Choline: Exploring the Growing Science on Its Benefits for Moms and Babies
Abstract
:1. Introduction
2. Food Sources of Choline
3. Choline Function
4. Choline Metabolism.
5. Choline and Fetal Development
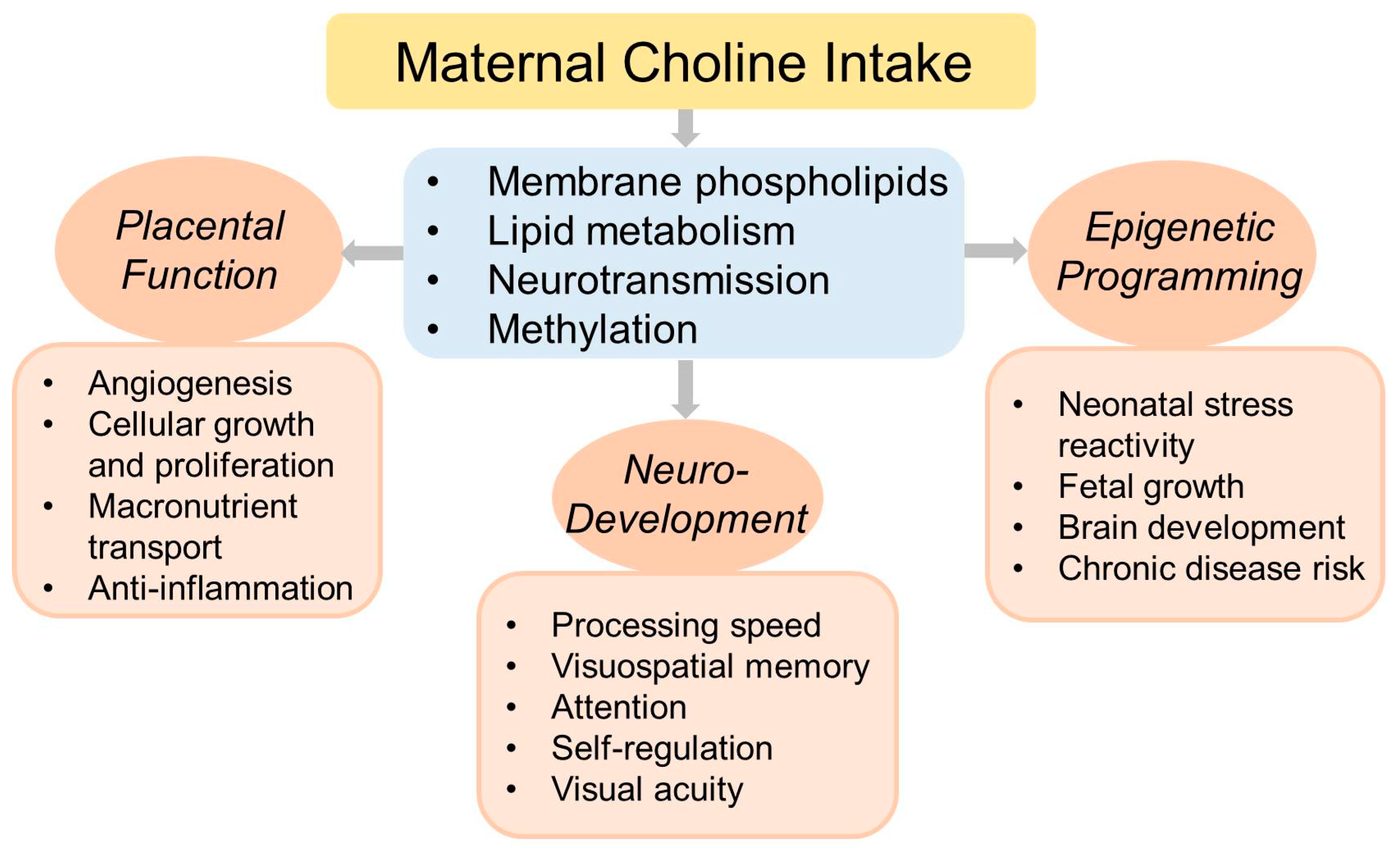
6. Choline and Pregnancy Outcomes
6.1. Epigenetic Programming of Postnatal Health
6.2. Placental Function
6.3. Macronutrient Metabolism and Energy Homeostasis
6.4. Neurodevelopment and Cognitive Function
6.5. Protection from Neural Insults
7. Choline Requirements during Pregnancy
8. Safety
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- AMA Wire. AMA Backs Global Health Experts in Calling Infertility a Disease. Available online: https://wire.ama-assn.org/ama-news/ama-backs-global-health-experts-calling-infertility-disease (accessed on 10 June 2019).
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Wallace, T.C.; Fulgoni, V.L. Usual Choline Intakes Are Associated with Egg and Protein Food Consumption in the United States. Nutrients 2017, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Patterson, Y.; Bhagwat, A.; Williams, R.; Howe, C.; Holden, M. USDA Database for The Choline Content of Common Foods; Release 2; Agricultural Research Service: Washington, DC, USA, 2008.
- Chester, D.; Goldman, J.; Ahuja, J.; Moshfegh, A. Dietary Intakes of Choline: What We Eat in America, NHANES 2007-Food Surveys Research Group Dietary Data Brief No. October 2011. Available online: http://ars.usda.gov/Services/docs.htm?docid=19476 (accessed on 10 June 2019).
- Dilger, R.N.; Garrow, T.A.; Baker, D.H. Betaine can partially spare choline in chicks but only when added to diets containing a minimal level of choline. J. Nutr. 2007, 137, 2224–2228. [Google Scholar] [CrossRef] [PubMed]
- Klatt, K.; Caudill, M. Folate, Choline, Vitamin B12 and Vitamin B6. In Biochemical, Physiological, & Molecular Aspects of Human Nutrition, 4th ed.; Stipanuk, M., Caudill, M., Eds.; Elsevier Saunders: St Louis, MO, USA, 2018. [Google Scholar]
- Li, Z.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewell, L.; Mason, A.; Vance, D.E. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- Morell, P.; Quarles, R. Characteristic Composition of Myelin. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Kwan, S.; King, J.; Caudill, M. Choline and Placental Trophoblast Development. In Human Placental Trophoblasts; Duttaroy, A., Basak, S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Park, E.I.; Garrow, T.A. Interaction between Dietary Methionine and Methyl Donor Intake on Rat Liver Betaine-homocysteine Methyltransferase Gene Expression and Organization of the Human Gene. J. Biol. Chem. 1999, 274, 7816–7824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Brenna, J.T.; Stabler, S.P.; Allen, R.H.; Gregory, J.F., 3rd; Caudill, M.A. Pregnancy alters choline dynamics: Results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am. J. Clin. Nutr. 2013, 98, 1459–1467. [Google Scholar] [CrossRef]
- Delong, C.J.; Shen, Y.-J.; Thomas, M.J.; Cui, Z. Molecular Distinction of Phosphatidylcholine Synthesis between the CDP-Choline Pathway and Phosphatidylethanolamine Methylation Pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef] [Green Version]
- Clandinin, M.; Chappell, J.; Leong, S.; Heim, T.; Swyer, P.; Chance, G. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef]
- A West, A.; Yan, J.; Jiang, X.; A Perry, C.; Innis, S.M.; A Caudill, M. Choline intake influences phosphatidylcholine DHA enrichment in nonpregnant women but not in pregnant women in the third trimester. Am. J. Clin. Nutr. 2013, 97, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a Preferred Carrier Form of Docosahexaenoic Acid to the Brain. J. Mol. Neurosci. 2001, 16, 201–204; discussion 215–221. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud-Roucher, V.; Kramer, A.; Silva, E.; Pantham, P.; Weintraub, S.T.; Jansson, T.; Powell, T.L. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a Is a Transporter for the Essential ω-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef] [PubMed]
- Ganz, A.B.; Klatt, K.C.; Caudill, M.A. Common Genetic Variants Alter Metabolism and Influence Dietary Choline Requirements. Nutrients 2017, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Ganz, A.B.; Shields, K.; Fomin, V.G.; Lopez, Y.S.; Mohan, S.; Lovesky, J.; Chuang, J.C.; Ganti, A.; Carrier, B.; Yan, J.; et al. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. FASEB J. 2016, 30, 3321–3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, A.B.; Cohen, V.V.; Swersky, C.C.; Stover, J.; Vitiello, G.A.; Lovesky, J.; Chuang, J.C.; Shields, K.; Fomin, V.G.; Lopez, Y.S.; et al. Genetic Variation in Choline-Metabolizing Enzymes Alters Choline Metabolism in Young Women Consuming Choline Intakes Meeting Current Recommendations. Int. J. Mol. Sci. 2017, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, K.-A.; Kozyreva, O.G.; Song, J.; Galanko, J.A.; Fischer, L.M.; Zeisel, S.H. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006, 20, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Filgueiras, C.C.; Manhães, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M.; Schambra, U.B. Morphogenetic Roles of Acetylcholine. Environ. Heal. Perspect. 1999, 107 (Suppl. 1), 65–69. [Google Scholar]
- Blusztajn, J.K.; Rinnofner, J. Intrinsic Cholinergic Neurons in the Hippocampus: Fact or Artifact? Front. Synaptic Neurosci. 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Mellott, T.J. Choline nutrition programs brain development via DNA and histone methylation. Central Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef]
- Kovacheva, V.P.; Mellott, T.J.; Davison, J.M.; Wagner, N.; Lopez-Coviella, I.; Schnitzler, A.C.; Blusztajn, J.K. Gestational Choline Deficiency Causes Global and Igf2 Gene DNA Hypermethylation by Up-regulation ofDnmt1Expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef] [PubMed]
- Mehedint, M.G.; Craciunescu, C.N.; Zeisel, S.H. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 12834–12839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehedint, M.G.; Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; King, J.H.; Grenier, J.K.; Yan, J.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation during Normal Murine Pregnancy Alters the Placental Epigenome: Results of an Exploratory Study. Nutrients 2018, 10, 417. [Google Scholar] [CrossRef]
- Medici, V.; Shibata, N.M.; Kharbanda, K.K.; Islam, M.S.; Keen, C.L.; Kim, K.; Tillman, B.; French, S.W.; Halsted, C.H.; LaSalle, J.M. Maternal choline modifies fetal liver copper, gene expression, DNA methylation, and neonatal growth in the tx-j mouse model of Wilson disease. Epigenetics 2014, 9, 286–296. [Google Scholar] [CrossRef]
- Kovacheva, V.P.; Davison, J.M.; Mellott, T.J.; Rogers, A.E.; Yang, S.; O’Brien, M.J.; Blusztajn, J.K. Raising gestational choline intake alters gene expression in DMBA-evoked mammary tumors and prolongs survival. FASEB J. 2009, 23, 1054–1063. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, J.A.; Karalis, K.P. Placental corticotropin-releasing hormone: Function and regulation. Am. J. Obstet. Gynecol. 1999, 180, S242–S246. [Google Scholar] [CrossRef]
- Levitt, N.S.; Lambert, E.V.; Woods, D.; Hales, C.N.; Andrew, R.; Seckl, J.R. Impaired Glucose Tolerance and Elevated Blood Pressure in Low Birth Weight, Nonobese, Young South African Adults: Early Programming of Cortisol Axis. J. Clin. Endocrinol. Metab. 2000, 85, 4611–4618. [Google Scholar] [PubMed]
- Xiong, F.; Zhang, L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 2013, 34, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L. Epigenetic mechanisms in developmental programming of adult disease. Drug Discov. Today 2011, 16, 1007–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, G.M.; Carmichael, S.L.; Yang, W.; Selvin, S.; Schaffer, D.M. Periconceptional Dietary Intake of Choline and Betaine and Neural Tube Defects in Offspring. Am. J. Epidemiol. 2004, 160, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Signore, C.; Ueland, P.M.; Troendle, J.; Mills, J.L. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am. J. Clin. Nutr. 2008, 87, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Shaw, G.M.; Finnell, R.H.; Blom, H.J.; Carmichael, S.L.; Vollset, S.E.; Yang, W.; Ueland, P.M. Choline and Risk of Neural Tube Defects in a Folate-fortified Population. Epidemiology 2009, 20, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.T.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 2012, 7, e43448. [Google Scholar] [CrossRef]
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Périnat. Epidemiol. 2012, 26, 328–335. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Goldman, B.D.; Fischer, L.M.; Da Costa, K.-A.; Reznick, J.S.; Zeisel, S.H. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2012, 96, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bar, H.Y.; Yan, J.; Jones, S.; Brannon, P.M.; West, A.A.; Perry, C.A.; Ganti, A.; Pressman, E.; Devapatla, S.; et al. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1). FASEB J. 2013, 27, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.G.; Hunter, S.K.; McCarthy, L.; Beuler, J.; Hutchison, A.K.; Wagner, B.D.; Leonard, S.; Stevens, K.E.; Freedman, R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 2013, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.G.; Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; Chambers, B.M.; Law, A.J.; Leonard, S.; Zerbe, G.O.; Freedman, R. Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence for CHRNA7 Moderation. Am. J. Psychiatry 2016, 173, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Freedman, R.; Hunter, S.K.; Law, A.J.; Wagner, B.D.; D’Alessandro, A.; Christians, U.; Noonan, K.; Wyrwa, A.; Hoffman, M.C. Higher Gestational Choline Levels in Maternal Infection Are Protective for Infant Brain Development. J. Pediatr. 2019, 208, 198–206.e2. [Google Scholar] [CrossRef]
- Bahnfleth, C.; Canfield, R.; Nevins, J.; Caudill, M.; Strupp, B. Prenatal Choline Supplementation Improves Child Color-location Memory Task Performance at 7 Y of Age (FS05-01-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1). [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. BioMed Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef]
- Jiang, X.; Jones, S.; Andrew, B.Y.; Ganti, A.; Malysheva, O.V.; Giallourou, N.; Brannon, P.M.; Roberson, M.S.; Caudill, M.A. Choline Inadequacy Impairs Trophoblast Function and Vascularization in Cultured Human Placental Trophoblasts. J. Cell. Physiol. 2014, 229, 1016–1027. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; King, J.H.; Yan, J.; Jiang, X.; Wei, E.; Fomin, V.G.; Roberson, M.S.; Caudill, M.A. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta 2017, 53, 57–65. [Google Scholar] [CrossRef]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Jiang, X.; Fomin, V.G.; Levine, S.P.; Wei, E.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Modulates Placental Markers of Inflammation, Angiogenesis, and Apoptosis in a Mouse Model of Placental Insufficiency. Nutrients 2019, 11, 374. [Google Scholar] [CrossRef]
- Hutzler, J.S.; Klein, H.R.; Brenna, J.T.; Kwan, S.T.C.; King, J.H.; Yan, J.; Wang, Z.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Modulates Placental Nutrient Transport and Metabolism in Late Gestation of Mouse Pregnancy. J. Nutr. 2017, 147, 2083–2092. [Google Scholar]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Klatt, K.C.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Alters Fetal Growth Patterns in a Mouse Model of Placental Insufficiency. Nutrients 2017, 9, 765. [Google Scholar] [CrossRef]
- Nam, J.; Greenwald, E.; Jack-Roberts, C.; Ajeeb, T.T.; Malysheva, O.V.; Caudill, M.A.; Axen, K.; Saxena, A.; Semernina, E.; Nanobashvili, K.; et al. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J. Nutr. Biochem. 2017, 49, 80–88. [Google Scholar] [CrossRef]
- Jack-Roberts, C.; Joselit, Y.; Nanobashvili, K.; Bretter, R.; Malysheva, O.V.; Caudill, M.A.; Saxena, A.; Axen, K.; Gomaa, A.; Jiang, X. Choline Supplementation Normalizes Fetal Adiposity and Reduces Lipogenic Gene Expression in a Mouse Model of Maternal Obesity. Nutrients 2017, 9, 899. [Google Scholar] [CrossRef]
- Nanobashvili, K.; Jack-Roberts, C.; Bretter, R.; Jones, N.; Axen, K.; Saxena, A.; Blain, K.; Jiang, X. Maternal Choline and Betaine Supplementation Modifies the Placental Response to Hyperglycemia in Mice and Human Trophoblasts. Nutrients 2018, 10, 1507. [Google Scholar] [CrossRef]
- Jansson, N.; Rosario, F.J.; Gaccioli, F.; Lager, S.; Jones, H.N.; Roos, S.; Jansson, T.; Powell, T.L. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 2013, 98, 105–113. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R87–R93. [Google Scholar] [CrossRef] [Green Version]
- Joselit, Y.; Nanobashvili, K.; Jack-Roberts, C.; Greenwald, E.; Malysheva, O.V.; Caudill, M.A.; Saxena, A.; Jiang, X. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr. Diabetes 2018, 8, 41. [Google Scholar] [CrossRef]
- Carlin, J.; George, R.; Reyes, T.M. Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS ONE 2013, 8, e63549. [Google Scholar] [CrossRef]
- McCann, J.C.; Hudes, M.; Ames, B.N. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 2006, 30, 696–712. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003, 27, 385–399. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Mudd, A.T.; Getty, C.M.; Dilger, R.N. Maternal Dietary Choline Status Influences Brain Gray and White Matter Development in Young Pigs. Curr. Dev. Nutr. 2018, 2, nzy015. [Google Scholar] [CrossRef] [Green Version]
- Getty, C.M.; Dilger, R.N. Moderate Perinatal Choline Deficiency Elicits Altered Physiology and Metabolomic Profiles in the Piglet. PLoS ONE 2015, 10, e0133500. [Google Scholar] [CrossRef]
- Mudd, A.T.; Getty, C.M.; Sutton, B.P.; Dilger, R.N. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr. Neurosci. 2016, 19, 425–433. [Google Scholar] [CrossRef]
- Albright, C.D.; Tsai, A.Y.; Friedrich, C.B.; Mar, M.-H.; Zeisel, S.H. Choline availability alters embryonic development of the hippocampus and septum in the rat. Dev. Brain Res. 1999, 113, 13–20. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, I.; Friday, W.B.; Munson, C.A.; Bachleda, A.; Weiss, E.R.; Alam, N.M.; Sha, W.; Zeisel, S.H.; Surzenko, N. Low availability of choline in utero disrupts development and function of the retina. FASEB J. 2019, 33, 9194–9209. [Google Scholar] [CrossRef]
- Gould, R.M.; Dawson, R.M. Incorporation of newly formed lecithin into peripheral nerve myelin. J. Cell Biol. 1976, 68, 480–496. [Google Scholar] [CrossRef]
- Cermak, J.M.; Holler, T.; Jackson, D.A.; Blusztajn, J.K. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998, 12, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guo-Ross, S.; Lewis, D.V.; Turner, D.; White, A.M.; Wilson, W.A.; Swartzwelder, H.S. Dietary Prenatal Choline Supplementation Alters Postnatal Hippocampal Structure and Function. J. Neurophysiol. 2004, 91, 1545–1555. [Google Scholar] [CrossRef]
- Glenn, M.J.; Gibson, E.M.; Kirby, E.D.; Mellott, T.J.; Blusztajn, J.K.; Williams, C.L. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 2007, 25, 2473–2482. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.; Meck, W.H.; Williams, C.L.; A Wilson, W.; Swartzwelder, H.; Iii, J.J. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Dev. Brain Res. 1999, 118, 159–167. [Google Scholar] [CrossRef]
- Mellott, T.J.; Huleatt, O.M.; Shade, B.N.; Pender, S.M.; Liu, Y.B.; Slack, B.E.; Blusztajn, J.K. Perinatal Choline Supplementation Reduces Amyloidosis and Increases Choline Acetyltransferase Expression in the Hippocampus of the APPswePS1dE9 Alzheimer’s Disease Model Mice. PLoS ONE 2017, 12, e0170450. [Google Scholar]
- Velazquez, R.; Ferreira, E.; Winslow, W.; Dave, N.; Piras, I.S.; Naymik, M.; Huentelman, M.J.; Tran, A.; Caccamo, A.; Oddo, S. Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatry 2019. [Google Scholar] [CrossRef]
- Thomas, J. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology Teratol. 2004, 26, 35–45. [Google Scholar] [CrossRef]
- Thomas, J.D.; Abou, E.J.; Dominguez, H.D. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology Teratol. 2009, 31, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.D.; Tran, T.D. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 2012, 22, 619–630. [Google Scholar] [CrossRef]
- Langley, E.A.; Krykbaeva, M.; Blusztajn, J.K.; Mellott, T.J. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav. Brain Res. 2015, 278, 210–220. [Google Scholar] [CrossRef]
- Orenbuch, A.; Fortis, K.; Taesuwan, S.; Yaffe, R.; Caudill, M.A.; Golan, H.M. Prenatal Nutritional Intervention Reduces Autistic-Like Behavior Rates Among. Front. Neurosci. 2019, 13, 383. [Google Scholar] [CrossRef]
- Velázquez, R.; Ash, J.A.; Powers, B.E.; Kelley, C.M.; Strawderman, M.; Luscher, Z.I.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 2013, 58, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Powers, B.E.; Kelley, C.M.; Velazquez, R.; Ash, J.A.; Strawderman, M.S.; Alldred, M.J.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation in a mouse model of Down syndrome: Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience 2017, 340, 501–514. [Google Scholar] [CrossRef]
- Ash, J.A.; Velázquez, R.; Kelley, C.M.; Powers, B.E.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol. Dis. 2014, 70, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Ginsberg, S.D.; Powers, B.; Alldred, M.J.; Saltzman, A.; Strupp, B.J.; Caudill, M.A. Maternal choline supplementation programs greater activity of the phosphatidylethanolamine N-methyltransferase (PEMT) pathway in adult Ts65Dn trisomic mice. FASEB J. 2014, 28, 4312–4323. [Google Scholar] [CrossRef]
- Kennedy, B.C.; Dimova, J.G.; Siddappa, A.J.M.; Tran, P.V.; Gewirtz, J.C.; Georgieff, M.K. Prenatal Choline Supplementation Ameliorates the Long-Term Neurobehavioral Effects of Fetal-Neonatal Iron Deficiency in Rats. J. Nutr. 2014, 144, 1858–1865. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.V.; Kennedy, B.C.; Pisansky, M.T.; Won, K.-J.; Gewirtz, J.C.; A Simmons, R.; Georgieff, M.K. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency–Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus. J. Nutr. 2016, 146, 484–493. [Google Scholar] [CrossRef]
- Strupp, B.J.; Powers, B.E.; Velazquez, R.; Ash, J.A.; Kelley, C.M.; Alldred, M.J.; Strawderman, M.; Caudill, M.A.; Mufson, E.J.; Ginsberg, S.D. Maternal choline supplementation: A potential prenatal treatment for Down syndrome and Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 97–106. [Google Scholar] [CrossRef]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcohol Clin. Exp. Res. 2013, 37, 1133–1142. [Google Scholar] [CrossRef]
- Otero, N.K.H.; Thomas, J.D.; Saski, C.A.; Xia, X.; Kelly, S.J. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol. Clin. Exp. Res. 2012, 36, 1701–1709. [Google Scholar] [CrossRef]
- Resseguie, M.; Song, J.; Niculescu, M.D.; Da Costa, K.-A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Stabler, S.P.; Allen, R.H.; et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Schaible, T.D.; Harris, R.A.; Dowd, S.E.; Smith, C.W.; Kellermayer, R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum. Mol. Genet. 2011, 20, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, J.W.; Maruoka, S.; Boon, K.; Garantziotis, S.; Li, Z.; Tomfohr, J.; Bailey, N.; Potts, E.N.; Whitehead, G.; Brass, D.M.; et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Investig. 2008, 118, 3462–3469. [Google Scholar] [CrossRef] [Green Version]
- Giudicelli, F.; Brabant, A.L.; Grit, I.; Parnet, P.; Amarger, V. Excess of methyl donor in the perinatal period reduces postnatal leptin secretion in rat and interacts with the effect of protein content in diet. PLoS ONE 2013, 8, e68268. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine- N -Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Klatt, K.C.; Caudill, M.A. Pressing the trimethylamine N-oxide narrative. AME Med. J. 2017, 2, 132. [Google Scholar] [CrossRef]

| Study | Design | Intervention/Choline Marker Measurement | Pregnancy and Child Health Outcomes | References |
|---|---|---|---|---|
| Shaw et al. 2004 | Case-control | Maternal dietary choline intake during the 3 months before conception | Reduced neural tube defect (NTD) risk with higher maternal choline intakes (n = 424 NTD and 440 control) | [43] |
| Signore et al. 2008 | Prospective cohort | Maternal serum total and free choline throughout gestation and cord blood choline concentrations | No association between child intelligence quotient (IQ) scores at 5 years of age and maternal or cord blood choline (n = 404 maternal-child pairs) | [44] |
| Shaw et al. 2009 | Prospective case-control | Serum total choline concentrations during the gestational week 15–18 | Reduced NTD risk with higher serum choline concentrations (n = 80 NTD and 409 control) | [45] |
| Wu et al. 2012 | Prospective cohort | Maternal plasma free choline at gestational week 16 | Better cognitive scores in 18-month-old infants with higher maternal plasma free choline levels (n = 154 maternal-child pairs) | [46] |
| Villamor et al. 2012 | Prospective cohort | Maternal dietary choline intake in the 1st and 2nd trimester of pregnancy | No association between cognitive performance in 3-year-old children and maternal choline intake (n = 1210) | [47] |
| Jiang et al. 2012 a | Randomized clinical trial (RCT) | Controlled feeding of 930 versus 480 mg choline/day for 12 weeks during 3rd trimester of pregnancy | Higher placental CRH promoter methylation and lower cord blood cortisol concentrations in the 930 mg/d (n = 13) versus 480 mg/day (n = 13) choline intake group. | [38] |
| Cheatham et al. 2012 | RCT | Phosphatidylcholine (PC) supplement (750 mg choline/day) from 2nd trimester of pregnancy to 90 days postpartum | No effect on development or memory in 10 or 12-month-old infants (intervention group n = 49, placebo group n = 50) | [48] |
| Boeke et al. 2013 | Prospective cohort | Maternal dietary choline intake in the 2nd trimester of pregnancy | Better visual memory in 7-year-old children with top interquartile dietary choline intake during pregnancy (n = 895) | [49] |
| Jiang et al. 2013 a | RCT | Controlled feeding of 930 versus 480 mg choline/day for 12 weeks during 3rd trimester of pregnancy | Lower placental sFlt1 mRNA expression and maternal serum sFLT1 levels in the 930 mg/day (n = 13) versus 480 mg/d (n = 13) choline intake group | [50] |
| Ross et al. 2013 b | RCT | PC supplement (900 mg choline/day) from 2nd trimester of pregnancy until delivery; 100mg/day of PC to infants until 3 months of age | Greater attention development in 5-week-old infants in the intervention group (n = 36) versus control (n = 40) | [51] |
| Ross et al. 2016 b | RCT | PC supplement (900 mg choline/day) from 2nd trimester of pregnancy until delivery; 100mg/day of PC to infants until 3 months of age | Reduced attentional problems and social withdrawal in children at 40 months of age in the intervention group (n = 23) versus control (n = 26) | [52] |
| Caudill et al. 2018 a | RCT | Controlled feeding of 930 versus 480 mg choline/day for 12 weeks during 3rd trimester of pregnancy | Faster information processing speed in infants during 4–13 months in the 930 (n = 12) versus 480 mg/day group (n = 12) | [53] |
| Jacobson et al. 2018 | RCT | 2 g choline/day or placebo from mid-pregnancy until delivery among heavy alcohol drinkers | Better eyeblink conditioning in infants at 6.5 months, higher novelty preference scores at 12 months, and more catch-up growth at both time points in the choline treated group (n = 32) versus control (n = 31) | [54] |
| Freedman et al. 2018 | Prospective cohort | Serum-free choline and betaine concentrations at week 16 of gestation | Improved development of cerebral inhibition in newborns and behavioral regulation in 1-year-old infants born to infected mothers (n = 66) with higher gestational serum choline concentrations. | [55] |
| Bahnfleth et al. 2019 a | RCT | Controlled feeding of 930 versus 480 mg choline/day for 12 weeks during 3rd trimester of pregnancy | Better performance on a task of color-location memory at age 7 years in the 930 mg choline/day (n = 11) versus 480 choline mg/day (n = 9) choline intake group. | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. https://doi.org/10.3390/nu11081823
Korsmo HW, Jiang X, Caudill MA. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients. 2019; 11(8):1823. https://doi.org/10.3390/nu11081823
Chicago/Turabian StyleKorsmo, Hunter W., Xinyin Jiang, and Marie A. Caudill. 2019. "Choline: Exploring the Growing Science on Its Benefits for Moms and Babies" Nutrients 11, no. 8: 1823. https://doi.org/10.3390/nu11081823
APA StyleKorsmo, H. W., Jiang, X., & Caudill, M. A. (2019). Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients, 11(8), 1823. https://doi.org/10.3390/nu11081823






