Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Participants
2.3. Dietary Intervention
2.4. Fecal Samples and Microbiota Profiling
2.5. Fecal Metabolite Profiling
2.6. Volatile Organic Compounds Profiling
2.7. Statistical Analyses
3. Results
3.1. Subjects
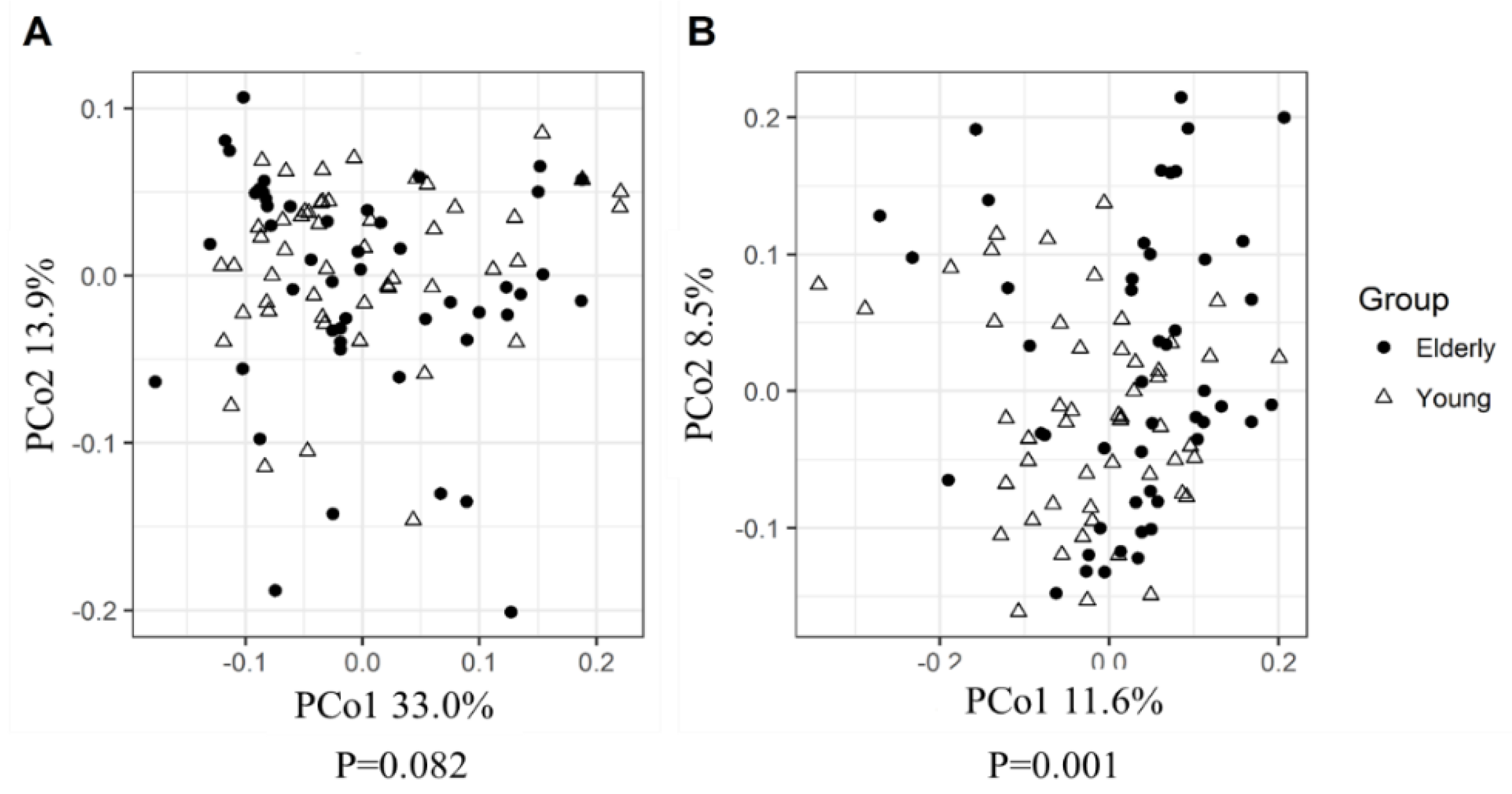
3.2. Young Adults and Elderly Showed Similar Fecal Microbiota Composition, SCFA- and Exhaled VOC Profiles
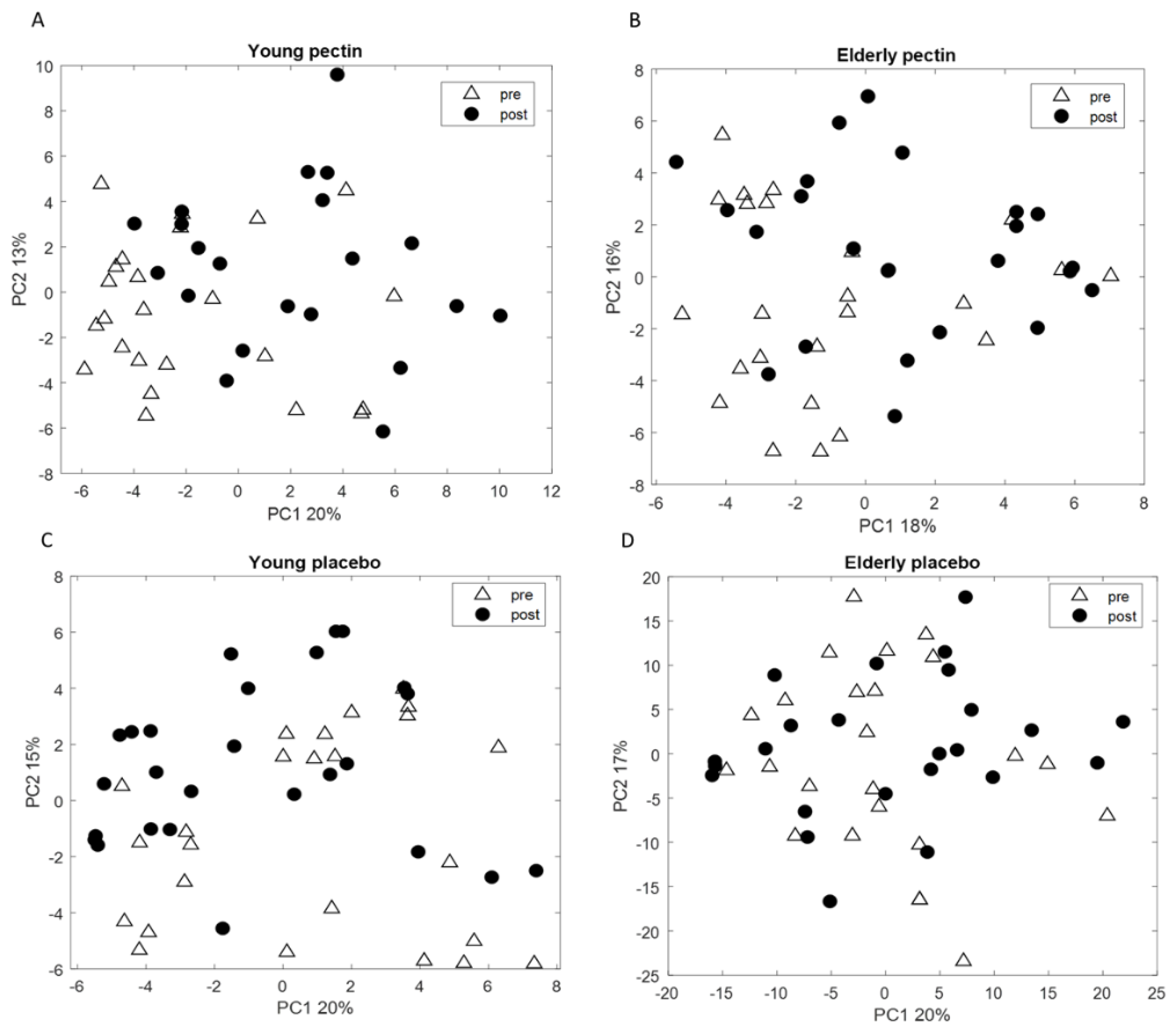
3.3. Four Weeks of Sugar Beet Pectin Supplementation Did Neither Alter Fecal Microbiota Composition, Nor SCFA- and Exhaled VOC Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO; US National Institute of Aging. Global Health and Aging; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- United Nations Department of Economic and Social Affairs. World Population Prospects; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2017. [Google Scholar]
- An, R.; Wilms, E.; Masclee, A.A.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Rochat, F.; Perruisseau-Carrier, G.; Rochat, I.; Schiffrin, E. Effects of oligosaccharide on the faecal flora and non-specific immune system in elderly people. Nutr. Res. 2002, 22, 13–25. [Google Scholar] [CrossRef]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [PubMed]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.E.; van den Heuvel, E.G.; Kosters, M.H.; Rastall, R.A.; Tuohy, K.M.; Gibson, G.R. A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br. J. Nutr. 2012, 107, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 121, pp. 91–119. [Google Scholar]
- Bianchi, F.; Larsen, N.; de Mello Tieghi, T.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.-J.; Kim, G.; Lim, M.Y.; Song, E.-J.; Jung, D.-H.; Kum, J.-S.; Nam, Y.-D.; Park, C.-S.; Seo, D.-H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 2018, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Jimenez, B.; Lodge, S. How to feed the mammalian gut microbiota: Bacterial and metabolic modulation by dietary fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 1600186. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 255. [Google Scholar] [CrossRef]
- Xu, L.; Yu, W.; Jiang, J.; Li, N. Clinical benefits after soluble dietary fiber supplementation: A randomized clinical trial in adults with slow-transit constipation. Zhonghua Yi Xue Za Zhi 2014, 94, 3813–3816. [Google Scholar]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef]
- Sagar, N.M.; Cree, I.A.; Covington, J.A.; Arasaradnam, R.P. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol. Res. Pract. 2015, 2015, 398585. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Greenman, J. Microbial volatile compounds in health and disease conditions. J. Breath Res. 2012, 6, 024001. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Mujagic, Z.; Smolinska, A.; Dallinga, J.; Jonkers, D.; Tigchelaar, E.; Dekens, J.; Zhernakova, A.; Ludwig, T.; Masclee, A. Volatile organic compounds in breath as markers for irritable bowel syndrome: A metabolomic approach. Aliment. Pharmacol. Ther. 2016, 44, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Tedjo, D.I.; Blanchet, L.; Bodelier, A.; Pierik, M.J.; Masclee, A.A.; Dallinga, J.; Savelkoul, P.H.; Jonkers, D.M.; Penders, J. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal. Chim. Acta 2018, 1025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Tigchelaar, E.; Smolinska, A.; Dallinga, J.W.; Moonen, E.J.; Dekens, J.A.; Wijmenga, C.; Zhernakova, A.; van Schooten, F.J. Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. J. Breath Res. 2013, 7, 037104. [Google Scholar] [CrossRef] [PubMed]
- Clooney, A.G.; Fouhy, F.; Sleator, R.D.; O’Driscoll, A.; Stanton, C.; Cotter, P.D.; Claesson, M.J. Comparing apples and oranges?: Next generation sequencing and its impact on microbiome analysis. PLoS ONE 2016, 11, e0148028. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Lorenz, A.; Proll, J. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J. Nutr. 2002, 132, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahu, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar] [CrossRef]
- Yao, C.; Muir, J.; Gibson, P. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Wilms, E.; Jonkers, D.M.; Savelkoul, H.F.; Elizalde, M.; Tischmann, L.; de Vos, P.; Masclee, A.A.; Troost, F.J. The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly. Nutrients 2019, 11, 1554. [Google Scholar] [CrossRef]
- Russo, F.; Linsalata, M.; Clemente, C.; Chiloiro, M.; Orlando, A.; Marconi, E.; Chimienti, G.; Riezzo, G. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr. Res. 2012, 32, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Nikkilä, J.; Jalanka-Tuovinen, J.; Immonen, O.; Rajilić-Stojanović, M.; Kekkonen, R.A.; Palva, A.; de Vos, W.M. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods 2010, 81, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Garcia, J.; Hermes, G.D.; Giatsis, C.; Sipkema, D.; Zoetendal, E.G.; Schaap, P.J.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2016, 5, 1791. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.; Dallinga, J.W.; van de Kant, K.D.; Jobsis, Q.; Moonen, E.J.; van Schayck, O.C.; Dompeling, E.; van Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Hauschild, A.-C.; Fijten, R.; Dallinga, J.; Baumbach, J.; Van Schooten, F. Current breathomics—A review on data pre-processing techniques and machine learning in metabolomics breath analysis. J. Breath Res. 2014, 8, 027105. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Shetty, S.; Blake, T. Tools for microbiome analysis in R. Microbiome Package; GitHub Inc.: San Francisco, CA, USA, 2017; Volume 88, pp. 2012–2017. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version. 2.0-10; Comprehensive R Archive Network: Vienna, Austria, 2013; Volume 2. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Hippe, B.; Zwielehner, J.; Liszt, K.; Lassl, C.; Unger, F.; Haslberger, A.G. Quantification of butyryl CoA: Acetate CoA-transferase genes reveals different butyrate production capacity in individuals according to diet and age. FEMS Microbiol. Lett. 2011, 316, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, F.; Sato, M.; Poco, S.E.; Hashimura, T.; Ikeda, T.; Kalfas, S.; Sundqvist, G.; Hoshino, E. Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: New York, NY, USA, 2015; Volume 3, pp. 1157–1160. [Google Scholar]
- López, R.; Smith, P.C.; Göstemeyer, G.; Schwendicke, F. Ageing, dental caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S145–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, T.S.B.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E. Extensive transmission of microbes along the gastrointestinal tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Yang, J.; Summanen, P.H.; Henning, S.M.; Hsu, M.; Lam, H.M.; Huang, J.; Tseng, C.-H.; Dowd, S.E.; Finegold, S.M.; Heber, D. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study. Front. Physiol. 2015, 6, 216. [Google Scholar] [CrossRef]
- Abtahi, F.; Naghshzan, A.; Zibaeenezhad, M.J.; Heydari, S.T.; Khosropanah, S.; Zamirian, M.; Aghasadeghi, K.; Moaref, A. The relationship between body mass index and pre-diabetes in teachers residing in shiraz-Iran 2009. Iran. Cardiovasc. Res. J. 2010, 4, 112–117. [Google Scholar]
- Kleessen, B.; Sykura, B.; Zunft, H.-J.; Blaut, M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 1997, 65, 1397–1402. [Google Scholar] [CrossRef]
- Tran, T.T.; Cousin, F.J.; Lynch, D.B.; Menon, R.; Brulc, J.; Brown, J.R.-M.; O’Herlihy, E.; Butto, L.F.; Power, K.; Jeffery, I.B. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 2019, 7, 39. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A. Unsaturated oligogalacturonic acids are generated by in vitro treatment of pectin with human faecal flora. Carbohydr. Res. 1998, 314, 237–244. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.-H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef]
- Khalid, T.; Richardson, P.; Probert, C.S. The liver breath! Breath volatile organic compounds for the diagnosis of liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 524–526. [Google Scholar] [CrossRef]
- Smolinska, A.; Bodelier, A.; Dallinga, J.; Masclee, A.; Jonkers, D.; van Schooten, F.J.; Pierik, M. The potential of volatile organic compounds for the detection of active disease in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 1244–1254. [Google Scholar] [CrossRef]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.; Wijmenga, C.; Van Schooten, F. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.; Ranieri, T.; Carratù, P.; Resta, O. Influence of age on exhaled breath profile analyzed by an electronic nose in normal subjects. Eur. Respir. J. 2015, 46, PA2093. [Google Scholar]
- Kistler, M.; Szymczak, W.; Fedrigo, M.; Fiamoncini, J.; Höllriegl, V.; Hoeschen, C.; Klingenspor, M.; de Angelis, M.H.; Rozman, J. Effects of diet-matrix on volatile organic compounds in breath in diet-induced obese mice. J. Breath Res. 2014, 8, 016004. [Google Scholar] [CrossRef]
- Ajibola, O.A.; Smith, D.; Španěl, P.; Ferns, G.A. Effects of dietary nutrients on volatile breath metabolites. J. Nutr. Sci. 2013, 2, e34. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Bergmann, A.; Steffens, M.; Trefz, P.; Ziller, M.; Miekisch, W.; Schubert, J.S.; Köhler, H.; Reinhold, P. Impact of food intake on in vivo VOC concentrations in exhaled breath assessed in a caprine animal model. J. Breath Res. 2015, 9, 047113. [Google Scholar] [CrossRef]
- Smolinska, A.; Baranska, A.; Dallinga, J.; Mensink, R.; Baumgartner, S.; van de Heijning, B.; van Schooten, F. Comparing patterns of volatile organic compounds exhaled in breath after consumption of two infant formulae with a different lipid structure: A randomized trial. Sci. Rep. 2019, 9, 554. [Google Scholar] [CrossRef]
- Raninen, K.J.; Lappi, J.E.; Mukkala, M.L.; Tuomainen, T.-P.; Mykkänen, H.M.; Poutanen, K.S.; Raatikainen, O.J. Fiber content of diet affects exhaled breath volatiles in fasting and postprandial state in a pilot crossover study. Nutr. Res. 2016, 36, 612–619. [Google Scholar] [CrossRef]



| Young Adult (n = 52) | Elderly (n = 48) | All Young Adults vs. All Elderly | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 27) | Pectin (n = 25) | p-Value | Placebo (n = 24) | Pectin (n = 24) | p-Value | p-Value | |
| Age (years) | 22.8 ± 4.1 | 23.4 ± 4.5 | 0.614 | 69.8 ± 2.4 | 69.5 ± 3.2 | 0.723 | <0.001 |
| Female (%) | 48.2 | 68.0 | 0.148 | 50.0 | 37.5 | 0.383 | 0.164 |
| BMI (kg/m2) | 22.6 ± 2.7 | 23.2 ± 2.7 | 0.444 | 26.2 ± 2.8 | 25.5 ± 2.6 | 0.334 | <0.001 |
| Medication (%) | 0 | 0 | 1.000 | 33.3 | 45.8 | 0.376 | <0.001 |
| PPI (%) | 0 | 0 | 1.000 | 12.5 | 12.5 | 1.000 | <0.001 |
| Statins (%) | 0 | 0 | 1.000 | 4.2 | 4.2 | 1.000 | <0.001 |
| Antihypertensives (%) | 0 | 0 | 1.000 | 8.3 | 12.5 | 0.637 | <0.001 |
| Other medication (%) | 0 | 0 | 1.000 | 12.5 | 16.7 | 0.683 | <0.001 |
| Young Adults (n = 52) | Elderly (n = 48) | p-Value * | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Acetic acid | 225.9 | 187.6 | 201.6 | 145.2 | 0.469 |
| Propionic acid | 71.1 | 66.4 | 58.1 | 53.2 | 0.281 |
| Butyric acid | 59.2 | 45.0 | 56.6 | 49.8 | 0.785 |
| Valeric acid | 8.4 | 6.4 | 9.3 | 6.7 | 0.473 |
| Isobutyric acid | 6.8 | 3.7 | 7.2 | 6.0 | 0.715 |
| Isovaleric acid | 10.6 | 5.6 | 11.1 | 9.0 | 0.729 |
| Intervention | Young Adults | Elderly | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value * (Placebo vs. Pectin) | Pre-Intervention | Post-Intervention | p-Value * (Placebo vs. Pectin) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Acetic acid | Placebo | 210.2 | 182.7 | 263.7 | 233.3 | 0.202 | 167.9 | 95.0 | 230.5 | 188.1 | 0.548 |
| Pectin | 242.8 | 195.1 | 237.8 | 222.4 | 235.3 | 178.0 | 268.4 | 155.2 | |||
| Butyric acid | Placebo | 56.1 | 41.5 | 77.5 | 55.8 | 0.066 | 44.1 | 25.8 | 56.3 | 46.8 | 0.280 |
| Pectin | 62.6 | 49.1 | 61.1 | 47.2 | 69.2 | 63.9 | 67.3 | 37.5 | |||
| Isobutyric acid | Placebo | 6.2 | 2.4 | 7.9 | 4.9 | 0.495 | 6.1 | 3.7 | 7.2 | 4.3 | 0.290 |
| Pectin | 7.5 | 4.8 | 8.2 | 5.3 | 8.3 | 7.5 | 7.8 | 4.0 | |||
| Isovaleric acid | Placebo | 10.1 | 3.5 | 12.4 | 8.0 | 0.654 | 9.6 | 6.0 | 10.9 | 6.2 | 0.364 |
| Pectin | 11.2 | 7.2 | 12.5 | 8.2 | 12.7 | 11.2 | 11.8 | 6.4 | |||
| Propionic acid | Placebo | 71.0 | 69.6 | 99.4 | 131.0 | 0.074 | 40.7 | 18.0 | 52.6 | 32.5 | 0.752 |
| Pectin | 71.2 | 64.3 | 66.8 | 49.0 | 75.6 | 69.4 | 81.8 | 43.8 | |||
| Valeric acid | Placebo | 7.1 | 5.1 | 10.7 | 13.5 | 0.113 | 7.9 | 4.3 | 9.4 | 7.2 | 0.391 |
| Pectin | 9.8 | 7.4 | 9.6 | 5.5 | 10.8 | 8.3 | 10.3 | 4.1 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Wilms, E.; Smolinska, A.; Hermes, G.D.A.; Masclee, A.A.M.; de Vos, P.; Schols, H.A.; van Schooten, F.J.; Smidt, H.; Jonkers, D.M.A.E.; et al. Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly. Nutrients 2019, 11, 2193. https://doi.org/10.3390/nu11092193
An R, Wilms E, Smolinska A, Hermes GDA, Masclee AAM, de Vos P, Schols HA, van Schooten FJ, Smidt H, Jonkers DMAE, et al. Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly. Nutrients. 2019; 11(9):2193. https://doi.org/10.3390/nu11092193
Chicago/Turabian StyleAn, Ran, Ellen Wilms, Agnieszka Smolinska, Gerben D.A. Hermes, Ad A.M. Masclee, Paul de Vos, Henk A. Schols, Frederik J. van Schooten, Hauke Smidt, Daisy M.A.E. Jonkers, and et al. 2019. "Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly" Nutrients 11, no. 9: 2193. https://doi.org/10.3390/nu11092193









