Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Red Lentil Flour and Experimental Diets
2.2. Study Design
2.3. Fecal Microbiome Analyses
2.4. Fecal SCFA and Branched-Chain Fatty Acid (BCFA) Analyses
2.5. Colon Histology
2.6. Cell Proliferation
2.7. Colonic mRNA Expression
2.8. Statistical Analysis
3. Results
3.1. Consumption of Lentil-Supplemented Diets Do Not Alter Food Intake or Body Weight Gain in C57Bl/6 Male Mice
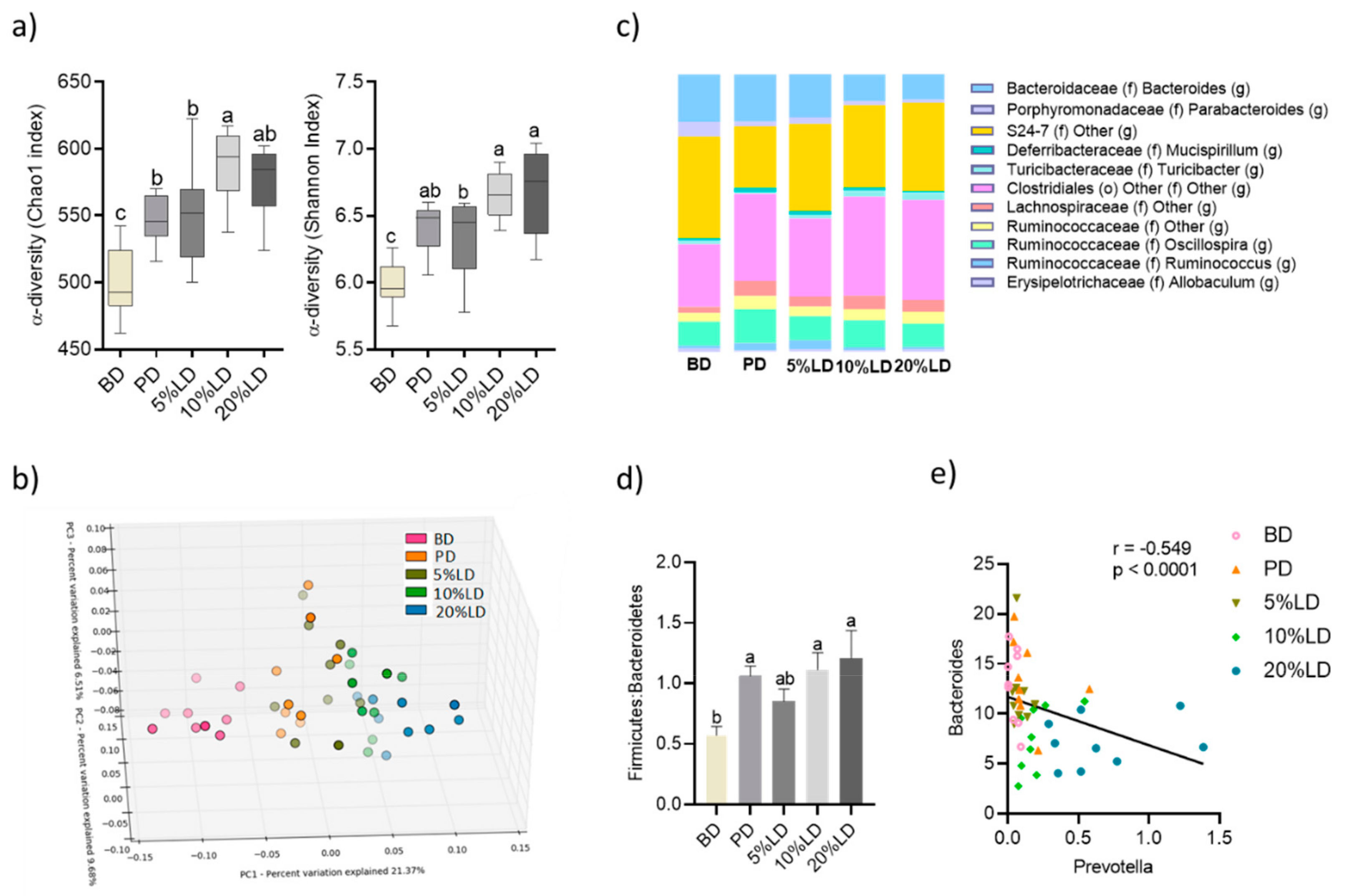
3.2. Consumption of Lentil-Supplemented Diets Modulate Fecal Microbial Community Structure and Activity
3.2.1. Microbial Community Diversity
3.2.2. Microbial Community Structure
3.2.3. Microbial Community Activity
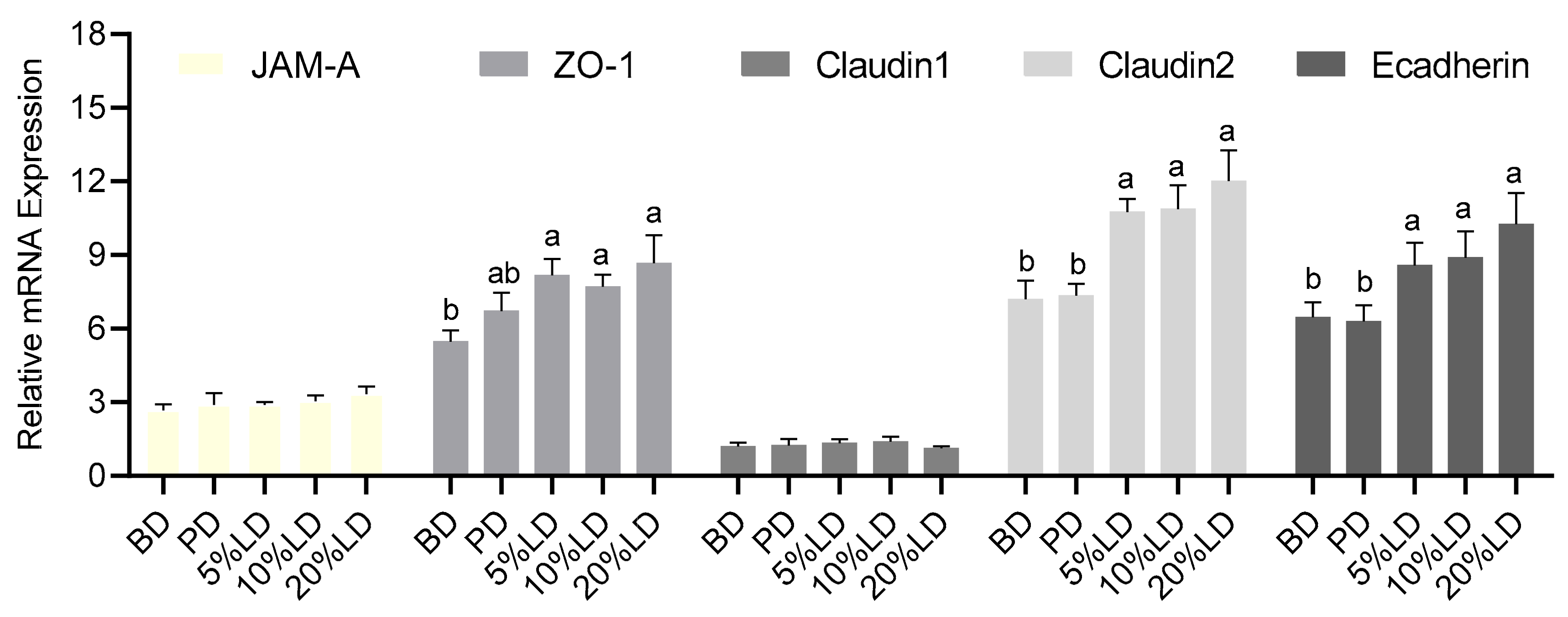
3.3. Consumption of Lentil-Supplemented Diets Alters Colon Barrier Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Willcox, G.; Fornite, S.; Herveux, L. Early Holocene cultivation before domestication in northern Syria. Veg. Hist. Archaeobot. 2008, 17, 313–325. [Google Scholar] [CrossRef]
- Oomah, B.; Ankit, P.; Rawson, A.; Singh, N.; Compos-Vega, R. Chemistry of pulses. In Pulse Foods: Processing, Quality and Neutraceutical Applications; Brijesh, K., Tiwari, A.G., McKenna, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nosworthy, M.G.; Neufeld, J.; Frohlich, P.; Young, G.; Malcolmson, L.; House, J.D. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 2017, 5, 896–903. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Curran, J.; Barr, S.I.; Slavin, J.; Puri, S.; Swaminathan, S.; Tapsell, L.; Patterson, C.A. Enhancing nutrition with pulses: Defining a recommended serving size for adults. Nutr. Rev. 2017, 75, 990–1006. [Google Scholar] [CrossRef]
- De Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Pissini Machado Reis, S.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Liu, Q.; Donner, E.; Hawke, A.; Kalinga, D.; Winberg, J.; Wolever, T.M.S. Investigating the relationship between lentil carbohydrate fractions and in vivo postprandial blood glucose response by use of the natural variation in starch fractions among 20 lentil varieties. Food Funct. 2017, 8, 3783–3791. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Reynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta-Gallegos, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Oomah, B.D.; Loarca-Pina, G. Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, T59–T65. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef]
- Jayalath, V.H.; de Souza, R.J.; Sievenpiper, J.L.; Ha, V.; Chiavaroli, L.; Mirrahimi, A.; Di Buono, M.; Bernstein, A.M.; Leiter, L.A.; Kris-Etherton, P.M.; et al. Effect of dietary pulses on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Am. J. Hypertens. 2014, 27, 56–64. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. B.m.j. 2011, 343, d6617. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food Outlook—Biannual Report on Global Food Markets; Food and Agriculture Organization of the United Nations: Quebec City, QC, Canada, 2016. [Google Scholar]
- Government of Canada. In Canada’s Food Guide. Available online: https://food-guide.canada.ca/en/ (accessed on 8 August 2019).
- Monk, J.M.; Lepp, D.; Wu, W.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J. Nutr. Biochem. 2017, 49, 89–100. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct. Foods 2017, 38 PartB, 663–674. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E.; et al. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef]
- Fernando, W.M.; Hill, J.E.; Zello, G.A.; Tyler, R.T.; Dahl, W.J.; Van Kessel, A.G. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef Microbes 2010, 1, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Monk, J.M.; Wu, W.; McGillis, L.H.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Robinson, L.E.; Power, K.A. Chickpea supplementation prior to colitis onset reduces inflammation in dextran sodium sulfate-treated C57Bl/6 male mice. Appl. Physiol. Nutr. Metab. 2018, 43, 893–901. [Google Scholar] [CrossRef]
- Monk, J.M.; Wu, W.; Hutchinson, A.L.; Pauls, P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation attenuates colitis-associated inflammation and colonic epithelial damage. J. Nutr. Biochem. 2018, 56, 215–223. [Google Scholar] [CrossRef]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Pauls, K.P.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760. [Google Scholar] [CrossRef]
- Feregrino-Perez, A.A.; Pinol-Felis, C.; Gomez-Arbones, X.; Guevara-Gonzalez, R.G.; Campos-Vega, R.; Acosta-Gallegos, J.; Loarca-Pina, G. A non-digestible fraction of the common bean (Phaseolus vulgaris L.) induces cell cycle arrest and apoptosis during early carcinogenesis. Plant Foods Hum. Nutr. 2014, 69, 248–254. [Google Scholar] [CrossRef]
- Vergara-Castaneda, H.A.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Guzman-Maldonado, H.; Feregrino-Perez, A.A.; Oomah, B.D.; Loarca-Pina, G. Non-digestible fraction of cooked bean (Phaseolus vulgaris L.) cultivar Bayo Madero suppresses colonic aberrant crypt foci in azoxymethane-induced rats. Food Funct. 2010, 1, 294–300. [Google Scholar] [CrossRef]
- Hayde, V.C.; Ramon, G.G.; Lorenzo, G.O.; Dave, O.B.; Rosalia, R.C.; Paul, W.; Guadalupe, L.P. Non-digestible fraction of beans (Phaseolus vulgaris L.) modulates signalling pathway genes at an early stage of colon cancer in Sprague-Dawley rats. Br. J. Nutr. 2012, 108 (Suppl. 1), S145–S154. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Garcia-Gasca, T.; Guevara-Gonzalez, R.; Ramos-Gomez, M.; Oomah, B.D.; Loarca-Pina, G. Human gut flora-fermented nondigestible fraction from cooked bean (Phaseolus vulgaris L.) modifies protein expression associated with apoptosis, cell cycle arrest, and proliferation in human adenocarcinoma colon cancer cells. J. Agric. Food Chem. 2012, 60, 12443–12450. [Google Scholar] [CrossRef]
- Chino, X.M.S.; Martínez, C.J.; Garzón, V.R.V.; González, I.; Treviño, S.V.; Bujaidar, E.M.; Ortiz, G.D.; Hoyos, R.B. Cooked Chickpea Consumption Inhibits Colon Carcinogenesis in Mice Induced with Azoxymethane and Dextran Sulfate Sodium. J. Am. Coll. Nutr. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible dry bean consumption (Phaseolus vulgaris L.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br. J. Nutr. 2012, 108 (Suppl. 1), S66–S73. [Google Scholar] [CrossRef]
- Dominguez-Uscanga, A.; Loarca-Pina, G.; Gonzalez de Mejia, E. Baked corn (Zea mays L.) and bean (Phaseolus vulgaris L.) snack consumption lowered serum lipids and differentiated liver gene expression in C57BL/6 mice fed a high-fat diet by inhibiting PPARgamma and SREBF2. J. Nutr. Biochem. 2017, 50, 1–15. [Google Scholar] [CrossRef]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. J. Nutr. Biochem 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Anderson, G.H.; Liu, Y.; Smith, C.E.; Liu, T.T.; Nunez, M.F.; Mollard, R.C.; Luhovyy, B.L. The acute effect of commercially available pulse powders on postprandial glycaemic response in healthy young men. Br. J. Nutr. 2014, 112, 1966–1973. [Google Scholar] [CrossRef] [Green Version]
- Saraf-Bank, S.; Esmaillzadeh, A.; Faghihimani, E.; Azadbakht, L. Effect of non-soy legume consumption on inflammation and serum adiponectin levels among first-degree relatives of patients with diabetes: A randomized, crossover study. Nutrition 2015, 31, 459–465. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Kendall, C.W.; Esfahani, A.; Wong, J.M.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 2017, 61, 1600129–1600149. [Google Scholar] [CrossRef]
- Van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- Hernandez-Salazar, M.; Osorio-Diaz, P.; Loarca-Pina, G.; Reynoso-Camacho, R.; Tovar, J.; Bello-Perez, L.A. In vitro fermentability and antioxidant capacity of the indigestible fraction of cooked black beans (Phaseolus vulgaris L.), lentils (Lens culinaris L.) and chickpeas (Cicer arietinum L.). J. Sci. Food Agric. 2010, 90, 1417–1422. [Google Scholar] [CrossRef]
- Gullon, P.; Gullon, B.; Tavaria, F.; Vasconcelos, M.; Gomes, A.M. In vitro fermentation of lupin seeds (Lupinus albus) and broad beans (Vicia faba): Dynamic modulation of the intestinal microbiota and metabolomic output. Food Funct. 2015, 6, 3316–3322. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Stephen, A.M.; Dahl, W.J.; Sieber, G.M.; van Blaricom, J.A.; Morgan, D.R. Effect of green lentils on colonic function, nitrogen balance, and serum lipids in healthy human subjects. Am. J. Clin. Nutr. 1995, 62, 1261–1267. [Google Scholar] [CrossRef]
- Siva, N.; Johnson, C.R.; Richard, V.; Jesch, E.D.; Whiteside, W.; Abood, A.A.; Thavarajah, P.; Duckett, S.; Thavarajah, D. Lentil (Lens culinaris Medikus) Diet Affects the Gut Microbiome and Obesity Markers in Rat. J. Agric. Food Chem. 2018, 66, 8805–8813. [Google Scholar] [CrossRef]
- Faris, M.A.; Takruri, H.R.; Shomaf, M.S.; Bustanji, Y.K. Chemopreventive effect of raw and cooked lentils (Lens culinaris L) and soybeans (Glycine max) against azoxymethane-induced aberrant crypt foci. Nutr. Res. 2009, 29, 355–362. [Google Scholar] [CrossRef]
- Jood, S.; Bishnoi, S.; Sharma, A. Chemical analysis and physico-chemical properties of chickpea and lentil cultivars. Food/Nahrung 1998, 42, 71–74. [Google Scholar] [CrossRef]
- Satya, S.; Kaushik, G.; Naik, S.N. Processing of food legumes: A boon to human nutrition. Mediterr. J. Nutr. Metab. 2010, 3, 183–195. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Vandenberg, A. Lentils (Lens culinaris Medikus Subspecies culinaris): A Whole Food for Increased Iron and Zinc Intake. J. Agric. Food Chem. 2009, 57, 5413–5419. [Google Scholar] [CrossRef]
- Chen, Y.; McGee, R.; Vandemark, G.; Brick, M.; Thompson, H.J. Dietary Fiber Analysis of Four Pulses Using AOAC 2011.25: Implications for Human Health. Nutrients 2016, 8, 829. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Han, H.; Baik, B.K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Xu, B.J.; Yuan, S.H.; Chang, S.K. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Duenas, M.; Sun, B.S.; Hernandez, T.; Estrella, I.; Spranger, M.I. Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.). J. Agric. Food Chem. 2003, 51, 7999–8004. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.Y.; Tang, Y.; Chen, P.X.; Liu, R.H.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Effect of Domestic Cooking on Carotenoids, Tocopherols, Fatty Acids, Phenolics, and Antioxidant Activities of Lentils (Lens culinaris). J. Agric. Food Chem. 2014, 62, 12585–12594. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Phytochemical profiles and health-promoting effects of cool-season food legumes as influenced by thermal processing. J. Agric. Food Chem. 2009, 57, 10718–10731. [Google Scholar] [CrossRef]
- Kan, L.J.; Nie, S.P.; Hu, J.L.; Wang, S.N.; Bai, Z.Y.; Wang, J.Q.; Zhou, Y.M.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Sung, M.K.; Park, M.Y. Nutritional modulators of ulcerative colitis: Clinical efficacies and mechanistic view. World J. Gastroenterol. 2013, 19, 994–1004. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.Y.; Liu, L.; Li, Y.X.; Xun, A.Y.; Zeng, W.S.; Jia, C.H.; Wei, X.X.; Feng, J.L.; Zhao, L.; et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010, 41, 288–294. [Google Scholar] [CrossRef]
- Palla, A.H.; Iqbal, N.T.; Minhas, K.; Gilani, A.H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 2016, 38, 153–166. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.I.; Kim, Y.; Kim, Y.S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2018, 58, 1603–1613. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Devel. Ther. 2015, 9, 3923–3934. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Power, K.A.L.D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 18, 61–69. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Ea-utils. Command-line Tools for Processing Biological Sequencing Data. Available online: https://expressionanalysis.github.io/ea-utils/ (accessed on 8 August 2019).
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Dabdoub, S.M.; Fellows, M.L.; Paropkari, A.D.; Mason, M.R.; Huja, S.S.; Tsigarida, A.A.; Kumar, P.S. PhyloToAST: Bioinformatics tools for species-level analysis and visualization of complex microbial datasets. Sci. Rep. 2016, 6, 29123. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega, R.L.; Knight, T.R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- UK, N. The Eatwell Guide. Available online: https://www.nhs.uk/live-well/eat-well/the-eatwell-guide (accessed on 8 August 2019).
- Brazil MoHo. Dietary Guidelines for the Brazilian Population. Available online: http//:bvsms.saude.gov.br/bvs/publicacoes/dietary_guidelines_brazilian_population.pdf (accessed on 8 August 2019).
- Tosh, S.; Farnworth, E.; Brummer, Y.; Duncan, A.; Wright, A.; Boye, J.; Marcotte, M.; Benali, M. Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients. Foods 2013, 2, 338. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Bjorck, I.; Backhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, A.C.; Fernandes, G.R.; da Silva, I.T.; Almeida-Pititto, B.; Gomes, E.P.; Pereira, A.D.; Ferreira, S.R. Enterotype May Drive the Dietary-Associated Cardiometabolic Risk Factors. Front. Cell. Infect. Microbiol. 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Simonyte Sjodin, K.; Hammarstrom, M.L.; Ryden, P.; Sjodin, A.; Hernell, O.; Engstrand, L.; West, C.E. Temporal and long-term gut microbiota variation in allergic disease: A prospective study from infancy to school age. Allergy 2019, 74, 176–185. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med J. 2016, 57, 5–14. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Turnbaugh PJ2. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Bergmann, H.; Rogoll, D.; Scheppach, W.; Melcher, R.; Richling, E. The Ussing type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol. Nutr. Food Res. 2009, 53, 1211–1225. [Google Scholar] [CrossRef]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.D.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. Rev. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, A.; Claustre, J.; Moro, F.; Chayvialle, J.A.; Cuber, J.C.; Plaisancie, P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 2000, 46, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Hartman, T.J.; Mitchell, D.C.; Lawrence, F.R.; Aukema, H.M. Pulse consumption in Canadian adults influences nutrient intakes. Br. J. Nutr. 2012, 108 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudo, A.; Slimani, N.; Ocke, M.C.; Naska, A.; Miller, A.B.; Kroke, A.; Bamia, C.; Karalis, D.; Vineis, P.; Palli, D.; et al. Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr. 2002, 5, 1179–1196. [Google Scholar] [CrossRef] [Green Version]
- Winham, D.M. Hutchins Perceptions of flatulence from bean consumption among adults in 3 feeding studies. A. M. Nutr. J. 2011, 10, 128. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127–fix136. [Google Scholar] [CrossRef]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Jakobsdottir, G.; Jadert, C.; Holm, L.; Nyman, M.E. Propionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serum. Br. J. Nutr. 2013, 110, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, M.T.; Vezza, T.; Diez-Echave, P.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salter, A.M. Animal Models in Nutrition Research. In Nutrition Research Methodologies. 2015. [Google Scholar] [CrossRef]
- Hugenholtz, F.; De Vos, W.M. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | BD | PD | 5%LD | 10%LD | 20%LD |
|---|---|---|---|---|---|
| Casein | 200 | 200 | 185 | 169 | 138 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 |
| Corn Starch | 378 | 378 | 352 | 327 | 277 |
| Maltodextrin | 132 | 132 | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 | 100 | 100 |
| Corn Oil | 70 | 70 | 69 | 69 | 68 |
| Cellulose | 70 | 63 | 61 | 52 | 35 |
| Citrus pectin 1 | 0 | 7 | 0 | 0 | 0 |
| Mineral Mix | 35 | 35 | 35 | 35 | 35 |
| Vitamin Mix | 10 | 10 | 10 | 10 | 10 |
| Choline Bitartrate | 3 | 3 | 3 | 3 | 3 |
| Lentil Powder 2 | 0 | 0 | 50 | 100 | 200 |
| Caloric Density (kcal/Kg) | 4022 | 4022 | 4006.5 | 4000 | 3984 |
| Lentil Powder (% kcal) | 0 | 0 | 4.4 | 8.7 | 17.4 |
| Taxonomy | BD | PD | 5% LD | 10% LD | 20% LD |
|---|---|---|---|---|---|
| Actinobacteria | 3.25 ± 2.22 | 1.58 ± 1.48 | 2.96 ± 3.20 | 2.08 ± 0.87 | 1.34 ± 1.20 |
| Bacteroidetes | 60.80 ± 7.78 | 47.24 ± 5.80 | 52.47 ± 8.42 | 47.36 ± 9.91 | 47.99 ± 15.23 |
| Bacteroidaceae (f);Bacteroides | 12.83 ± 3.76 a | 13.35 ± 3.94 a | 12.10 ± 3.78 a | 7.51 ± 3.20 b | 7.10 ± 2.50 b |
| Porphyromonadaceae (f); Parabacteroides | 4.02 ± 1.34 a | 1.35 ± 0.35 b.c | 1.88 ± 0.50 b | 1.15 ± 0.49 c | 0.87 ± 0.31 c |
| Prevotellaceae (f);Prevotella | 0.04 ± 0.04 a | 0.15 ± 0.17 b.c | 0.08 ± 0.05 a.b | 0.20 ± 0.14 c | 0.67 ± 0.39 d |
| Cyanobacteria | 0.33 ± 0.30 | 0.15 ± 0.18 | 0.13 ± 0.09 | 0.14 ± 0.09 | 0.15 ± 0.10 |
| Deferribacteres | 0.63 ± 0.55 a.b | 1.23 ± 0.57 a | 1.00 ± 0.74 a.b | 0.82 ± 0.49 a.b | 0.34 ± 0.26 b |
| Deferribacteraceae (f); Mucispirillum | 0.63 ± 0.55 a.b | 1.23 ± 0.57 a | 1.00 ± 0.74 a.b | 0.82 ± 0.49 a.b | 0.34±0.26 b |
| Firmicutes | 33.26 ± 8.73 a | 48.85 ± 5.63 b | 42.52 ± 9.64 a.b | 48.92 ± 9.90 b | 48.88 ± 15.71 b |
| Clostridiaceae (f);Clostridium | 0.07 ± 0.07a.b | 0.04 ± 0.03 a | 0.19 ± 0.31 a.b | 0.15 ± 0.18 b | 0.21 ± 0.30 b |
| Clostridiaceae (f); Other | 0.10 ± 0.09 a | 0.40 ± 0.41 b | 0.12 ± 0.15 a | 0.11 ± 0.10 a | 0.07 ± 0.03 a |
| Lachnospiraceae (f);Coprococcus | 0.07 ± 0.04 a | 0.75 ± 1.73 b | 0.74 ± 0.79 b | 0.38 ± 0.23 b | 0.38 ± 0.24 b |
| Lachnospiraceae (f);Dorea | 0.02 ± 0.01 a | 0.03 ± 0.03 a | 0.25 ± 0.59 b | 0.37 ± 0.44 b | 0.43 ± 0.60 b |
| Lachnospiraceae (f); Other | 1.66 ± 1.09 a | 4.23 ± 1.80 b | 2.90 ± 2.19 a.b | 3.79 ± 1.91 b | 3.34 ± 1.61 b |
| Lachnospiraceae (f);Roseburia | 0.00 ± 0.00 a | 0.09±0.24a.b | 0.07 ± 0.06 b.c | 0.17 ± 0.11 c | 0.25 ± 0.21 c |
| Ruminococcaceae (f);Oscillospira | 6.49 ± 1.64 a | 9.60 ± 2.31 b | 6.76 ± 1.82 a | 7.53 ± 1.99 a.b | 6.53 ± 1.99 a |
| Ruminococcaceae (f);Ruminococcus | 0.81 ± 0.66 a | 2.09 ± 2.07 b | 2.57 ± 4.03 a | 0.83 ± 0.27 a.b | 0.71 ± 0.17 a |
| Streptococcaceae (f);Lactococcus | 0.14 ± 0.06 a | 0.08 ± 0.04 a.b | 0.08 ± 0.05 b.c | 0.05 ± 0.03 b.c | 0.05 ± 0.02 c |
| Turicibacteraceae (f);Turicibacter | 1.08 ± 1.09 a.b | 0.53 ± 0.63 a | 1.18 ± 0.95 a.b | 1.70 ± 0.40 b.c | 2.19 ± 1.11 c |
| Proteobacteria | 0.69 ± 0.42 | 0.29 ± 0.29 | 0.39 ± 0.30 | 0.24 ± 0.07 | 0.27 ± 0.14 |
| TM7 | 0.08 ± 0.04 a | 0.14 ± 0.12 a.b | 0.17 ± 0.10 b.c | 0.24 ± 0.09 c | 0.32 ± 0.24 c |
| F16 (f); Other | 0.08 ± 0.04 a | 0.14 ± 0.12 a.b | 0.17 ± 0.10 b.c | 0.24 ± 0.09 c | 0.32 ± 0.24 c |
| Tenericutes | 0.23 ± 0.24 | 0.46 ± 0.59 | 0.22 ± 0.27 | 0.15 ± 0.12 | 0.22 ± 0.12 |
| Verrucomicrobia | 0.67 ± 1.39 | 0.03 ± 0.04 | 0.08 ± 0.12 | 0.01 ± 0.02 | 0.46 ± 0.72 |
| Colonic Measurement | BD | PD | 5% LD | 10% LD | 20% LD |
|---|---|---|---|---|---|
| Colon weight [mg] | 22.9 ± 1.1 | 23.0 ± 1.0 | 21.0 ± 1.3 | 23.5 ± 0.9 | 25.0 ± 1.0 |
| Colon length [mm] | 75.0 ± 1.7 | 77.0 ± 1.4 | 73.6 ± 1.9 | 76.2 ± 1.4 | 76.6 ± 1.5 |
| Proximal crypt length [µm] | 173.1 ± 1.9 | 159.2 ± 10.9 | 175.7 ± 4.2 | 177.2 ± 8.9 | 176.5 ± 4.7 |
| Distal crypt length [µm] | 160.2 ± 3.3 | 156.5 ± 5.7 | 148.7 ± 3.8 | 149.7 ± 3.4 | 151.9 ± 2.8 |
| Proximal goblet cell # | 21.1 ± 0.7 | 18.5 ± 1.3 | 19.7 ± 1.0 | 22.4 ± 1.0 | 21.3 ± 0.5 |
| Distal goblet cell # | 7.0 ± 0.3 | 6.6 ± 0.3 | 6.9 ± 0.2 | 6.5 ± 0.2 | 6.9 ± 0.2 |
| Mucus layer thickness [µm] | 24.9 ± 2.7 | 24.8 ± 2.0 | 29.4 ± 4.2 | 33.5 ± 5.1 | 36.2 ± 4.7 |
| Mucin content [AB intensity/µm2] | 0.43 ± 0.03 | 0.45 ± 0.03 | 0.41 ± 0.02 | 0.45 ± 0.03 | 0.45 ± 0.03 |
| Proliferation index | 7.6 ± 0.4 | 9.1 ± 0.9 | 11.8 ± 1.3 | 10.7 ± 1.3 | 9.6 ± 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, D.; Monk, J.M.; Lepp, D.; Wu, W.; McGillis, L.; Roberton, K.; Brummer, Y.; Tosh, S.M.; Power, K.A. Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice. Nutrients 2019, 11, 1853. https://doi.org/10.3390/nu11081853
Graf D, Monk JM, Lepp D, Wu W, McGillis L, Roberton K, Brummer Y, Tosh SM, Power KA. Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice. Nutrients. 2019; 11(8):1853. https://doi.org/10.3390/nu11081853
Chicago/Turabian StyleGraf, Daniela, Jennifer M. Monk, Dion Lepp, Wenqing Wu, Laurel McGillis, Kyle Roberton, Yolanda Brummer, Susan M. Tosh, and Krista A. Power. 2019. "Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice" Nutrients 11, no. 8: 1853. https://doi.org/10.3390/nu11081853
APA StyleGraf, D., Monk, J. M., Lepp, D., Wu, W., McGillis, L., Roberton, K., Brummer, Y., Tosh, S. M., & Power, K. A. (2019). Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl/6 Male Mice. Nutrients, 11(8), 1853. https://doi.org/10.3390/nu11081853