Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Analytical Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Kiortsis, D.N.; Catalán, V. Precision medicine: Diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018, 6, 164–166. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An Endocrine Society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Connett, J.E.; Inabnet, W.B.; Billington, C.J.; Thomas, A.J.; Leslie, D.B.; Chong, K.; Jeffery, R.W.; et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA 2013, 309, 2240–2249. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Davies, S.W.; Efird, J.T.; Guidry, C.A.; Penn, R.I.; Sawyer, R.G.; Schirmer, B.D.; Hallowell, P.T. Long-term diabetic response to gastric bypass. J. Surg. Res. 2014, 190, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Still, C.D.; Wood, G.C.; Benotti, P.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Ibele, A.; Seiler, J.; Irving, B.A.; Celaya, M.P.; et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: A retrospective cohort study. Lancet Diabetes Endocrinol. 2014, 2, 38–45. [Google Scholar] [CrossRef]
- Zhang, R.; Borisenko, O.; Telegina, I.; Hargreaves, J.; Ahmed, A.R.; Sanchez Santos, R.; Pring, C.; Funch-Jensen, P.; Dillemans, B.; Hedenbro, J.L. Systematic review of risk prediction models for diabetes after bariatric surgery. Br. J. Surg. 2016, 103, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Almulaifi, A.; Tsou, J.J.; Ser, K.H.; Lee, Y.C.; Chen, S.C. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: Predicting the success by ABCD score. Surg. Obes. Relat. Dis. 2015, 11, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Mirshahi, T.; Still, C.D.; Hirsch, A.G. Association of DiaRem Score with cure of type 2 diabetes following bariatric surgery. JAMA Surg. 2016, 151, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.F.; Scopinaro, N.; Cordera, R. Adipokine pattern after bariatric surgery: Beyond the weight loss. Obes. Surg. 2016, 26, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Ramírez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Salvador, J.; Frühbeck, G. Adipokines in the treatment of diabetes mellitus and obesity. Expert Opin. Pharmacother. 2009, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, P.; Christ-Crain, M.; Stoeckli, R.; Reusch, C.E.; Lutz, T.A.; Muller, B.; Keller, U. Increase in high molecular weight adiponectin by bariatric surgery-induced weight loss. Diabetes Obes. Metab. 2008, 10, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.F.; Gradaschi, R.; Andraghetti, G.; Scopinaro, N.; Cordera, R. Serum leptin and adiponectin concentration in type 2 diabetes patients in the short and long term following biliopancreatic diversion. Obes. Surg. 2016, 26, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutritents 2019, 11, 454. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7, 6619. [Google Scholar] [CrossRef]
- Vega, G.L.; Grundy, S.M. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J. Obes. 2013, 2013, 409679. [Google Scholar] [CrossRef]
- Jung, C.H.; Rhee, E.J.; Choi, J.H.; Bae, J.C.; Yoo, S.H.; Kim, W.J.; Park, C.Y.; Mok, J.O.; Kim, C.H.; Lee, W.Y.; et al. The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy korean male adults. Korean Diabetes J. 2010, 34, 237–243. [Google Scholar] [CrossRef]
- Musil, F.; Blaha, V.; Ticha, A.; Hyspler, R.; Haluzik, M.; Lesna, J.; Smahelova, A.; Sobotka, L. Effects of body weight reduction on plasma leptin and adiponectin/leptin ratio in obese patients with type 1 diabetes mellitus. Physiol. Res. 2015, 64, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Maehata, E.; Yano, M.; Taniyama, M.; Suzuki, S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism 2005, 54, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Yano, M.; Yamakado, M.; Maehata, E.; Suzuki, S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism 2006, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, C.; Maachi, M.; Nguyen, T.H.; Coussieu, C.; Gharakhanian, S.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I.; Rozenbaum, W.; Capeau, J.; et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003, 17, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Tiliscan, C.; Arama, V.; Mihailescu, R.; Munteanu, D.; Iacob, D.G.; Popescu, C.; Catana, R.; Negru, A.; Lobodan, A.; Arama, S.S. Association of adiponectin/leptin ratio with carbohydrate and lipid metabolism parameters in HIV-infected patients during antiretroviral therapy. Endocr. Res. 2018, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibañez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Muruzábal, F.J.; Frühbeck, G.; Gómez-Ambrosi, J.; Archanco, M.; Burrell, M.A. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Lancha, A.; Frühbeck, G.; Gómez-Ambrosi, J. Peripheral signalling involved in energy homeostasis control. Nutr. Res. Rev. 2012, 25, 223–248. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef]
- Lasa, A.; Miranda, J.; Bullo, M.; Casas, R.; Salas-Salvado, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutierrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Oda, N.; Iniamura, S.; Fujita, T.; Uchida, Y.; Inagaki, K.; Kakizawa, H.; Hayakawa, N.; Suzuki, A.; Takeda, J.; Horikawa, Y.; et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metab. Clin. Exp. 2008, 57, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Masquio, D.C.; de Piano, A.; Sanches, P.L.; Corgosinho, F.C.; Campos, R.M.; Carnier, J.; da Silva, P.L.; Caranti, D.A.; Tock, L.; Oyama, L.M.; et al. The effect of weight loss magnitude on pro-/anti-inflammatory adipokines and carotid intima-media thickness in obese adolescents engaged in interdisciplinary weight loss therapy. Clin. Endocrinol. 2013, 79, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Raselli, S.; Grigore, L.; Garlaschelli, K.; Dozio, E.; Magni, P.; Catapano, A.L. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007, 38, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Naruse, M.; Usui, T.; Tagami, T.; Suganami, T.; Yamada, K.; Kuzuya, H.; Shimatsu, A.; Ogawa, Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care 2004, 27, 2488–2490. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Salvador, J.; Rotellar, F.; Silva, C.; Catalán, V.; Rodríguez, A.; Gil, M.J.; Frühbeck, G. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes. Surg. 2006, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, Y.J. Prediction of Diabetes Remission in Morbidly Obese Patients After Roux-en-Y Gastric Bypass. Obes. Surg. 2016, 26, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, M.; Mari, A.; Anselmino, M.; Baldi, S.; Barsotti, E.; Guarino, D.; Camastra, S.; Bellini, R.; Berta, R.D.; Ferrannini, E. The role of β-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2011, 96, E1372–E1379. [Google Scholar] [CrossRef]
- Mallipedhi, A.; Min, T.; Prior, S.L.; MacIver, C.; Luzio, S.D.; Dunseath, G.; Bracken, R.M.; Islam, S.; Barry, J.D.; Caplin, S.; et al. Association between the preoperative fasting and postprandial C-peptide AUC with resolution of type 2 diabetes 6 months following bariatric surgery. Metabolism 2015, 64, 1556–1563. [Google Scholar] [CrossRef][Green Version]
- Lee, W.J.; Chong, K.; Ser, K.H.; Chen, J.C.; Lee, Y.C.; Chen, S.C.; Su, Y.H.; Tsai, M.H. C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes. Surg. 2012, 22, 293–298. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin. Endocrinol. 2008, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Escuredo, J.M.; Gómez-Ambrosi, J.; Catalán, V.; Domingo, P.; Giralt, M.; Frühbeck, G.; Villarroya, F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 2015, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell. Signal. 2001, 13, 827–833. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J.; Salvador, J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before Surgery | After Surgery |
|---|---|---|
| n (males, females) | 25 (11, 14) | 25 (11, 14) |
| Age (years) | 50 ± 2 | 51 ± 2 |
| BMI (kg/m2) | 44.2 ± 1.3 | 33.6 ± 1.6 *** |
| Body fat (%) | 49.9 ± 1.5 | 39.6 ± 2.0 *** |
| Waist (cm) | 128 ± 3 | 107 ± 3 *** |
| Waist-to-hip ratio | 1.00 ± 0.01 | 0.97 ± 0.02 ** |
| SBP (mmHg) | 128 ± 3 | 120 ± 3 ** |
| DBP (mmHg) | 80 ± 2 | 73 ± 1 *** |
| Fasting glucose (mg/dL) | 133 ± 7 | 115 ± 9 |
| Fasting insulin (μU/mL) | 21.3 ± 2.5 | 8.0 ± 1.2 *** |
| HOMA | 6.6 ± 0.8 | 2.1 ± 0.3 *** |
| QUICKI | 0.302 ± 0.006 | 0.363 ± 0.010 *** |
| HbA1c (%) | 7.2 ± 0.2 | 6.4 ± 0.2 ** |
| Triglycerides (mg/dL) | 140 ± 10 | 97 ± 11 ** |
| Total cholesterol (mg/dL) | 185 ± 7 | 159 ± 7 * |
| LDL-cholesterol (mg/dL) | 111 ± 6 | 90 ± 5 * |
| HDL-cholesterol (mg/dL) | 46 ± 2 | 51 ± 2 * |
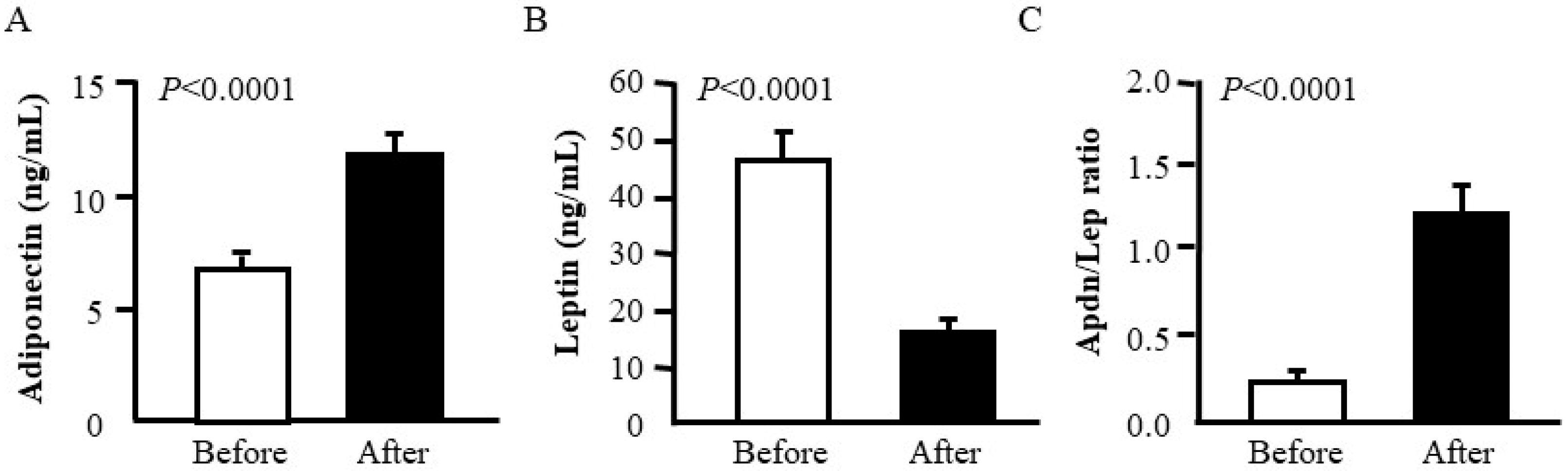
| Leptin (ng/mL) | 45.3 ± 5.6 | 15.1 ± 2.4 *** |
| Adiponectin (µg/mL) | 6.73 ± 0.67 | 11.68 ± 0.81 *** |
| Adpn/Lep ratio | 0.21 ± 0.03 | 1.20 ± 0.19 *** |
| Uric acid (mg/dL) | 5.8 ± 0.3 | 4.5 ± 0.2 *** |
| Creatinine (mg/dL) | 0.80 ± 0.04 | 0.77 ± 0.03 * |
| CRP (mg/L) | 8.5 ± 1.6 | 2.1 ± 1.0 * |
| Fibrinogen (mg/dL) | 385 ± 21 | 348 ± 23 |
| von Willebrand factor (%) | 152 ± 11 | 138 ± 14 |
| Homocysteine (μmol/L) | 10.2 ± 1.2 | 9.3 ± 1.1 |
| AST (IU/L) | 15 ± 1 | 18 ± 2 |
| ALT (IU/L) | 21 ± 2 | 27 ± 5 |
| AST/ALT | 0.78 ± 0.07 | 0.87± 0.07 |
| γ-GT (IU/L) | 35 ± 6 | 16 ± 2 ** |
| Adpn/Lep Ratio Before Surgery | Adpn/Lep Ratio After Surgery | |||
|---|---|---|---|---|
| r | p | r | p | |
| Δ BMI | −0.36 | 0.077 | 0.58 | 0.002 |
| Δ BF | −0.02 | 0.912 | 0.79 | <0.001 |
| Δ Waist | −0.14 | 0.526 | 0.72 | <0.001 |
| Δ WHR | 0.37 | 0.087 | 0.58 | 0.004 |
| Δ Adiponectin | Δ Leptin | Δ Adpn/Lep Ratio | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Δ BMI | −0.49 | 0.013 | 0.73 | <0.001 | −0.64 | <0.001 |
| Δ BF | −0.48 | 0.014 | 0.25 | 0.234 | −0.80 | <0.001 |
| Δ Waist | −0.45 | 0.030 | 0.58 | 0.004 | −0.76 | <0.001 |
| Δ WHR | −0.06 | 0.788 | 0.06 | 0.783 | −0.52 | 0.011 |
| Responders | Non-Responders | p (R vs. NR) | ||||
|---|---|---|---|---|---|---|
| Before BS | After BS | Before BS | After BS | Before BS | After BS | |
| n | 18 | 18 | 7 | 7 | - | - |
| Age | 49 ± 3 | 50 ± 3 | 58 ± 2 | 59 ± 2 | 0.014 | 0.014 |
| BMI (kg/m2) | 45.2 ± 1.7 | 33.8 ± 1.6 *** | 41.4 ± 1.9 | 32.3 ± 2.6 ** | 0.226 | 0.601 |
| Body fat (%) | 50.7 ± 1.9 | 40.2 ± 2.5 *** | 49.2 ± 2.9 | 38.4 ± 4.1 * | 0.273 | 0.701 |
| Waist (cm) | 127 ± 4 | 106 ± 4 *** | 105 ± 4 | 110 ± 6 ** | 0.731 | 0.730 |
| Waist-to-hip ratio | 0.99 ± 0.01 | 0.96 ± 0.02 | 1.02 ± 0.02 | 0.95 ± 0.02 * | 0.246 | 0.836 |
| SBP (mmHg) | 127 ± 4 | 117 ± 3 * | 130 ± 4 | 126 ± 7 ** | 0.679 | 0.220 |
| DBP (mmHg) | 81 ± 3 | 72 ± 2 *** | 77 ± 4 | 74 ± 3 * | 0.439 | 0.628 |
| Fasting glucose (mg/dL) | 125 ± 6 | 97 ± 4 *** | 166 ± 16 | 172 ± 25 | 0.004 | 0.002 |
| Fasting insulin (μU/mL) | 21.9 ± 3.2 | 9.5 ± 1.5 ** | 19.1 ± 4.9 | 4.7 ± 1.0 * | 0.649 | 0.008 |
| HOMA | 6.3 ± 1.0 | 2.3 ± 1.4 ** | 7.8 ± 2.2 | 1.8 ± 0.5 ** | 0.487 | 0.516 |
| QUICKI | 0.304 ± 0.007 | 0.358 ± 0.012 *** | 0.298 ± 0.017 | 0.362 ± 0.020 * | 0.728 | 0.869 |
| HbA1c (%) | 7.3 ± 0.6 | 6.1 ± 0.4 ** | 8.1 ± 0.5 | 6.7 ± 0.2 | 0.005 | 0.172 |
| Triglycerides (mg/dL) | 141 ± 12 | 84 ± 10 *** | 129 ± 28 | 135 ± 30 | 0.422 | 0.041 |
| Total cholesterol (mg/dL) | 194 ± 10 | 150 ± 7 *** | 162 ± 8 | 184 ± 9 | 0.222 | 0.025 |
| LDL-cholesterol (mg/dL) | 122 ± 8 | 86 ± 7 ** | 90 ± 9 | 100 ± 6 | 0.190 | 0.263 |
| HDL-cholesterol (mg/dL) | 45 ± 3 | 48 ± 2 | 45 ± 2 | 57 ± 4 * | 0.513 | 0.058 |
| Leptin (ng/mL) | 48.8 ± 6.9 | 16.9 ± 3.1 *** | 38.1 ± 10.8 | 11.8 ± 3.1 * | 0.415 | 0.830 |
| Adiponectin (µg/mL) | 6.49 ± 0.90 | 11.80 ± 1.06 *** | 7.34 ± 0.64 | 11.39 ± 1.14 | 0.582 | 0.356 |
| Adpn/Lep ratio | 0.17 ± 0.03 | 1.11 ± 0.21 *** | 0.32 ± 0.09 | 1.43 ± 0.44 * | 0.150 | 0.478 |
| Uric acid (mg/dL) | 5.9 ± 0.4 | 4.4 ± 0.3 ** | 5.3 ± 0.3 | 4.6 ± 0.3 | 0.325 | 0.759 |
| Creatinine (mg/dL) | 0.78 ± 0.04 | 1.29 ± 0.54 | 0.80 ± 0.09 | 0.74 ± 0.07 * | 0.551 | 0.811 |
| CRP (mg/L) | 9.7 ± 4.2 | 3.0 ± 1.6 | 7.3 ± 3.1 | 0.9 ± 0.2 * | 0.447 | 0.352 |
| Fibrinogen (mg/dL) | 378 ± 24 | 373 ± 24 | 357 ± 72 | 271 ± 32 | 0.828 | 0.154 |
| von Willebrand factor (%) | 159 ± 23 | 140 ± 18 | 161 ± 28 | 136 ± 27 * | 0.940 | 0.971 |
| Homocysteine (μmol/L) | 7.92 ± 1.67 | 9.47 ± 1.44 | 11.30 ± 1.70 | 9.13 ± 1.11 | 0.722 | 0.838 |
| AST (IU/L) | 16 ± 2 | 17 ± 2 | 18 ± 1 | 31 ± 3 | 0.913 | 0.262 |
| ALT (IU/L) | 23 ± 2 | 26 ± 5 | 23 ± 3 | 26 ± 12 | 0.273 | 0.656 |
| AST/ALT | 0.70 ± 0.04 | 0.79 ± 0.06 | 1.00 ± 0.18 | 1.07 ± 0.21 | 0.135 | 0.240 |
| γ-GT (IU/L) | 32 ± 5 | 15 ± 2 ** | 43 ± 18 | 24 ± 7 | 0.439 | 0.233 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unamuno, X.; Izaguirre, M.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; et al. Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients 2019, 11, 2069. https://doi.org/10.3390/nu11092069
Unamuno X, Izaguirre M, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, et al. Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients. 2019; 11(9):2069. https://doi.org/10.3390/nu11092069
Chicago/Turabian StyleUnamuno, Xabier, Maitane Izaguirre, Javier Gómez-Ambrosi, Amaia Rodríguez, Beatriz Ramírez, Sara Becerril, Víctor Valentí, Rafael Moncada, Camilo Silva, Javier Salvador, and et al. 2019. "Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass" Nutrients 11, no. 9: 2069. https://doi.org/10.3390/nu11092069
APA StyleUnamuno, X., Izaguirre, M., Gómez-Ambrosi, J., Rodríguez, A., Ramírez, B., Becerril, S., Valentí, V., Moncada, R., Silva, C., Salvador, J., Portincasa, P., Frühbeck, G., & Catalán, V. (2019). Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients, 11(9), 2069. https://doi.org/10.3390/nu11092069