Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. The 16HBE Cell Line
2.1.2. Primary Bronchial Epithelial Cells
2.2. Preparation Cigarette Smoke Extract
2.3. CSE and Vitamin D Exposure
2.4. IL-8 ELISA
2.5. qPCR
2.6. Wound Healing Assay
2.7. Statistics
3. Results
3.1. Both 1,25(OH)2D and 25(OH)D Reduce CSE-Induced IL-8 by 16HBE Cells
3.2. Both 1,25(OH)2D and 25(OH)D are Able to Induce Cathelicidin Expression in 16HBE Cells
3.3. Effect of CSE and Vitamin D on Airway Remodeling and Wound Healing in 16HBE Cells
3.4. Effect of CSE and Vitamin D on Vitamin D Metabolism
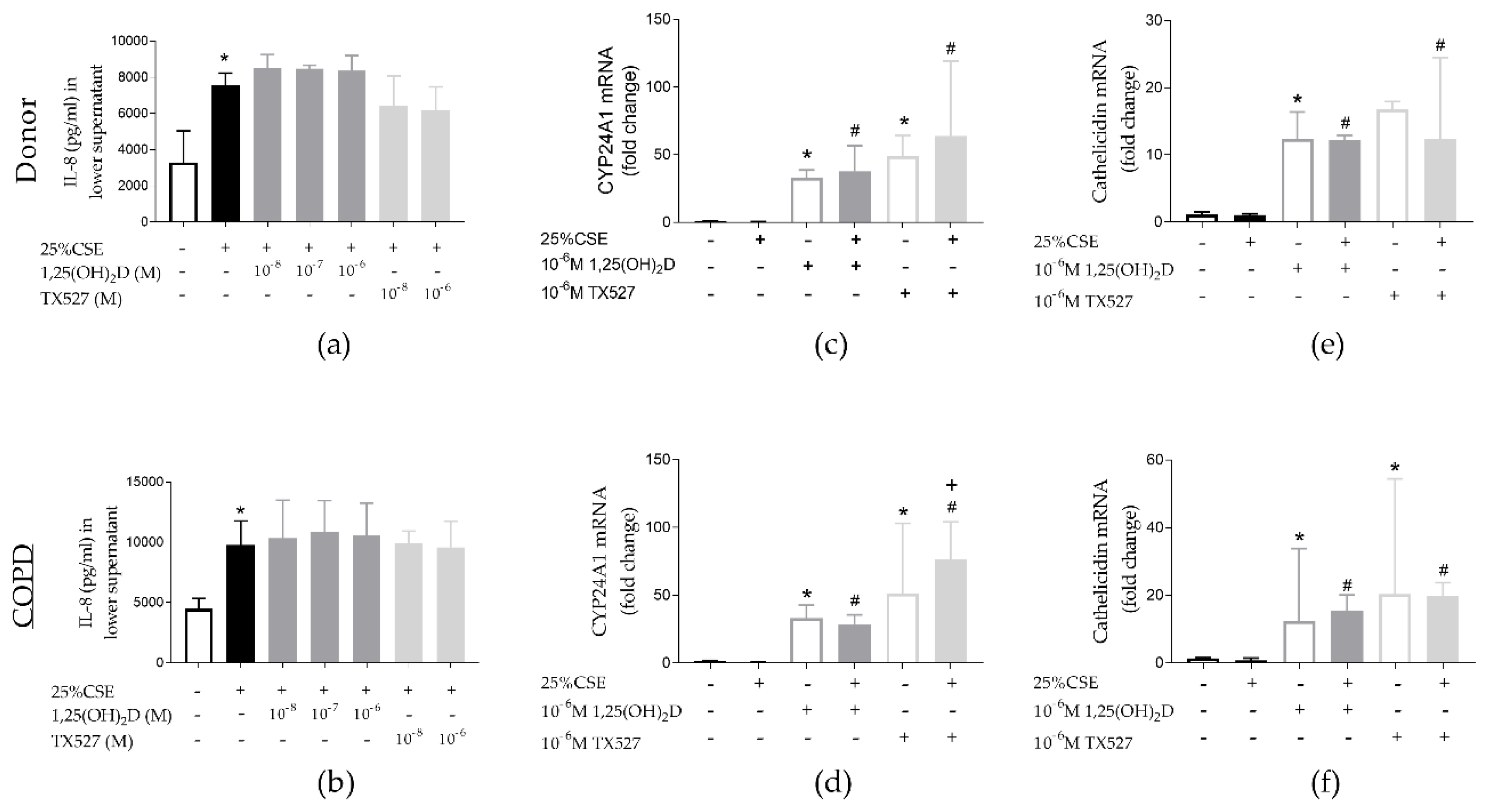
3.5. Effect of CSE and Vitamin D on PBEC from Unused Donor Lungs and COPD Explant Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease; Gold Report. Fontana, CA, USA, 2019; Volume 2019, pp. 1–155. Available online: https://goldcopd.org/ (accessed on 15 July 2019).
- Gao, W.; Li, L.; Wang, Y.; Zhang, S.; Adcock, I.M.; Barnes, P.J.; Huang, M.; Yao, X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 2015, 20, 722–729. [Google Scholar] [CrossRef]
- Phillips, J.; Kluss, B.; Richter, A.; Massey, E.D. Exposure of bronchial epithelial cells to whole cigarette smoke: Assessment of cellular responses. ATLA Altern. Lab. Anim. 2005, 33, 239–248. [Google Scholar] [CrossRef]
- Heulens, N.; Korf, H.; Janssens, W. Innate immune modulation in chronic obstructive pulmonary disease: Moving closer toward vitamin D therapy. J. Pharmacol. Exp. Ther. 2015, 353, 360–368. [Google Scholar] [CrossRef]
- Grzela, K.; Litwiniuk, M.; Zagorska, W.; Grzela, T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: The Role of Matrix Metalloproteinase-9. Arch. Immunol. Ther. Exp. 2016, 64, 47–55. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; Broekman, W.; Daniel, N.M.; van der Vlugt, L.; van Schadewijk, A.; Taube, C.; Hiemstra, P. Cigarette smoke modulates repair and innate immunity following injury to airway epithelial cells. PLoS ONE 2016, 11, e0166255. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gyseman, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Janssens, W.; Lehouck, A.; Carremans, C.; Bouillon, R.; Mathieu, C.; Decramer, M. Pulmonary Perspective Vitamin D Beyond Bones in Chronic Obstructive Pulmonary Disease Time to Act. Am. J. Respir. Crit. Care Med. 2009, 179, 630–636. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D Decreases Respiratory Syncytial Virus Induction of NF-κB-Linked Chemokines and Cytokines in Airway Epithelium While Maintaining the Antiviral State. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef]
- Heulens, N.; Korf, H.; Mathyssen, C.; Everaerts, S.; De Smidt, E.; Dooms, C.; Yserbyt, J.; Gyseman, C.; Gayan-Ramirez, G.; Mathieu, C.; et al. 1,25-Dihydroxyvitamin D Modulates Antibacterial and Inflammatory Response in Human Cigarette Smoke-Exposed Macrophages. PLoS ONE 2016, 11, e0160482. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef]
- Menezes, R.J.; Cheney, R.T.; Husain, A.; Tretiakova, M.; Loewen, G.; Johnson, C.S.; Jayaprakash, V.; Moysich, K.B.; Salgia, R.; Reid, M.E. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1104–1110. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-mediated hypercalcemia: Mechanisms, diagnosis, and treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis HHS Public Access. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Butler, A.; Walton, G.M.; Sapey, E. Neutrophilic Inflammation in the Pathogenesis of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 392–404. [Google Scholar] [CrossRef]
- Beeh, K.M.; Kornmann, O.; Buhl, R.; Culpitt, S.V.; Giembycz, M.A.; Barnes, P.J. Neutrophil chemotactic activity of sputum from patients with COPD: Role of interleukin 8 and leukotriene B4. Chest 2003, 123, 1240–1247. [Google Scholar] [CrossRef]
- Comer, D.M.; Elborn, J.S.; Ennis, M. Cigarette Smoke, Airway Epithelial Cells and Host Defence. Inflamm Cell Signal 2014, 1, e203. [Google Scholar]
- Mortaz, E.; Henricks, P.A.J.; Kraneveld, A.D.; Givi, M.E.; Folkerts, G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1104–1110. [Google Scholar] [CrossRef]
- Kode, A.; Yang, S.R.; Rahman, I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir. Res. 2006, 7, 132. [Google Scholar] [CrossRef]
- Glader, P.; Möller, S.; Lilja, J.; Wieslander, E.; Löfdahl, C.-G.; von Wachenfeldt, K. Cigarette smoke extract modulates respiratory defence mechanisms through effects on T-cells and airway epithelial cells. Respir. Med. 2006, 100, 818–827. [Google Scholar] [CrossRef]
- Mulligan, J.K.; Nagel, W.; O’Connell, B.P.; Wentzel, J.; Atkinson, C.; Schlosser, R.J. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2014, 134, 342–349. [Google Scholar] [CrossRef]
- Takano, Y.; Mitsuhashi, H.; Ueno, K. 1α,25-Dihydroxyvitamin D₃ inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids 2011, 76, 1305–1309. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Amatngalim, G.D.; Van Der Does, A.M.; Taube, C. Antimicrobial peptides and innate lung defenses: Role in infectious and noninfectious lung diseases and therapeutic applications. Chest 2016, 149, 545–551. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; Schrumpf, J.A.; Henic, A.; Dronkers, E.; Verhoosel, R.M.; Soledad, S.R.; Haagsam, H.P.; Funetes, M.E.; Sridhar, S.; Aarbiou, J.; et al. Antibacterial Defense of Human Airway Epithelial Cells from Chronic Obstructive Pulmonary Disease Patients Induced by Acute Exposure to Nontypeable Haemophilus influenzae: Modulation by Cigarette Smoke. J. Innate Immun. 2017, 9, 359–374. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.H.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef]
- Rockett, K.A.; Brookes, R.; Udalova, I.; Vidal, V.; Hill, A.V.S.; Kwiatkowski, D. 1, 25-Dihydroxyvitamin D3 inducesnitric oxide synthase and surpresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 1998, 66, 5314–5321. [Google Scholar]
- Serré, J.; Mathyssen, C.; Ajime, T.T.; Korf, H.; Maes, K.; Heulens, N.; Gysemans, C.; Mathieu, C.; Vanaudenaerde, B.; Janssens, W.; et al. Airway Infection with Nontypeable Haemophilus influenza is more rapidly eradicated in vitamin D deficient mice. J. Steroid Biochem. Mol. Biol. 2018. [Google Scholar]
- Hogg, J.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The Nature of Small-Airway Obstruction in Chronic Obsructive Pulmonary Disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Thorley, A.J.; Tetley, T.D. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 409–428. [Google Scholar]
- Chung, K.F. The Role of Airway Smooth Muscle in the Pathogenesis of Airway Wall Remodeling in Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2005, 2, 347–354. [Google Scholar] [CrossRef]
- Schamberger, A.C.; Staab-Weijnitz, C.A.; Mise-Racek, N.; Eickelberg, O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci. Rep. 2015, 5, 8163. [Google Scholar] [CrossRef]
- Eurlings, I.M.J.; Reynaert, N.L.; Van Den Beucken, T.; Gosker, H.R.; de Theije, C.C.; Verhamme, F.M.; Bracke, K.R.; Wouters, E.F.M.; Dentner, M.A. Cigarette smoke extract induces a phenotypic shift in epithelial cells; involvement of HIF1alpha in mesenchymal transition. PLoS ONE 2014, 9, e107757. [Google Scholar] [CrossRef]
- Milara, J.; Peiró, T.; Serrano, A.; Cortijo, J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax 2013, 68, 410–420. [Google Scholar] [CrossRef]
- Russell, R.E.; Culpitt, S.V.; DeMatos, C.; Donnelly, L.; Smith, M.; Wiggins, J.; Barnes, P.J. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2002, 26, 602–609. [Google Scholar] [CrossRef]
- Perotin, J.-M.; Adam, D.; Vella-Boucaud, J.; Delepine, G.; Sandu, S.; Jonvel, A.-C.; Prevost, A.; Berthiot, G.; Pison, C.; Lebargy, F.; et al. Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir. Res. 2014, 15, 151. [Google Scholar] [CrossRef]
- Silverstein, P. Smoking and wound healing. Am. J. Med. 1992, 93 (Suppl. 1), 22–24. [Google Scholar] [CrossRef]
- Heilborn, J.D.; Frohm Nilsson, M.; Kratz, G.; Weber, G.; Sørensen, O.; Borregaard, N.; Ståhle-Bäckdahl, M. The Cathelicidin Anti-Microbial Peptide LL-37 is Involved in Re-Epithelialization of Human Skin Wounds and is Lacking in Chronic Ulcer Epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef]
- Fisher, K.; Agrawal, D. Vitamin D regulating TGF-β induced epithelial-mesenchymal transition. Respir. Res. 2014, 15, 146. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin-D-Mediated Human Antimbacterial Response. Science 2006, 311, 23–26. [Google Scholar] [CrossRef]
- Ishii, M.; Yamaguchi, Y.; Nakamura, T.; Akishita, M. The Vitamin D Receptors May Function as Antiinflammatory Effects in Patients with COPD. Chest 2015, 148, 690A. [Google Scholar] [CrossRef]
- Buonfiglio, L.G.V.; Cano, M.; Pezzulo, A.A.; Vangas Calderon, D.G.; Zabner, J.; Gerke, A.K.; Comellas, A.P. Effect of vitamin D 3 on the antimicrobial activity of human airway surface liquid: Preliminary results of a randomised placebo-controlled double- blind trial. Open Respir. Res. 2017, 4, e000211. [Google Scholar] [CrossRef]
- Pohl, C.; Hermanns, M.I.; Uboldi, C.; Bock, M.; Fuchs, S.; Dei-Anang, J.; Mayer, E.; Kehe, K.; Kummer, W.; Kirkpatrick, C.J. Barrier functions and paracellular integrity in human cell culture models of the proximal respiratory unit. Eur. J. Pharm. Biopharm. 2009, 72, 339–349. [Google Scholar] [CrossRef]
- Cozens, A.L.; Yezzi, M.J.; Kunzelmann, K.; Ohrui, T.; Chin, L.; Eng, K.; Finkbeiner, W.E.; Widdicombe, J.H.; Gruenert, D.C. CFfR Expression and Chloride Secretion in Polarized Immortal Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Bio. 1994, 10, 38–47. [Google Scholar] [CrossRef]
- Banerjee, B.; Kicic, A.; Musk, M.; Sutanto, E.N.; Stick, S.M.; Chambers, D.C. Successful establishment of primary small airway cell cultures in human lung transplantation.pdf. Respir. Res. 2009, 10, 99. [Google Scholar] [CrossRef][Green Version]
- Forbes, B. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm. Sci. Technol. Today 2000, 3, 18–27. [Google Scholar] [CrossRef]





| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | TGGTATCGTGGAAGGACTCA | CCAGTAGAGGCAGGGATGAT |
| VDR | GATTGGAGAAGCTGGACGAG | GTTCGTGTGAATGATGGTGGA |
| CYP27B1 | CGCACTGTCCCAAAGCTG | CGGAGCTTGGCAGACATC |
| CYP24A1 | GTGACCATCATCCTCCCAAA | AGTATCTGCCTCGTGTTGTATG |
| Cathelicidin | GGGCTCCTTTGACATCAGTT | AGCAGGGCAAATCTCTTGTT |
| iNOS | TTCAGTATCACAACCTCAGCAAG | TGGACCTGCAAGTTAAAATCCC |
| Fibronectin | AAACCAATTCTTGGAGGAGG | CCATAAAGGGCAACCAAGAG |
| E-cadherin | GAAGGTGACAGAGCCTCTGGAT | GATCGGTTACCGTGATCAAAATC |
| MMP-9 | GCACGACGTCTTCCAGTACC | CAGGATGTCATAGGTCACGTAGC |
| Donor | COPD | p-Value | |
|---|---|---|---|
| Age (years) | 57 (55–58) | 57 (53–60) | 0.873 |
| gender (M/F) | 1/3 | 1/3 | >0.999 |
| FEV1% | NA | 24.5 (21.0–25.8) | NA |
| FEV1%/FVC% | NA | 34 (24.75–30.25) | NA |
| FVC% | NA | 62.5 (56.5–64.0) | NA |
| DLco% | NA | 36 (27–39) | NA |
| Smoking history (Y/N/U) | 1/2/1 | 4/0/0 | |
| VitD supplementation (Y/N/U) | 0/0/4 | 3/1/0 | NA |
| Serum 25(OH)D pretransplant µg/L | NA | 27 (21–43) | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathyssen, C.; Serré, J.; Sacreas, A.; Everaerts, S.; Maes, K.; Verleden, S.; Verlinden, L.; Verstuyf, A.; Pilette, C.; Gayan-Ramirez, G.; et al. Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract. Nutrients 2019, 11, 2138. https://doi.org/10.3390/nu11092138
Mathyssen C, Serré J, Sacreas A, Everaerts S, Maes K, Verleden S, Verlinden L, Verstuyf A, Pilette C, Gayan-Ramirez G, et al. Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract. Nutrients. 2019; 11(9):2138. https://doi.org/10.3390/nu11092138
Chicago/Turabian StyleMathyssen, Carolien, Jef Serré, Annelore Sacreas, Stephanie Everaerts, Karen Maes, Stijn Verleden, Lieve Verlinden, Annemieke Verstuyf, Charles Pilette, Ghislaine Gayan-Ramirez, and et al. 2019. "Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract" Nutrients 11, no. 9: 2138. https://doi.org/10.3390/nu11092138
APA StyleMathyssen, C., Serré, J., Sacreas, A., Everaerts, S., Maes, K., Verleden, S., Verlinden, L., Verstuyf, A., Pilette, C., Gayan-Ramirez, G., Vanaudenaerde, B., & Janssens, W. (2019). Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract. Nutrients, 11(9), 2138. https://doi.org/10.3390/nu11092138






