–21) (6) | <0.001 |
| Belching | ||||
| Baseline | 67 (20–84) | 71 (42–84) (3) | ||
| 2 weeks | 64 (36–81) (5) | 0.791 | 44 (15–60) (6) | <0.001 |
| 4 weeks | 59 (27–78) (2) | 0.246 | 26 (12–50) (6) | <0.001 |
| Headache | ||||
| Baseline | 31 (15–48) | 23 (7–67) (2) | ||
| 2 weeks | 30 (6–46) (5) | 0.378 | 24 (5–48) (6) | 0.009 |
| 4 weeks | 25 (8–35) (2) | 0.845 | 16 (4–36) (6) | 0.001 |
| Back Pain | ||||
| Baseline | 45 (19–66) | 37 (8–73) (2) | ||
| 2 weeks | 34 (7–54) (5) | 0.227 | 27 (6–53) (6) | 0.007 |
| 4 weeks | 32 (5–55) (2) | 0.249 | 20 (1–42) (6) | <0.001 |
| Leg Pain | ||||
| Baseline | 3 (0–16) | 4 (0–24) (2) | ||
| 2 weeks | 4 (1–12) (6) | 0.452 | 2 (0–16) (6) | 0.178 |
| 4 weeks | 4 (1–12) (2) | 0.924 | 2 (0–16) (6) | 0.006 |
| Muscle/Joint Pain | ||||
| Baseline | 21 (9–70) | 44 (12–70) (2) | ||
| 2 weeks | 25 (2–82) (3) | 0.290 | 30 (6–64) (6) | 0.022 |
| 4 weeks | 47 (8–74) (2) | 0.753 | 27 (6–50) (6) | <0.001 |
| Urinary Emergency | ||||
| Baseline | 20 (3–56) | 49 (18–73) (2) | ||
| 2 weeks | 21 (2–53) (6) | 0.401 | 26 (4–64) (6) | <0.001 |
| 4 weeks | 16 (1–43) (2) | 0.131 | 21 (1–47) (6) | <0.001 |
| Tiredness | ||||
| Baseline | 67 (39–91) | 65 (46–97) (2) | ||
| 2 weeks | 70 (46–84) (5) | 0.458 | 50 (26–73) (6) | <0.001 |
| 4 weeks | 65 (41–83) (2) | 0.794 | 48 (22–71) (6) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. https://doi.org/10.3390/nu11071662
Nilholm C, Roth B, Ohlsson B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients. 2019; 11(7):1662. https://doi.org/10.3390/nu11071662
Chicago/Turabian StyleNilholm, Clara, Bodil Roth, and Bodil Ohlsson. 2019. "A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients" Nutrients 11, no. 7: 1662. https://doi.org/10.3390/nu11071662
APA StyleNilholm, C., Roth, B., & Ohlsson, B. (2019). A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients, 11(7), 1662. https://doi.org/10.3390/nu11071662






