Alcohol Consumption Reduces the Beneficial Influence of Protein Intake on Muscle Mass in Middle-Aged Korean Adults: A 12-Year Community-Based Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Intake
2.3. Measurement of Alcohol Consumption
2.4. Measurement of Body Composition
2.5. Covariates
2.6. Statistical Analysis
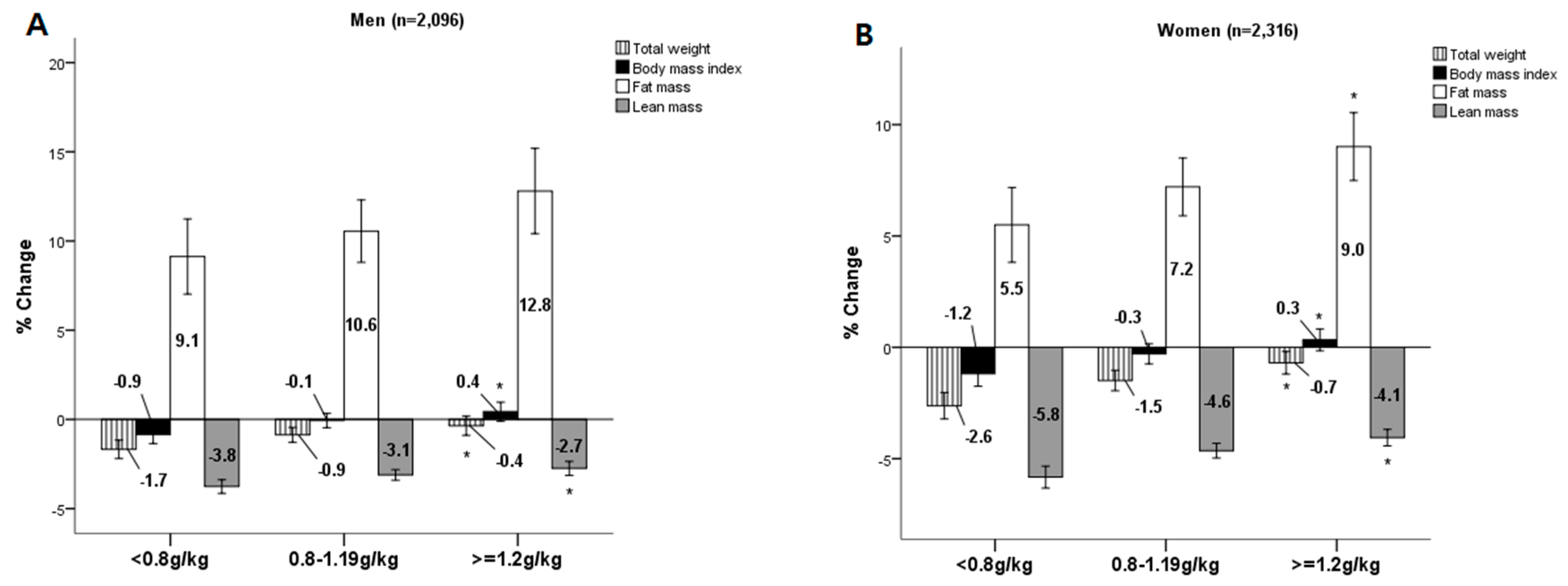
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.; Officer, A.; Cassels, A. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Chen, L.K.; Lee, W.J.; Peng, L.N.; Liu, L.K.; Arai, H.; Akishita, M.; Asian Working Group for, S. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, e761–e767. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med. J. 2012, 3, e0024. [Google Scholar] [CrossRef]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 68, 677–681. [Google Scholar] [CrossRef]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Kimball, S.R.; Lang, C.H. Acute alcohol-induced decrease in muscle protein synthesis in female mice is REDD-1 and mTOR-independent. Alcohol Alcohol. 2015, 51, 242–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lang, C.H.; Frost, R.A.; Svanberg, E.; Vary, T.C. Metabolism. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am. J. Physiol. Metab. 2004, 286, E916–E926. [Google Scholar]
- Lang, C.H.; Pruznak, A.M.; Nystrom, G.J.; Vary, T.C. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutr. Metab. 2009, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Bohannon, R.W.; Petr, M.; Kohlikova, E.; Holmerova, I. Alcohol consumption as a risk factor for sarcopenia−A meta-analysis. BMC Geriatr. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Lim, H.J.; Lee, Y.J.; Lee, H.S.; Linton, J.A.; Lee, J.W.; Kang, H.T. Associations between high-risk alcohol consumption and sarcopenia among postmenopausal women. Menopause 2017, 24, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Ha, Y.C.; Lee, Y.K.; Hana, C.; Yoo, M.J.; Koo, K.H. High prevalence of sarcopenia among binge drinking elderly women: A nationwide population-based study. BMC Geriatr. 2017, 17, 114. [Google Scholar] [CrossRef]
- Korzick, D.H.; Sharda, D.R.; Pruznak, A.M.; Lang, C.H. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R887–R898. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; Ko, G.E. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Clarys, J.P.; Martin, A.D.; Drinkwater, D.T. Gross tissue weights in the human body by cadaver dissection. Hum. Biol. 1984, 56, 459–473. [Google Scholar] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C.J.C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.J. Dietary reference intakes for energy, carbohdrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Acad. Nutr. Diet. 2002, 102, 1621. [Google Scholar]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhu, K.; Devine, A.; Kerr, D.A.; Binns, C.W.; Prince, R.L. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J. Bone Miner. Res. 2009, 24, 1827–1834. [Google Scholar] [CrossRef]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kroger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkila, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J.; Kwok, T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J. Nutr. Health Aging 2014, 18, 171–177. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Suominen, M.; Jyvakorpi, S.; Pitkala, K.; Finne-Soveri, H.; Hakala, P.; Mannisto, S.; Soini, H.; Sarlio-Lahteenkorva, S.J. Nutritional guidelines for older people in Finland. J. Nutr. Health Aging 2014, 18, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Koreal Health Statistics 2015; Ministry of Health and Welfare: Sejong, Korea, 2016.
- Adachi, J.; Asano, M.; Ueno, Y.; Niemelä, O.; Ohlendieck, K.; Peters, T.J.; Preedy, V.R. Alcoholic muscle disease and biomembrane perturbations. J. Nutr. Biochem. 2003, 14, 616–625. [Google Scholar] [CrossRef]
- Ronis, M.J.; Wands, J.R.; Badger, T.M.; De La Monte, S.M.; Lang, C.H.; Calissendorff, J. Alcohol-Induced Disruption of Endocrine Signaling. Alcohol. Clin. Exp. Res. 2007, 31, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Paice, A.; Mantle, D.; Dhillon, A.S.; Palmer, T.N.; Peters, T.J. Alcoholic myopathy: Biochemical mechanisms. Drug Alcohol Depend. 2001, 63, 199–205. [Google Scholar] [CrossRef]
- Lang, C.H.; Pruznak, A.M.; Deshpande, N.; Palopoli, M.M.; Frost, R.A.; Vary, T.C. Alcohol Intoxication Impairs Phosphorylation of S6K1 and S6 in Skeletal Muscle Independently of Ethanol Metabolism. Alcohol. Clin. Exp. Res. 2004, 28, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, T.H.; Hwang, H.J. The relationship of physical activity (PA) and walking with sarcopenia in Korean males aged 60 years and older using the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. Arch. Gerontol. Geriatr. 2013, 56, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Duboeuf, F.; Marchand, F.; Delmas, P.D. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am. J. Clin. Nutr. 2004, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Mumenthaler, M.S.; Taylor, J.L.; O’Hara, R.; Yesavage, J.A. Gender differences in moderate drinking effects. Alcohol Res. Health 1999, 23, 55–64. [Google Scholar] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur. J. Clin. Nutr. 2013, 67, 395–400. [Google Scholar] [CrossRef] [PubMed]
| Protein Intake | Men (n = 2096) | Women (n = 2316) | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤0.8 g/kg BW | 0.81‒1.19 g/kg BW | ≥1.2 g/kg BW | p for Trend 1 | ≤0.8 g/kg BW | 0.81‒1.19 g/kg BW | ≥1.2 g/kg BW | p for Trend | |
| n = 538 | n = 980 | n = 533 | n = 601 | n = 972 | n = 743 | |||
| Demographics and lifestyles (%) | ||||||||
| Age (years, mean ± SD) | 50.5 ± 8.0 | 49.7 ± 7.7 | 50.5 ± 8.2 | 0.073 | 52.4 ± 8.6 | 51.1 ± 8.4 | 49.6 ± 8.2 | 0.000 |
| Residential area (city) | 61.6 | 74.4 | 64.0 | 0.301 | 51.4 | 65.5 | 60.3 | 0.003 |
| Educational (≥College) | 19.9 | 27.8 | 27.1 | 0.005 | 4.0 | 7.5 | 11.5 | 0.000 |
| Household income (≥3,000,000 KRW) | 21.7 | 31.0 | 27.5 | 0.024 | 11.2 | 16.2 | 22.5 | 0.000 |
| Marital status (married) | 96.9 | 96.8 | 97.4 | 0.673 | 85.2 | 87.5 | 90.7 | 0.002 |
| Smoking (yes) | 45.5 | 42.3 | 47.8 | 0.475 | 2.9 | 2.0 | 2.3 | 0.547 |
| Chronic disease (yes) | 1.5 | 1.1 | 1.9 | 0.665 | 5.7 | 4.4 | 3.9 | 0.132 |
| Dental health status (poor) | 36.3 | 39.2 | 40.1 | 0.189 | 45.5 | 43.0 | 37.7 | 0.004 |
| Regular physical activity (yes) | 12.5 | 19.2 | 22.0 | 0.000 | 14.1 | 17.3 | 20.1 | 0.004 |
| Alcohol drinking (yes) 2 | 71.6 | 73.7 | 76.3 | 0.074 | 24.7 | 25.9 | 28.2 | 0.142 |
| Heavy drinking (yes) | 13.7 | 14.6 | 18.1 | 0.019 | 1.5 | 1.4 | 1.2 | 0.336 |
| Drinking frequency (≥1 time/week) | 49.1 | 52.8 | 58.9 | 0.001 | 7.0 | 7.6 | 9.2 | 0.136 |
| Binge drinker (yes) 3 | 42.2 | 49.4 | 48.4 | 0.033 | 6.7 | 7.1 | 5.7 | 0.421 |
| Dietary intake (Mean ± SD) | ||||||||
| Energy (kcal/day) | 1528.9 ± 271.5 | 1961.5 ± 312.4 | 2628.2 ± 574.4 | 0.000 | 1345.7 ± 291.4 | 1737.9 ± 296.0 | 2442.1 ± 617.0 | 0.000 |
| Carbohydrate (% of energy) | 74.0 ± 5.0 | 69.0 ± 5.3 | 65.0 ± 6.4 | 0.000 | 76.3 ± 5.3 | 71.9 ± 5.5 | 68.2 ± 6.7 | 0.000 |
| Fat (% of energy) | 12.4 ± 4.1 | 16.0 ± 4.1 | 19.0 ± 4.8 | 0.000 | 10.4 ± 4.2 | 13.7 ± 4.4 | 16.6 ± 5.0 | 0.000 |
| Protein (% of energy) | 12.3 ± 1.7 | 13.8 ± 1.8 | 15.3 ± 2.3 | 0.000 | 11.9 ± 1.9 | 13.3 ± 1.8 | 14.7 ± 2.2 | 0.000 |
| Protein (g/day) | 46.6 ± 9.2 | 67.3 ± 11.3 | 99.2 ± 22.7 | 0.000 | 39.4 ± 8.2 | 58.4 ± 9.4 | 88.6 ± 24.1 | 0.000 |
| Protein (g/kg body weight) | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.5 ± 0.3 | 0.000 | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.6 ± 0.4 | 0.000 |
| Body composition (Mean ± SD) | ||||||||
| Weight (kg) | 71.0 ± 9.5 | 68.4 ± 9.0 | 65.4 ± 8.7 | 0.000 | 61.4 ± 8.1 | 59.1 ± 7.4 | 56.2 ± 7.2 | 0.000 |
| Body Mass Index (kg/m2) | 25.1 ± 2.8 | 24.4 ± 2.7 | 23.5 ± 2.7 | 0.000 | 25.7 ± 3.0 | 24.8 ± 2.8 | 23.6 ± 2.7 | 0.000 |
| Fat mass (kg) | 16.1 ± 4.7 | 15.1 ± 4.5 | 13.6 ± 4.2 | 0.000 | 20.1 ± 4.9 | 18.8 ± 4.4 | 17.1 ± 4.3 | 0.000 |
| Lean mass (kg) | 54.80 ± 6.2 | 53.3 ± 6.1 | 51.8 ± 5.9 | 0.000 | 41.2 ± 4.3 | 40.3 ± 4.3 | 39.1 ± 4.1 | 0.000 |
| Skeletal muscle mass index (%) 4 | 40.3 ± 2.3 | 40.7 ± 2.4 | 41.4 ± 2.5 | 0.000 | 35.2 ± 2.6 | 35.6 ± 2.4 | 26.4 ± 2.5 | 0.000 |
| Alcohol Consumption Status | <0.8 g/kg BW | 0.8‒1.19 g/kg BW | ≥1.2 g/kg BW | p for Trend | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case(n)/Person-Months | Reference | Case(n)/Person-Months | HR | 95% CI | Case(n)/Person-Months | HR | 95% CI | ||
| Men (n = 2096) | 51/81,130 | 1.00 | 88/135,815 | 0.70 | (0.47, 1.05) | 32/74,611 | 0.24 | (0.12, 0.51) | 0.000 |
| Non-drinkers | 13/22,991 | 1.00 | 24/35,727 | 0.85 | (0.39, 1.84) | 10/17,684 | 0.28 | (0.07, 1.09) | 0.064 |
| Drinkers | 43/57,861 | 1.00 | 64/99,950 | 0.66 | (0.41, 1.07) | 22/56,784 | 0.23 | (0.10, 0.54) | 0.001 |
| Women (n = 2316) | 89/82,518 | 1.00 | 87/135,645 | 0.54 | (0.38, 0.76) | 42/104,027 | 0.29 | (0.16, 0.53) | 0.000 |
| Non-drinkers | 74/61,493 | 1.00 | 67/99,664 | 0.48 | (0.32, 0.70) | 31/74,485 | 0.23 | (0.11, 0.45) | 0.000 |
| Drinkers | 15/20,737 | 1.00 | 19/35,141 | 0.90 | (0.40, 2.03) | 11/29,260 | 0.64 | (0.18, 2.25) | 0.478 |
| Total (n = 4412) Quantity of Drinking | |||||||||
| Non-drinkers | 87/84,484 | 1.00 | 91/135,391 | 0.55 | (0.39, 0.77) | 41/92,169 | 0.24 | (0.13, 0.44) | 0.000 |
| Light-to-moderate drinkers | 49/64,233 | 1.00 | 65/109,753 | 0.68 | (0.44, 1.07) | 25/68,138 | 0.27 | (0.12, 0.58) | 0.158 |
| Heavy drinkers | 7/12,135 | 1.00 | 14/20,919 | 0.68 | (0.22, 2.12) | 6/14,312 | 0.20 | (0.03, 1.50) | 0.024 |
| Frequency of drinking | |||||||||
| <1 time/week | 112/117,898 | 1.00 | 124/189,519 | 0.60 | (0.44, 0.81) | 54/125,281 | 0.27 | (0.16, 0.47) | 0.000 |
| ≥1 time/week | 34/45,750 | 1.00 | 51/81,941 | 0.71 | (0.42, 1.20) | 20/53,357 | 0.28 | (0.11, 0.70) | 0.007 |
| Presence of binge drinking | |||||||||
| Social drinkers 2 | 119/123,801 | 1.00 | 128/194,703 | 0.59 | (0.44, 0.78) | 63/136,522 | 0.28 | (0.17, 0.47) | 0.000 |
| Binge drinkers 3 | 27/39,847 | 1.00 | 47/76,757 | 0.77 | (0.41, 1.43) | 11/42,116 | 0.26 | (0.08, 0.81) | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, E.; Joung, H. Alcohol Consumption Reduces the Beneficial Influence of Protein Intake on Muscle Mass in Middle-Aged Korean Adults: A 12-Year Community-Based Prospective Cohort Study. Nutrients 2019, 11, 2143. https://doi.org/10.3390/nu11092143
So E, Joung H. Alcohol Consumption Reduces the Beneficial Influence of Protein Intake on Muscle Mass in Middle-Aged Korean Adults: A 12-Year Community-Based Prospective Cohort Study. Nutrients. 2019; 11(9):2143. https://doi.org/10.3390/nu11092143
Chicago/Turabian StyleSo, Eunjin, and Hyojee Joung. 2019. "Alcohol Consumption Reduces the Beneficial Influence of Protein Intake on Muscle Mass in Middle-Aged Korean Adults: A 12-Year Community-Based Prospective Cohort Study" Nutrients 11, no. 9: 2143. https://doi.org/10.3390/nu11092143
APA StyleSo, E., & Joung, H. (2019). Alcohol Consumption Reduces the Beneficial Influence of Protein Intake on Muscle Mass in Middle-Aged Korean Adults: A 12-Year Community-Based Prospective Cohort Study. Nutrients, 11(9), 2143. https://doi.org/10.3390/nu11092143





