Perinatal Whole Blood Zinc Status and Cytokines, Adipokines, and Other Immune Response Proteins
Abstract
:1. Introduction
2. Materials and Methods
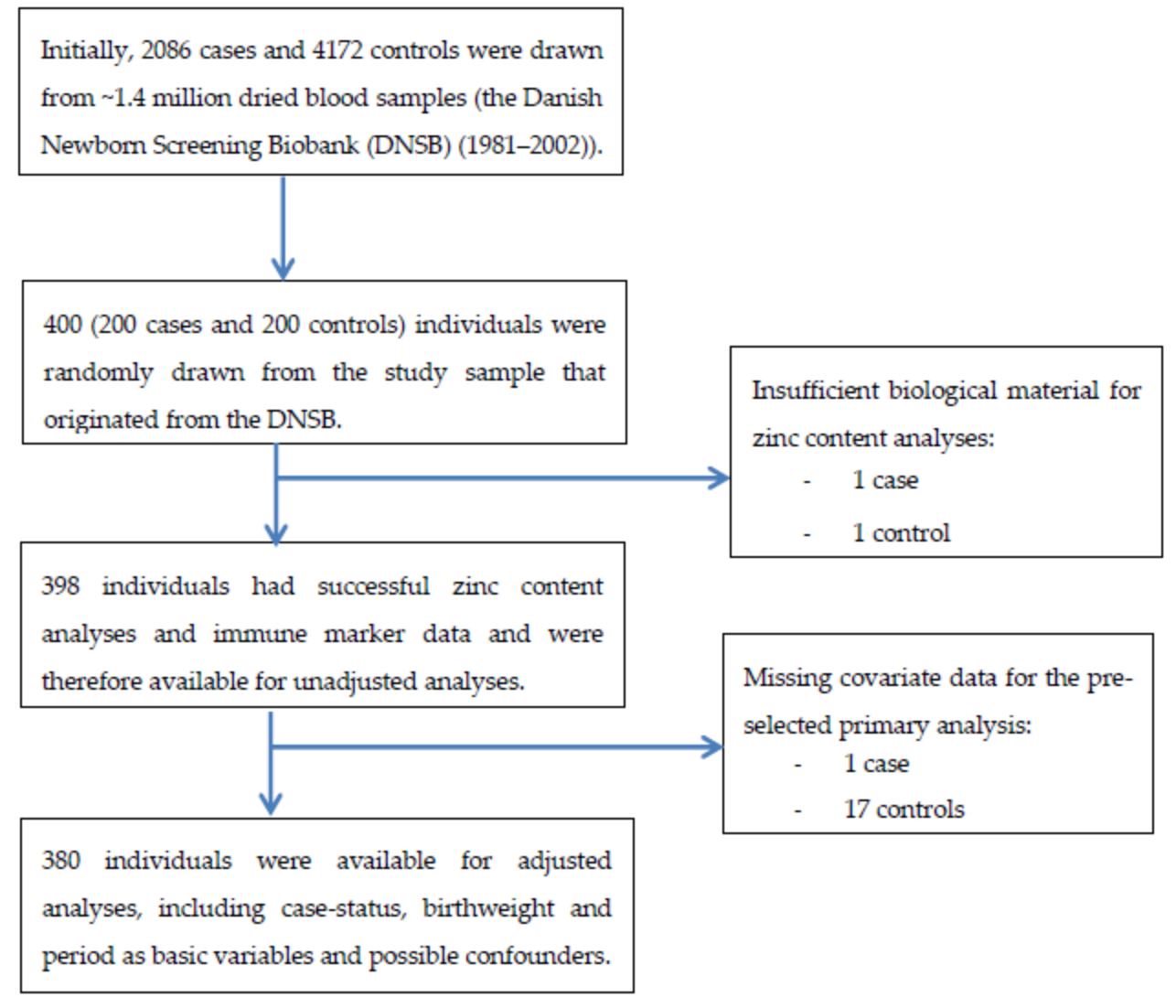
2.1. Study Design and Sampling
2.2. Exposure Assessment
Assessment of Whole Blood Zinc Content
2.3. Outcome Assessment
2.4. Other Variables
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Unadjusted Models and Adjusted Models
3.2. Unadjusted Models with Statification on Case Status
4. Discussion
4.1. Comparison with Other Studies
4.2. Strengths and Limitations
4.3. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetherolf, M.M.; Boyd, S.D.; Winkler, D.D.; Winge, D.R. Oxygen-dependent activation of Cu, Zn-superoxide dismutase-1. Metallomics 2017, 9, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Ohly, P.; Dohle, C.; Abel, J.; Seissler, J.; Gleichmann, H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia 2000, 43, 1020–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessells, K.R.; Singh, G.M.; Brown, K.H. Estimating the global prevalence of inadequate zinc intake from national food balance sheets: Effects of methodological assumptions. PLoS One 2012, 7, e50565. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Zavaleta, N.; Shankar, A.H.; Merialdi, M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr. 1998, 68, 499S–508S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driessen, C.; Hirv, K.; Kirchner, H.; Rink, L. Zinc regulates cytokine induction by superantigens and lipopolysaccharide. Immunology 1995, 84, 272–277. [Google Scholar] [PubMed]
- Diana, A.; Haszard, J.J.; Purnamasari, D.M.; Nurulazmi, I.; Luftimas, D.E.; Rahmania, S.; Nugraha, G.I.; Erhardt, J.; Gibson, R.S.; Houghton, L. Iron, zinc, vitamin A and selenium status in a cohort of Indonesian infants after adjusting for inflammation using several different approaches. Br. J. Nutr. 2017, 118, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Meftah, S.; Abdallah, J.; Kaplan, J.; Brewer, G.J.; Bach, J.F.; Dardenne, M. Serum thymulin in human zinc deficiency. J. Clin. Investig. 1988, 82, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.K.; Choi, B.Y. The Relationship between Zinc Status and Inflammatory Marker Levels in Rural Korean Adults Aged 40 and Older. PLoS One 2015, 10, e0130016. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.J.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Prasad, A.S.; Beck, F.W.J.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Prasad, A.S.; Penland, J.G.; Beck, F.W.J.; Kaplan, J.; Egger, N.G.; Alcock, N.W.; Carroll, R.M.; Ramanujam, V.M.S.; Dayal, H.H.; et al. Zinc deficiency in Mexican American children: Influence of zinc and other micronutrients on T cells, cytokines, and antiinflammatory plasma proteins. Am. J. Clin. Nutr. 2008, 88, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; West, C.E.; van der Ven-Jongekrijg, J.; van der Meer, J.W.M.; Muhilal. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur. J. Clin. Nutr. 2004, 58, 1498–1504. [Google Scholar] [CrossRef] [Green Version]
- Padgett, L.E.; Broniowska, K.A.; Hansen, P.A.; Corbett, J.A.; Tse, H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N. Y. Acad. Sci. 2013, 1281, 16–35. [Google Scholar] [CrossRef] [Green Version]
- Kyvsgaard, J.N.; Overgaard, A.J.; Jacobsen, L.D.; Thorsen, S.U.; Pipper, C.B.; Hansen, T.H.; Husted, S.; Mortensen, H.B.; Pociot, F.; Svensson, J. Low perinatal zinc status is not associated with the risk of type 1 diabetes in children. Pediatr. Diabetes 2016, 18, 637–642. [Google Scholar] [CrossRef]
- Samuelsson, U.; Oikarinen, S.; Hyöty, H.; Ludvigsson, J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr. Diabetes 2011, 12, 156–164. [Google Scholar] [CrossRef]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal Innate Immune Development: From Birth to Adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Cunningham-Rundles, S.; Lin, H.; Ho-Lin, D.; Dnistrian, A.; Cassileth, B.R.; Perlman, J.M. Role of nutrients in the development of neonatal immune response. Nutr. Rev. 2009, 67 (Suppl. 2), S152–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørgaard-Pedersen, B.; Hougaard, D.M. Storage policies and use of the Danish Newborn Screening Biobank. J. Inherit. Metab. Dis. 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Thorsen, S.U.; Cohen, A.S.; Lundqvist, M.; Frederiksen, P.; Pipper, C.B.; Pociot, F.; Thygesen, L.C.; Ascherio, A.; Svensson, J.; et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: Results from two large Danish population-based studies. Diabetologia 2016, 59, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Eising, S.; Svensson, J.; Skogstrand, K.; Nilsson, A.; Lynch, K.; Andersen, P.S.; Lernmark, Å.; Hougaard, D.M.; Pociot, F.; Nørgaard-Pedersen, B.; et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: Design and feasibility of an unselected case–control study. Paediatr. Perinat. Epidemiol. 2007, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Cerqueira, C.; Kjærsgaard, P.; Lyngsøe, L.; Hertel, N.T.; Madsen, M.; Mortensen, H.B.; Johannesen, J. Danish Registry of Childhood and Adolescent Diabetes. Clin. Epidemiol. 2016, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Langer, E.K.; Johnson, K.J.; Shafer, M.M.; Gorski, P.; Overdier, J.; Musselman, J.; Ross, J.A. Characterization of the elemental composition of newborn blood spots using sector-field inductively coupled plasma-mass spectrometry. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Capiau, S.; Stove, V.V.; Lambert, W.E.; Stove, C.P. Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Anal. Chem. 2013, 85, 404–410. [Google Scholar] [CrossRef]
- Skogstrand, K.; Thorsen, P.; Nørgaard-Pedersen, B.; Schendel, D.E.; Sørensen, L.C.; Hougaard, D.M. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005, 51, 1854–1866. [Google Scholar] [CrossRef]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skogstrand, K. Multiplex assays of inflammatory markers, a description of methods and discussion of precautions—Our experience through the last ten years. Methods 2012, 56, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Pipper, C.B.; Ellervik, C.; Pociot, F.; Kyvsgaard, J.N.; Svensson, J. Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients 2019, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Pipper, C.B.; Ritz, C.; Bisgaard, H. A versatile method for confirmatory evaluation of the effects of a covariate in multiple models. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2012, 61, 315–326. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. Biom. Z. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Banupriya, N.; Vishnu Bhat, B.; Benet, B.D.; Sridhar, M.G.; Parija, S.C. Efficacy of zinc supplementation on serum calprotectin, inflammatory cytokines and outcome in neonatal sepsis—A randomized controlled trial. J. Matern.-Fetal Neonatal Med. 2017, 30, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip 14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Westcott, J. Zinc and breastfed infants: If and when is there a risk of deficiency? Adv. Exp. Med. Biol. 2002, 503, 69–75. [Google Scholar] [PubMed]
- Chaffee, B.W.; King, J.C. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 118–137. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, U.; Gulturk, S.; Aker, A.; Guvenal, T.; Imir, G.; Erselcan, T. Correlation between birth weight, leptin, zinc and copper levels in maternal and cord blood. J. Physiol. Biochem. 2007, 63, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Virmani, D.; Jaipal, M.; Gupta, S.; Sankar, M.J.; Bhatia, S.; Agarwal, A.; Devgan, V.; Deorari, A.; Paul, V.K.; et al. Poor zinc status in early infancy among both low and normal birth weight infants and their mothers in Delhi. Neonatology 2013, 103, 54–59. [Google Scholar] [CrossRef]
- Gómez, T.; Bequer, L.; Mollineda, A.; González, O.; Diaz, M.; Fernández, D. Serum zinc levels of cord blood: Relation to birth weight and gestational period. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2015, 30, 180–183. [Google Scholar] [CrossRef] [PubMed]
| Variables | Cases (n = 199) | Controls (n = 199) |
|---|---|---|
| Sex 1 | ||
| Female, n/% of total | 103/51.8 | 95/47.7 |
| Gestational age 2, | ||
| Median/interquartile range (IQR), weeks | 40.0/1.0 | 40.0/2.0 |
| Birth weight 3, | ||
| Median/IQR, grams | 3500/630 | 3500/744 |
| Maternal age 4, | ||
| Median/IQR, years | 29.0/6.0 | 28.0/8.0 |
| Season of blood sampling, n/% from total | ||
| Winter | 41/20.6 | 40/20.1 |
| Spring | 49/24.6 | 49/24.6 |
| Summer | 59/29.6 | 60/30.2 |
| Autumn | 50/25.1 | 50/25.1 |
| Period of blood sampling, n/% from total | ||
| 1991–1993 | 109/54.8 | 109/54.8 |
| 1994–1998 | 90/45.2 | 90/45.2 |
| Human leukocyte antigen (HLA)-risk groups 5, n/% from total | ||
| High/moderate 6 | 151/82.5 | 68/40.0 |
| Low/protective 7 | 32/17.5 | 102/60.0 |
| Outcome | Variable | Univariate Model | p-Value | Multivariate Model | p-Value |
|---|---|---|---|---|---|
| IL-1β | WB-Zinc content | 1.00 (0.89; 1.12) | 1.00 | 0.97 (0.87; 1.09) | 0.64 |
| IL-4 | WB-Zinc content | 0.99 (0.92; 1.06) | 0.73 | 0.97 (0.90; 1.04) | 0.37 |
| IL-6 | WB-Zinc content | 1.08 (0.99; 1.19) | 0.10 | 1.07 (0.96; 1.19) | 0.21 |
| IL-8 | WB-Zinc content | 0.98 (0.92; 1.04) | 0.49 | 1.02 (0.97; 1.07) | 0.52 |
| IL-10 | WB-Zinc content | 0.98 (0.83; 1.16) | 0.81 | 1.01 (0.86; 1.19) | 0.91 |
| IL-12 | WB-Zinc content | 1.08 (0.98; 1.20) | 0.12 | 1.02 (0.93; 1.12) | 0.61 |
| IFN-γ | WB-Zinc content | 1.02 (0.93; 1.11) | 0.71 | 1.02 (0.92; 1.12) | 0.73 |
| TNF-α | WB-Zinc content | 1.07 (0.98; 1.16) | 0.15 | 1.01 (0.93; 1.10) | 0.85 |
| TGF-β | WB-Zinc content | 1.06 (0.97; 1.14) | 0.20 | 1.01 (0.93; 1.09) | 0.83 |
| Adiponectin | WB-Zinc content | 1.02 (0.96; 1.10) | 0.50 | 0.99 (0.93; 1.06) | 0.86 |
| Leptin | WB-Zinc content | 0.97 (0.90; 1.05) | 0.48 | 0.99 (0.93; 1.07) | 0.90 |
| CRP | WB-Zinc content | 0.91 (0.82; 1.01) | 0.08 | 0.97 (0.87; 1.08) | 0.55 |
| MBL | WB-Zinc content | 1.04 (0.93; 1.17) | 0.44 | 1.05 (0.93; 1.18) | 0.41 |
| sTREM-1 | WB-Zinc content | 0.99 (0.89; 1.11) | 0.90 | 0.95 (0.85; 1.05) | 0.31 |
| Outcome | Variable | Univariate Model (Cases) | p-Value | Univariate Model (Controls) | p-Value |
|---|---|---|---|---|---|
| IL-1β | WB-Zinc content | 0.97 (0.83; 1.14) | 0.74 | 1.03 (0.89; 1.20) | 0.66 |
| IL-4 | WB-Zinc content | 0.99 (0.90; 1.09) | 0.86 | 0.98 (0.90; 1.08) | 0.71 |
| IL-6 | WB-Zinc content | 1.12 (0.99; 1.26) | 0.84 | 1.05 (0.91; 1.22) | 0.50 |
| IL-8 | WB-Zinc content | 0.99 (0.91; 1.08) | 0.80 | 0.97 (0.90; 1.05) | 0.43 |
| IL-10 | WB-Zinc content | 0.97 (0.76; 1.22) | 0.78 | 0.99 (0.78; 1.27) | 0.96 |
| IL-12 | WB-Zinc content | 1.07 (0.95; 1.20) | 0.25 | 1.10 (0.96; 1.26) | 0.17 |
| IFN-γ | WB-Zinc content | 1.04 (0.92; 1.16) | 0.54 | 0.99 (0.87; 1.14) | 0.94 |
| TNF-α | WB-Zinc content | 1.03 (0.91; 1.17) | 0.61 | 1.11 (0.99; 1.24) | 0.08 |
| TGF-β | WB-Zinc content | 1.04 (0.95; 1.13) | 0.40 | 1.07 (0.95; 1.21) | 0.24 |
| Adiponectin | WB-Zinc content | 1.00 (0.94; 1.07) | 0.95 | 1.05 (0.94; 1.18) | 0.38 |
| Leptin | WB-Zinc content | 0.98 (0.88; 1.08) | 0.66 | 0.98 (0.89; 1.06) | 0.57 |
| CRP | WB-Zinc content | 0.93 (0.81; 1.07) | 0.32 | 0.88 (0.76; 1.04) | 0.13 |
| MBL | WB-Zinc content | 1.05 (0.89; 1.24) | 0.59 | 1.04 (0.88; 1.24) | 0.65 |
| sTREM-1 | WB-Zinc content | 0.95 (0.82; 1.10) | 0.49 | 1.05 (0.91; 1.20) | 0.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyvsgaard, J.N.; Ellervik, C.; Lindkvist, E.B.; Pipper, C.B.; Pociot, F.; Svensson, J.; Thorsen, S.U. Perinatal Whole Blood Zinc Status and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients 2019, 11, 1980. https://doi.org/10.3390/nu11091980
Kyvsgaard JN, Ellervik C, Lindkvist EB, Pipper CB, Pociot F, Svensson J, Thorsen SU. Perinatal Whole Blood Zinc Status and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients. 2019; 11(9):1980. https://doi.org/10.3390/nu11091980
Chicago/Turabian StyleKyvsgaard, Julie Nyholm, Christina Ellervik, Emilie Bundgaard Lindkvist, Christian Bressen Pipper, Flemming Pociot, Jannet Svensson, and Steffen Ullitz Thorsen. 2019. "Perinatal Whole Blood Zinc Status and Cytokines, Adipokines, and Other Immune Response Proteins" Nutrients 11, no. 9: 1980. https://doi.org/10.3390/nu11091980
APA StyleKyvsgaard, J. N., Ellervik, C., Lindkvist, E. B., Pipper, C. B., Pociot, F., Svensson, J., & Thorsen, S. U. (2019). Perinatal Whole Blood Zinc Status and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients, 11(9), 1980. https://doi.org/10.3390/nu11091980






