1. Introduction
Metabolic syndrome is a world wide problem; it is a medical term referring to lifestyle related risk factors for arteriosclerosis-related diseases [
1]. Obesity and insulin resistance are at the core of most cases of metabolic syndrome; therefore, lifestyle changes, including diet, are strongly recommended. Dietary fiber is one of the best candidates for improving the components of metabolic syndrome [
2]. Some dietary fibers reduce the postprandial rise in plasma glucose [
3], reduce body weight [
4], and improve lipid metabolism [
5,
6].
Dietary fiber is characterized by water solubility and has different functions. Insoluble dietary fiber has shown favorable results in the treatment of irritable bowel disease [
7] and induced a significant increase in fecal bulk, a reduction in intestinal transit time, and a significant increase in the frequency of bowel movements in healthy people [
8]. In another study, soluble dietary fiber inhibited carbohydrate and lipid digestion, lipid absorption, reduced the postprandial glucose response, and improved serum lipid levels [
9].
Paramylon (PM) is a new source of dietary fiber generated as a characteristic cellular reserve material in
Euglena gracilis; it is a β-1, 3-glucan arranged in a triple helix and is considered to be an insoluble dietary fiber [
10]. Beta-glucan is commonly found in nature, in cereals, fungi and algae, but the structural and physical properties of β-glucans are quite different among the sources. Evidence of any beneficial effects of PM supplementation has not yet been reported. Anti-diabetic effects were not observed in genetically diabetic rats fed a 2% PM diet [
11] and recent experiments have shown that PM did not improve obesity or pro-inflammatory status in mice fed a high fat diet [
12,
13]. The lack of evidence for the beneficial effects of PM is suggested to be caused by insufficient dosage; a recent report suggested that the amount of PM used in the diets of previous studies were insufficient to detect any effectiveness [
14]. The dosage of dietary fiber administered via experimental diets is usually 5%, and significant effects have been observed on glucose and lipid metabolism in animal experiments at this dosage [
15,
16,
17]. The cellulose content in the AIN-93 diet is also 5% [
18]. Therefore, experiments using adequate amounts of PM in the diet are needed to elucidate the functions of PM as a new dietary fiber.
The purpose of this study was to investigate the effects of 2.5% and 5% PM, extracted from Euglena gracilis, in diet-induced obese mice fed a high-fat diet; we measured visceral fat accumulation, cholesterol and glucose metabolism and serum serum secretary immunoglobulin A (sIgA) concentrations. This is the first study to report on the preventive effects of dietary PM against obesity.
4. Discussion
We investigated the effects of PM extracted from Euglena gracilis EOD-1 on both lipid and glucose metabolism and abdominal fat accumulation in diet-induced obese mice. This is the first study to report on the preventive effects of dietary PM against obesity. The mechanism involves a reduction in both abdominal fat accumulation and serum LDL-cholesterol concentrations and an improvement in postprandial glucose concentrations. We also determined the characteristics of PM as a dietary fiber source; lipid absorption was hardly influenced by PM intake, and the intestinal fermentability of PM was low. Examination using electron microscopy revealed that PM recovered from feces retained its shape and was resistant to bacterial degradation.
Growth data showed that the reduction in the food efficiency ratio in mice fed PM was dose-dependent; a significant difference was also observed between the control and 5% PM group. It is suggested that reduced lipid accumulation in the abdominal fat organs leads to a reduction in the food efficiency ratio. Significant dose dependent reductions in total, retroperitoneal and mesenteric fat weight were observed in mice fed 5% PM. Dietary fiber has been reported to reduce abdominal fat accumulation through the inhibition of dietary fat absorption [
24]. A significant decrease in apparent digestibility of fat was observed with 5% PM supplementation; however, the difference in fat digestibility between the control and 5% PM group was less than 1%. The contribution of PM supplementation to the inhibition of fat absorption may be weak. The lack of reduction in hepatic lipid accumulation is suggested to be partially due to a poor reduction in apparent lipid digestibility.
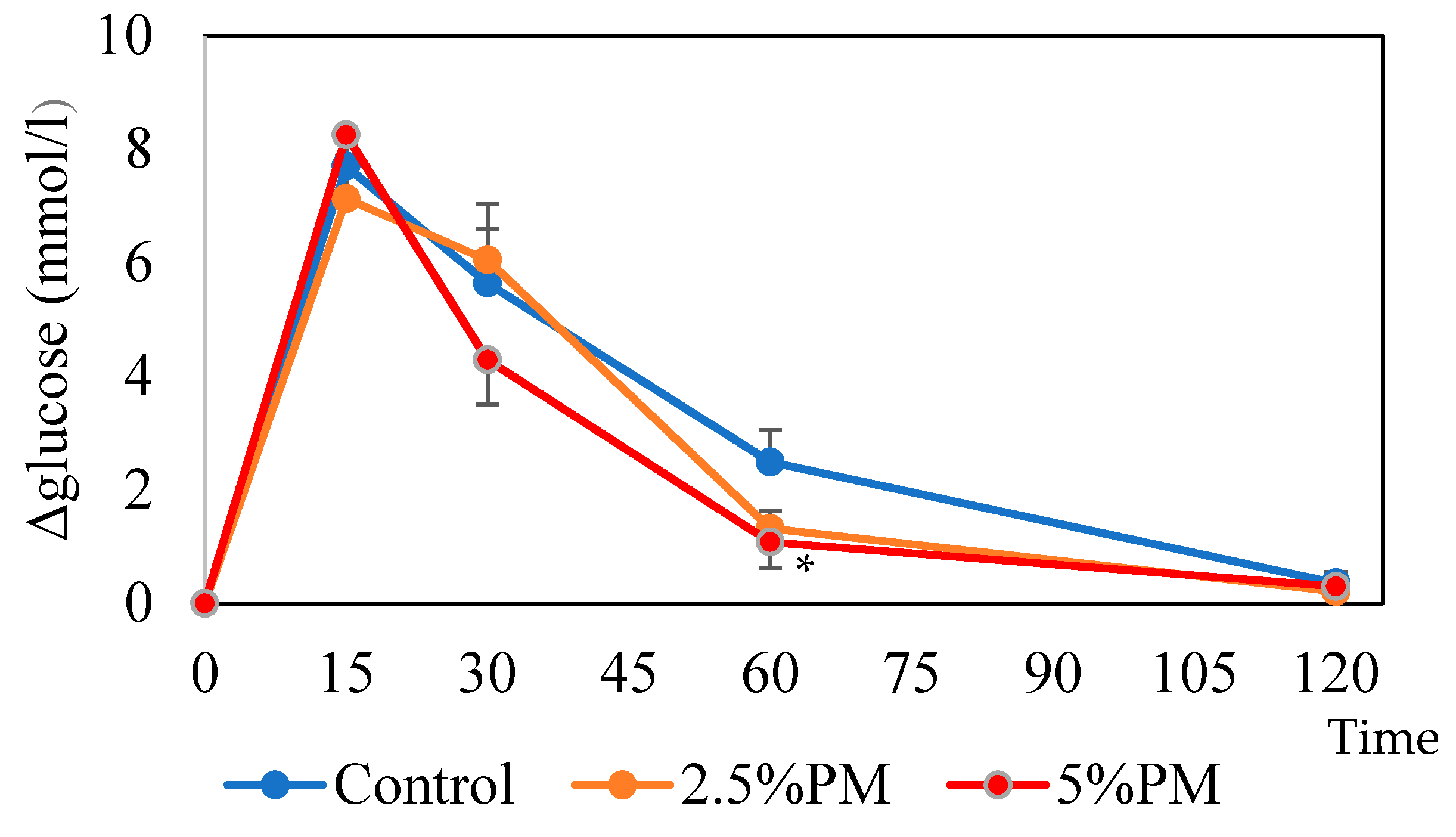
Sixty minutes after oral glucose administration in mice, the blood glucose level was significantly lower in the 5% PM group compared with the control group. It is suggested that insulin resistance occurs in mice fed PM due to the retardation of gastric emptying [
16]. It is also suggested that an increase in colonic fermentation in PM-fed mice may lower the increase in blood glucose due to the increase in SCFAs [
12,
13]. The production of incretins, such as glucagon-like peptide-1 (GLP-1), promotes insulin secretion and may contribute to improved insulin sensitivity [
25]. Significant increases in the concentration of cecal propionate were observed in the 2.5% and 5% PM supplementation groups; however, the contribution of propionate production to the total SCFA concentrations was relatively small. Further studies are needed to clarify the mechanism of improved glucose tolerance by PM intake.
It has been reported that curdlan, produced by
Alcaligenes faecalis var.
myxogenes, is also a β-1,3 glucan, but it is composed of linear single chains [
26]. Dietary curdlan is reported to be degraded and fermented by intestinal bacteria in the cecum, and may act to improve the intestinal environment via modification in the lower intestine [
27]. Therefore, the physiological characteristics of PM and curdlan differ, even though they are both β-1,3 glucans.
Serum sIgA concentrations were dose dependently increased in the PM-supplemented mice, in addition, significant differences were observed between the control group and the 2.5% and 5% PM supplementation groups. The insoluble PM particle itself may exert a direct stimulatory effect on intestinal epithelial cells, which may promote slgA content [
28]. PM might promote intestinal immunological activity:
Euglena gracilis EOD-1 biomass ingestion led to the production of a PM-specific IgA antibody and increased salivary IgA antibody titers in humans [
29]. Our data supports these previous reports.
PPARα is involved in many aspects of lipid metabolism [
30,
31], including fatty acid β-oxidation, synthesis, transport, storage, and lipoprotein metabolism during fasting [
32,
33]. Activation of PPARα by endogenous ligands or synthetic agonists moderately lowers LDL levels [
34]. Serum LDL-cholesterol concentrations were dose dependently reduced in mice supplemented with PM, and significant differences were observed between the 0% group and 5% PM supplementation group. The reduction in serum LDL-cholesterol might be caused by increasing hepatic PPARα expression; this is supported by the significant negative correlation of PPARα mRNA expression vs. LDL-cholesterol levels. Significant positive correlations between retroperitoneal and mesenteric fat weights vs. LDL-cholesterol levels were observed, suggesting that dose dependent reductions in serum LDL-cholesterol levels might cause a reduction in abdominal fat weights. However, we measured only PPARα mRNA expression; qualitative analysis of protein levels will be needed to help elucidate the mechanism of PM’s activation of PPARα mRNA expression.
Liver triacylglycerol production and secretion into VLDL are mainly determined by the fatty acid synthesis rate, which is controlled to a large extent at the level of transcription by both PPARα [
35], which stimulates fatty acid β-oxidation, and SREBP-1c [
36], which controls fatty acid synthesis. Our results indicated that activation of PPARα might decrease triglyceride accumulation in the abdominal fat tissues by reducing secretion into VLDL, whereas SREBP-1c may not be involved in fatty acid synthesis activation after PM supplementation. Previous reports showed that PPARα regulates the expression of fibroblast growth factor 21 (FGF21) during starvation [
37,
38]. FGF21 acts as an endocrine hormone targeting various functions, including glucose and lipid metabolism [
39]. Our results indicated that mRNA expression of hepatic FGF21 in the 2.5 and 5% PM groups increased by 1.5 times compared to the control group; however, the differences were not statistically significant. Further analyses of serum FGF21 concentrations are needed to confirm the relationship between PPARα mRNA expression and serum FGF21 secretion. It has been reported that PPAR activators inhibit the activation of inflammatory response genes by interfering with nuclear factor-κB (NF-κB) and transcription factor activator protein 1 (AP1) signaling pathways, thereby promoting insulin sensitivity [
40]. Improvements in insulin sensitivity caused by PM supplementation might activate PPARα by the same mechanism. Although PPARα is suggested to be activated by high levels of fatty acids present in the fasted liver [
41], the exact nature of the endogenous activation signal remains unknown [
42]. Further research is needed to elucidate the endogenous ligands promoted by direct PM stimulation of the epithelial cell.