Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Studies
2.3. Drug-Metabolizing Enzyme Activity Assays
2.4. Determination of Oxidative Stress in the Liver
2.5. APAP and APAP Conjugates in the Plasma, Liver, and Urine
2.6. In Vitro APAP–GSH Formation
2.7. Immunoblotting Analysis
2.8. Fecal β-Glucuronidase Activity
2.9. Statistical Analysis
3. Results
3.1. Drug-Metabolizing Enzyme Activity, Oxidative Stress, Membrane Transporters, and Liver Function Index in Normal Rats
3.2. Apoptosis and Autophagy in the Liver
3.3. APAP and APAP Conjugates in the Plasma, Liver, and Urine
3.4. Drug-Metabolizing Enzyme Activity in APAP-Treated Rats
3.5. Membrane Transporters’ Expression
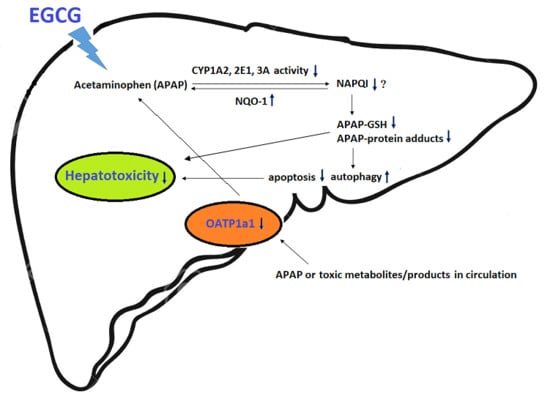
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Wei, Y.; Zhang, J. Novel mechanisms of anticancer activities of green tea component epigallocatechin-3-gallate. Anticancer Agents Med. Chem. 2014, 14, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Sarkar, J.; Chakraborti, T.; Pramanik, P.K.; Chakraborti, S. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed. Pharmacother. 2016, 78, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Hung, M.W.; Fung, M.L. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Fujita, K.; Yamazaki, Y.; Kamataki, T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. 2001, 479, 197–206. [Google Scholar] [CrossRef]
- Azman, N.A.; Peiró, S.; Fajarí, L.; Julià, M.P. Radical scavenging of white tea and its flavonoid constituents by electron paramagnetic resonance (EPR) spectroscopy. J. Agric. Food Chem. 2014, 62, 5743–5748. [Google Scholar] [CrossRef]
- Yang, C.S.; Pan, E. The effects of green tea polyphenols on drug metabolism. Expert Opin. Drug Metab. Toxicol. 2012, 8, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Arffa, M.L.; Zapf, M.A.; Kothari, A.N.; Chang, V.; Gupta, G.N.; Ding, X.; Al-Gayyar, M.M.; Syn, W.; Elsherbiny, N.M.; Kuo, P.C.; et al. Epigallocatechin-3-gallate upregulates miR-221 to inhibit osteopontin-dependent hepatic fibrosis. PLoS ONE 2016, 11, e0167435. [Google Scholar] [CrossRef]
- Yu, S.J.; Jiang, R.; Mazzu, Y.Z.; Wei, C.B.; Sun, Z.L.; Zhang, Y.Z.; Zhou, L.D.; Zhang, Q.H. Epigallocatechin-3-gallate prevents triptolide-induced hepatic injury by restoring the Th17/Treg balance in mice. Am. J. Chin. Med. 2016, 44, 1221–1236. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [Green Version]
- James, K.D.; Kennett, M.J.; Lambert, J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018, 111, 302–309. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.; Larson, V.A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.H.L. Paracetamol (acetaminophen) poisoning. Pharmacol. Ther. 1993, 17260, 91–120. [Google Scholar] [CrossRef]
- James, L.P.; Mayeux, P.R.; Hinson, J.A. Acetaminophen-induced hepatotoxicity. Drug Metab. Dispos. 2003, 31, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Mossanen, J.C.; Tacke, F. Acetaminophen-induced acute liver injury in mice. Lab. Anim. 2015, 49, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef]
- Ni, H.M.; Bockus, A.; Boggess, N.; Jaeschke, H.; Ding, W.X. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 2012, 55, 222–232. [Google Scholar] [CrossRef]
- Keppler, D.; Konig, J. Hepatic secretion of conjugated drugs and endogenous substances. Semin. Liver Dis. 2000, 20, 265–272. [Google Scholar] [CrossRef]
- Slitt, A.L.; Cherrington, N.J.; Maher, J.M.; Klaassen, C.D. Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metab. Dispos. 2003, 31, 1176–1186. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Augustine, L.M.; Cherrington, N.J.; Manautou, J.E. Influence of acetaminophen vehicle on regulation of transporter gene expression during hepatotoxicity. J. Toxicol. Environ. Health A 2007, 70, 1870–1872. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.S. Green-tea polyphenols downregulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef]
- Yao, H.T.; Yang, Y.C.; Chang, C.H.; Yang, H.T.; Yin, M.C. Protective effects of (-)-epigallocatechin-3-gallate against acetaminophen-induced liver injury in rats. Biomedicine 2015, 5, 15–21. [Google Scholar] [CrossRef]
- Liu, T.T.; Liang, N.S.; Li, Y.; Yang, F.; Lu, Y.; Meng, Z.Q.; Zhang, L.S. Effects of long-term tea polyphenols consumption on hepatic microsomal drug metabolizing enzymes and liver function in Wistar rats. World J. Gastroenterol. 2003, 9, 2742–2744. [Google Scholar] [CrossRef]
- Carleton, H.M. Carleton’s Histological Techniques, 5th ed.; Oxford University Press: London, UK, 1980. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Yao, H.T.; Hsu, Y.R.; Lii, C.K.; Lin, A.H.; Chang, K.H.; Yang, H.T. Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in Wistar rats. Food Chem. Toxicol. 2014, 70, 120–127. [Google Scholar] [CrossRef]
- Hanioka, N.; Jinno, H.; Tanaka-Kagawa, T.; Nishimura, T.; Ando, M. Determination of UDP-glucuronosyltransferase UGT1A6 activity in human and rat liver microsomes by HPLC with UV detection. J. Pharm. Biomed. Anal. 2001, 25, 65–75. [Google Scholar] [CrossRef]
- Dierickx, C.; Vanhoof, H. Massive rotator cuff tears treated by a deltoid muscular inlay flap. Acta Orthopaedica Belgica 1994, 60, 94–100. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar]
- Tsvetkov, P.; Asher, G.; Reiss, V.; Shaul, Y.; Sachs, L.; Lotem, J. Inhibition of NAD(P)H: quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc. Natl. Acad. Sci. USA 2005, 15, 5535–5540. [Google Scholar] [CrossRef]
- Guan, X.; Hoffman, B.; Dwivedi, C.; Matthees, D.P. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J. Pharm. Biomed. Anal. 2003, 31, 251–261. [Google Scholar] [CrossRef]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984, 44, 508–5091. [Google Scholar]
- Uehiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissue by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Yao, H.T.; Luo, M.N.; Lii, C.C. Soy isoflavones reduce acetaminophen-induced liver injury by inhibiting cytochrome P-450-mediated bioactivation and glutathione depletion and increasing urinary drug excretion in rats. J. Funct. Foods 2016, 26, 135–143. [Google Scholar]
- Acharya, M.; Lau Cam, C.A. Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat. J. Biomed. Sci. 2010, 17, S35–S46. [Google Scholar] [CrossRef]
- Allameh, A.; Alikhani, N. Acetaminophen-glutathione conjugate formation in a coupled cytochrome P-450-glutathione S-transferase assay system mediated by subcellular preparations from adult and weanling rat tissues. Toxicol. In Vitro 2002, 16, 637–641. [Google Scholar] [CrossRef]
- Yen, C.C.; Liu, Y.T.; Lin, Y.J.; Yang, Y.C.; Chen, C.C.; Yao, H.T.; Chen, H.W.; Lii, C.K. Bioavailability of the diterpenoid 14-deoxy-11,12-didehydroandrographolide in rats and up-regulation of hepatic drug-metabolizing enzyme and drug transporter expression. Phytomedicine 2019, 61, 152841. [Google Scholar] [CrossRef]
- Yao, H.T.; Chiang, M.T. Chitosan shifts the fermentation site toward the distal colon and increases the fecal short-chain fatty acids concentrations in rats. Int. J. Vitam. Nutr. Res. 2006, 76, 57–64. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Hong, J.; Tian, S.; Lee, M.J.; Stark, R.E.; Ho, C.T.; Yang, C.S. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005, 18, 1762–1769. [Google Scholar] [CrossRef]
- Zhou, J.; Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Bay, B.H.; Yang, C.S.; Yen, P.M. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE 2014, 9, e87161. [Google Scholar] [CrossRef]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar]
- Choi, J.S.; Burm, J.P. Effects of oral epigallocatechin gallate on the pharmacokinetics of nicardipine in rats. Arch. Pharm. Res. 2009, 32, 1721–1725. [Google Scholar] [CrossRef]
- Cohen, S.D.; Pumford, N.R.; Khairallah, E.A.; Boekelheide, K.; Pohl, L.R.; Amouzadeh, H.R.; Hinson, J.A. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997, 143, 1–12. [Google Scholar] [CrossRef]
- Qiu, Y.; Benet, L.Z.; Burlingame, A.L. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J. Biol. Chem. 1998, 273, 17940–17953. [Google Scholar] [CrossRef]
- Yamaura, K.; Shimada, M.; Nakayama, N.; Ueno, K. Protective effects of goldenseal (Hydrastis canadensis L.) on acetaminophen-induced hepatotoxicity through inhibition of CYP2E1 in rats. Pharmacogn. Res. 2011, 3, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kaneko, T.; Wang, Y.; Qin, L.Q.; Wang, P.Y.; Sato, A. Troglitazone enhances the hepatotoxicity of acetaminophen by inducing CYP3A in rats. Toxicology 2002, 176, 91–100. [Google Scholar] [CrossRef]
- Laine, J.E.; Auriola, S.; Pasanen, M.; Juvonen, R.O. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 2009, 39, 11–21. [Google Scholar] [CrossRef]
- Roušar, T.; Nýdlová, E.; Česla, P.; Staňková, P.; Kučera, O.; Pařík, P.; Červinková, Z. Purified acetaminophen-glutathione conjugate is able to induce oxidative stress in rat liver mitochondria. Physiol. Res. 2012, 61, S103–S109. [Google Scholar]
- Manov, I.; Bashenko, Y.; Hirsh, M.; Iancu, T.C. Involvement of the multidrug resistance P-glycoprotein in acetaminophen-induced toxicity in hepatoma-derived HepG2 and Hep3B cells. Basic Clin. Pharmacol. Toxicol. 2006, 99, 213–224. [Google Scholar] [CrossRef]
- Gregus, Z.; Madhu, C.; Klaassen, C.D. Species variation in toxification and detoxification of acetaminophen in vivo: A comparative study of biliary and urinary excretion of acetaminophen metabolites. J. Pharmacol. Exp. Ther. 1988, 244, 91–99. [Google Scholar]
- Roth, M.; Timmermann, B.N.; Hagenbuch, B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab. Dispos. 2011, 39, 920–926. [Google Scholar] [CrossRef]
- Frejnagel, S.; Juskiewicz, J. Dose-dependent effects of polyphenolic extracts from green tea, blue-berried honeysuckle, and chokeberry on rat caecal fermentation processes. Planta Med. 2011, 77, 888–893. [Google Scholar] [CrossRef]




| Control | 1× EGCG | 3× EGCG | |
|---|---|---|---|
| Phase I Enzymes (pmol/min/mg protein) | |||
| Testosterone 6β-hydroxylase (CYP3A) | 525.2 ± 63.0 | 535.7 ± 44.2 | 281.1 ± 75.3 * |
| Nitrophenol 6-hydroxylase (CYP2E1) | 304.7 ± 31.0 | 308.3 ± 38.4 | 208.9 ± 28.2 * |
| Methoxyresorufin O-demethylase (CYP1A2) | 34.5 ± 0.3 | 35.9 ± 5.3 | 24.5 ± 2.9 * |
| Phase II Enzymes (nmol/min/mg protein) | |||
| UDP-glucurosyltransferase | 34.5 ± 0.3 | 35.9 ± 5.3 | 24.5 ± 2.9 * |
| Sulfotransferase | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 |
| Glutathione S-transferase | 209.1 ± 10.0 | 178.1 ± 9.4 * | 158.2 ± 6.4 * |
| Oxidative Stress Status | |||
| GSH (nmol/mg protein) | 46.2 ± 0.9 | 43.8 ± 3.2 | 42.9 ± 1.7 * |
| GSSG (nmol/mg protein) | 0.7 ± 0.1 | 0.3 ± 0.0 * | 0.3 ± 0.1 * |
| GSH/GSSG | 69.1 ± 9.6 | 163.8 ± 27.6 * | 139.6 ± 38.1 * |
| GSH peroxidase (nmol/min/mg protein) | 83.0 ± 4.1 | 87.0 ± 16.3 | 88.1 ± 7.9 |
| TBARS (nmol/g protein) | 116.5 ± 18.0 | 98.3 ± 0.4 | 75.6 ± 6.9 * |
| ROS (nmol/mg protein) | 0.96 ± 0.11 | 0.71 ± 0.03 * | 0.74 ± 0.10 * |
| Liver Function Index | |||
| Alanine aminotransferase (U/L) | 22.4 ± 3.2 | 23.7 ± 4.0 | 21.0 ± 4.4 |
| 4 h | 12 h | |||
|---|---|---|---|---|
| APAP | APAP + EGCG | APAP | APAP + EGCG | |
| Plasma | ||||
| APAP (μg/mL) | 259.4 ± 130.3 | 187.9 ± 18.1 | 303.1 ± 61.0 | 387.5 ± 62.9 |
| APAP–glucuronide (μg/mL) | 101.2 ± 5.2 | 82.2 ± 6.9 * | 59.5 ± 9.3 | 51.4 ± 10.9 |
| APAP–sulfate (μg/mL) | 224.2 ± 102.9 | 163.8 ± 23.9 | 29.1 ± 14.1 | 19.8 ± 8.7 |
| APAP–GSH (μg/mL) | 12.1 ± 9.4 | 11.9 ± 6.1 | 32.5 ± 10.9 | 11.0 ± 9.7 * |
| Liver | ||||
| APAP (μg/g liver) | 115.7 ± 33.1 | 128.9 ± 55.6 | 77.3 ± 31.9 | 95.9 ± 33.0 |
| APAP–glucuronide (μg/g liver) | 618.1 ± 211.7 | 450.2 ± 89.1 | 432.5 ± 181.3 | 520.3 ± 157.8 |
| APAP–sulfate (μg/g liver) | 52.5 ± 15.7 | 69.4 ± 16.1 | 49.3 ± 18.0 | 44.9 ± 14.9 |
| APAP–GSH (μg/g liver) | 438.6 ± 88.2 | 447.8 ± 140.2 | 645.2 ± 230.6 | 344.2 ± 122.2 * |
| APAP–protein adducts (mg/g liver) | 1.8 ± 0.4 | 1.1 ± 0.2 * | 3.5 ± 1.0 | 2.1 ± 0.4 * |
| Urine | ||||
| APAP (mg/12 h) | 3.3 ± 0.7 | 5.4 ± 0.7 * | ||
| APAP–glucuronide (mg/12 h) | 144.5 ± 61.5 | 155.7 ± 14.2 | ||
| APAP–sulfate (mg/12 h) | 209.2 ± 39.1 | 189.6 ± 13.5 | ||
| Control | APAP | APAP + EGCG | |
|---|---|---|---|
| Phase I Enzymes (pmol/min/mg protein) | |||
| Midazolam 1-hydroxylation (CYP3A) | 216.1 ± 60.3 | 124.3 ± 26.9 * | 153.6 ± 48.5 |
| Nitrophenol 6-hydroxylase (CYP2E1) | 517.6 ± 55.1 | 388.4 ± 74.8 * | 436.4 ± 91.3 |
| Methoxyresorufin O-demethylase (CYP1A2) | 28.0 ± 2.9 | 28.0 ± 2.8 | 26.0 ± 1.9 |
| Phase II Enzymes (nmol/min/mg protein) | |||
| UDP-glucurosyltransferase | 48.0 ± 6.8 | 28.9 ± 11.3 * | 25.9 ± 3.6 |
| Sulfotransferase | 0.73 ± 0.16 | 0.62 ± 0.1 | 0.44 ± 0.13 # |
| Glutathione S-transferase | 146.5 ± 43.5 | 103.9 ± 15.4 * | 130.1 ± 30.1 |
| NADPH: quinine oxidoreductase-1 | 462.4 ± 132.8 | 279.5 ± 91.3 * | 383.5 ± 82.4 # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.-T.; Li, C.-C.; Chang, C.-H. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients 2019, 11, 1862. https://doi.org/10.3390/nu11081862
Yao H-T, Li C-C, Chang C-H. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients. 2019; 11(8):1862. https://doi.org/10.3390/nu11081862
Chicago/Turabian StyleYao, Hsien-Tsung, Chien-Chun Li, and Chen-Hui Chang. 2019. "Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats" Nutrients 11, no. 8: 1862. https://doi.org/10.3390/nu11081862
APA StyleYao, H. -T., Li, C. -C., & Chang, C. -H. (2019). Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients, 11(8), 1862. https://doi.org/10.3390/nu11081862